β-Elemene Attenuates Renal Fibrosis in the Unilateral Ureteral Obstruction Model by Inhibition of STAT3 and Smad3 Signaling via Suppressing MyD88 Expression
Abstract
:1. Introduction
2. Results
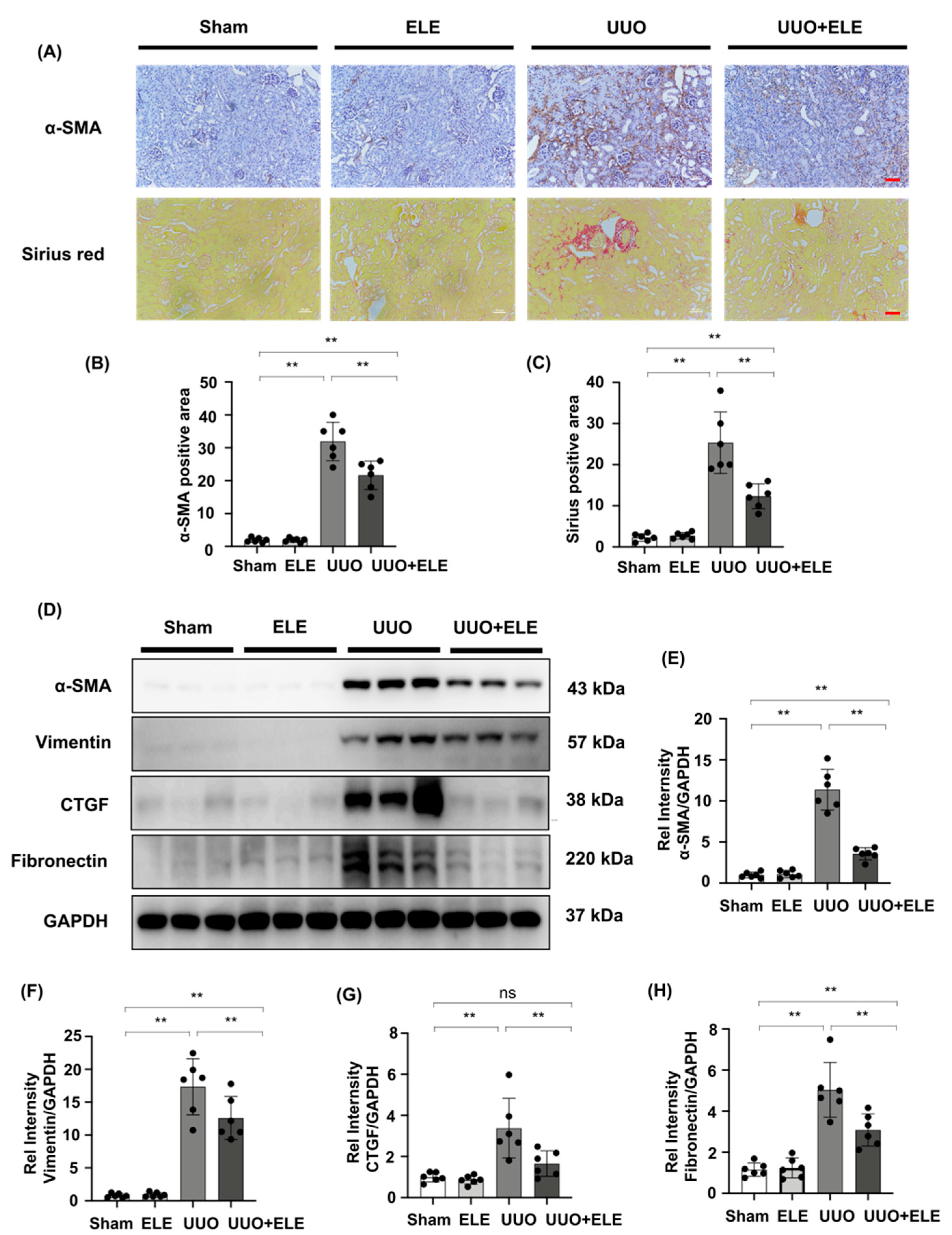
2.1. β-Elemene Ameliorated Histopathological Alterations in UUO Mice
2.2. β-Elemene Attenuated the Expression of Fibrosis-Related Markers in UUO Mice
2.3. β-Elemene Inhibited TGF-β Stimulated Fibroblast Activation in NRK49F Cells
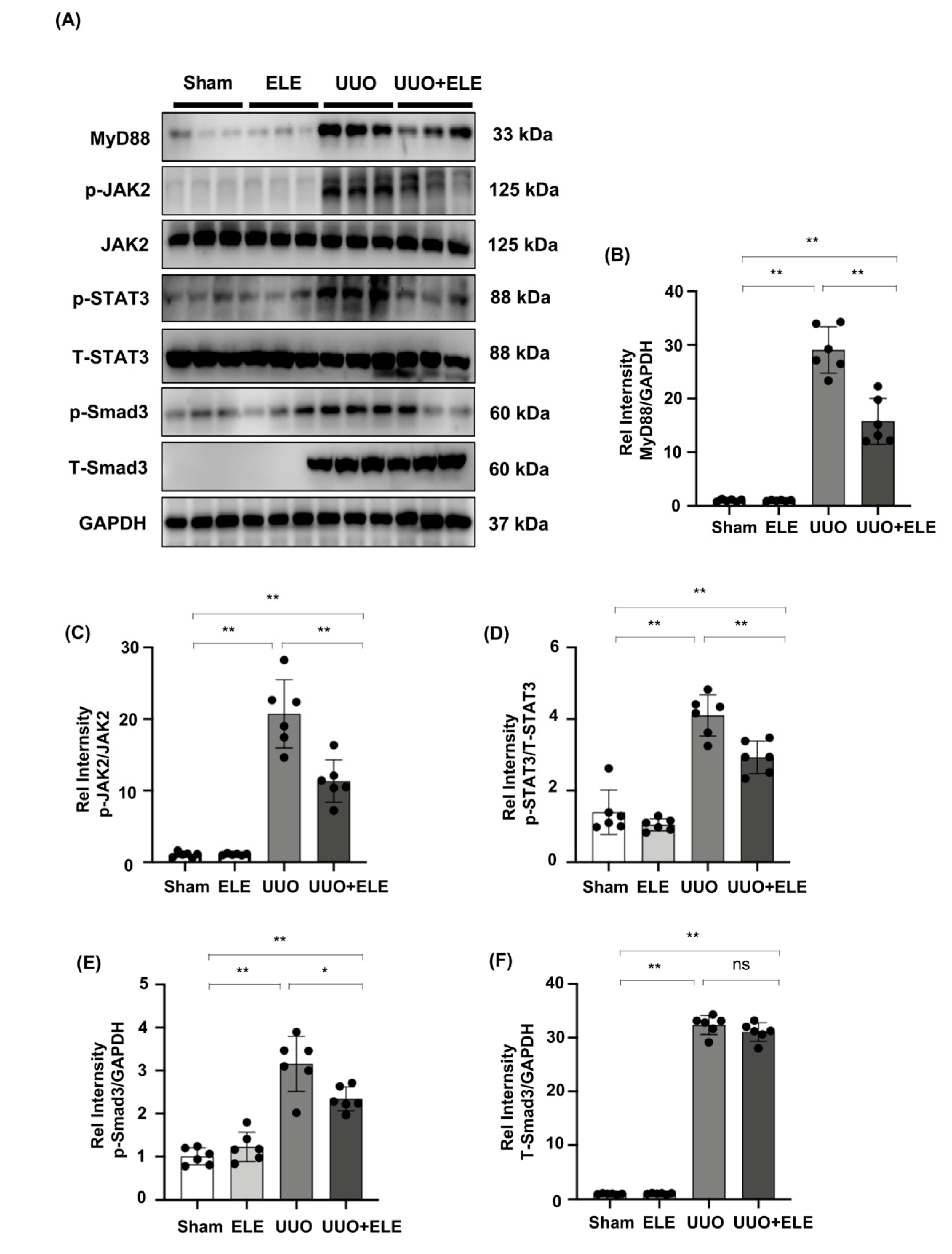
2.4. β-Elemene Inhibited the Phosphorylation of JAK2/STAT3 and Smad3 and Suppressed the Expression of MyD88 in the UUO Model
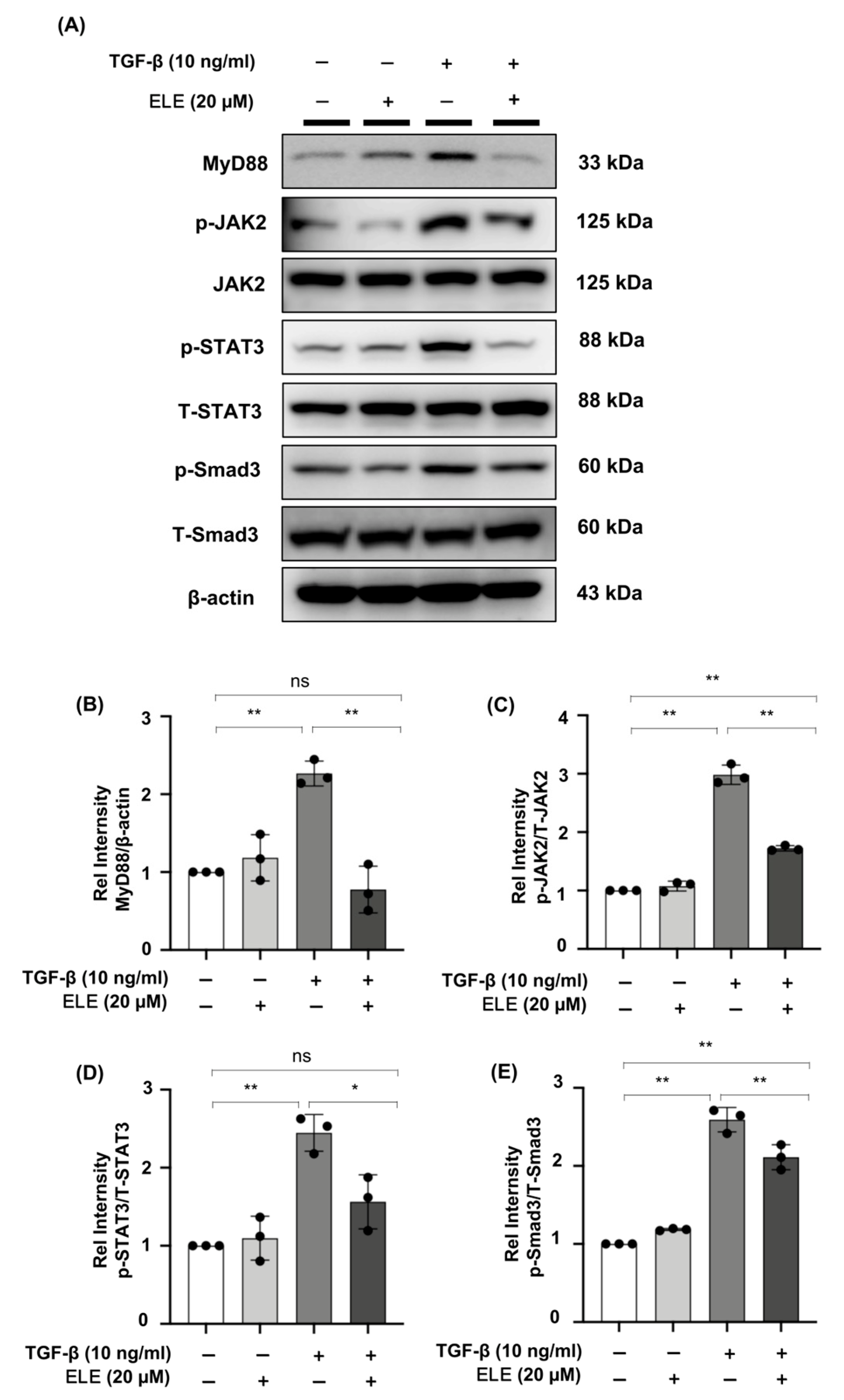
2.5. β-Elemene Down-Regulated the Phosphorylation of JAK2/STAT3 and Smad3 and Inhibited the Expression of MyD88 in TGF-β-Stimulated NRK49F Cells
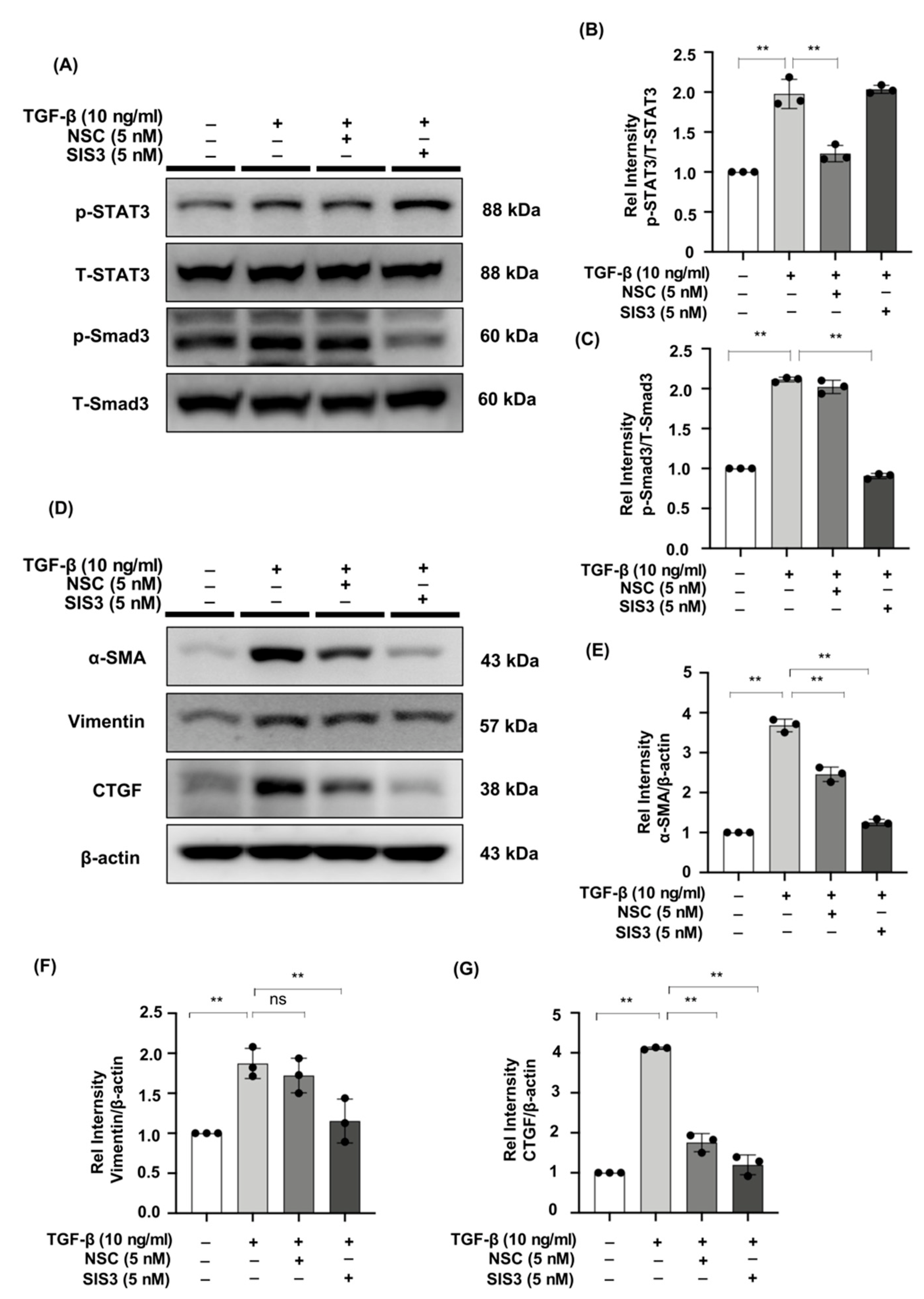
2.6. The STAT3 Inhibitor, NSC, and the Smad3 Inhibitor, SIS3 Reduced the Expression of Fibrotic Markers in TGF-β-Stimulated NRK49F Cells
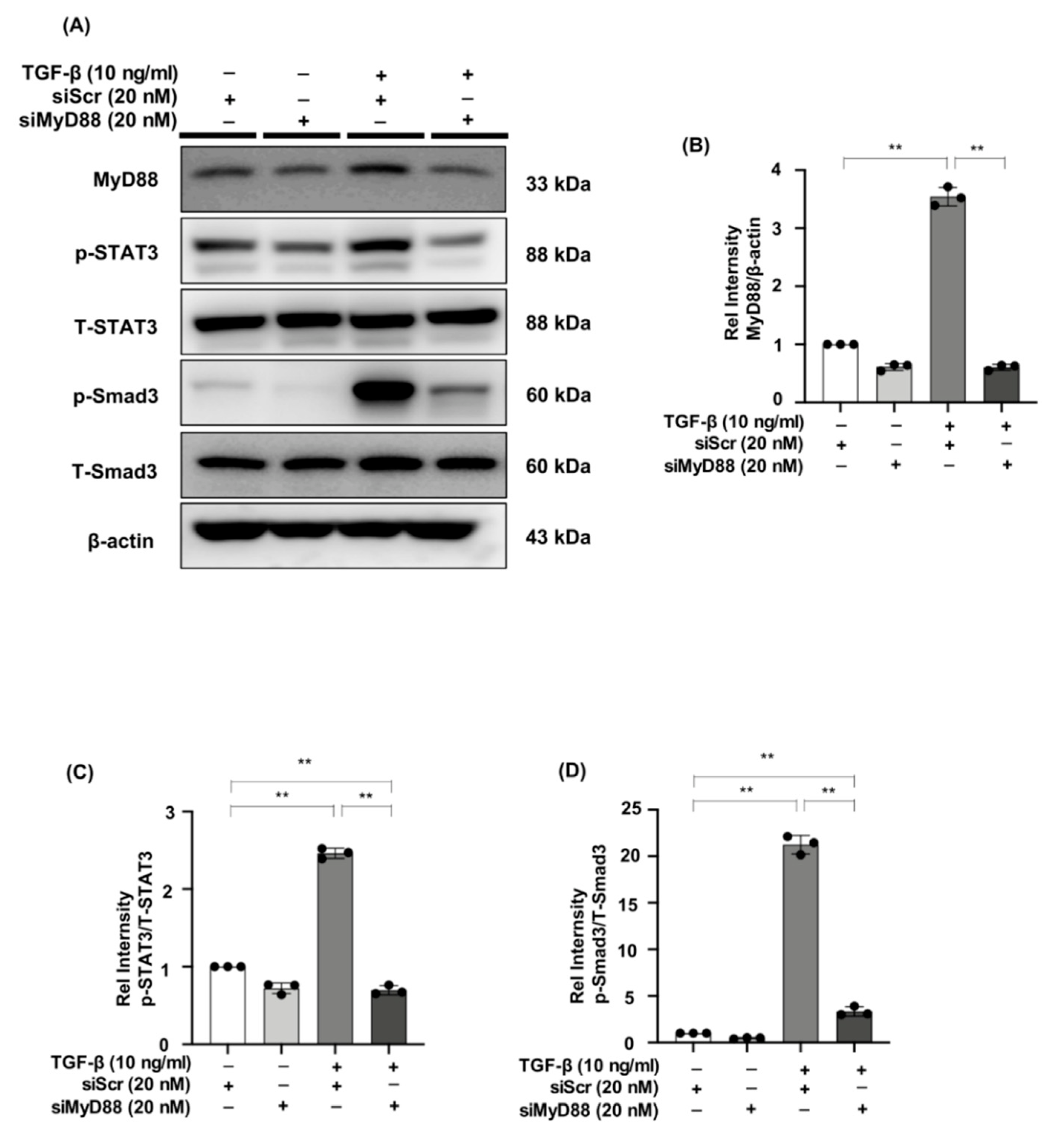
2.7. MyD88 Silencing Suppresses STAT3 and Smad3 Phosphorylation in TGF-β Stimulated NRK49F Cells
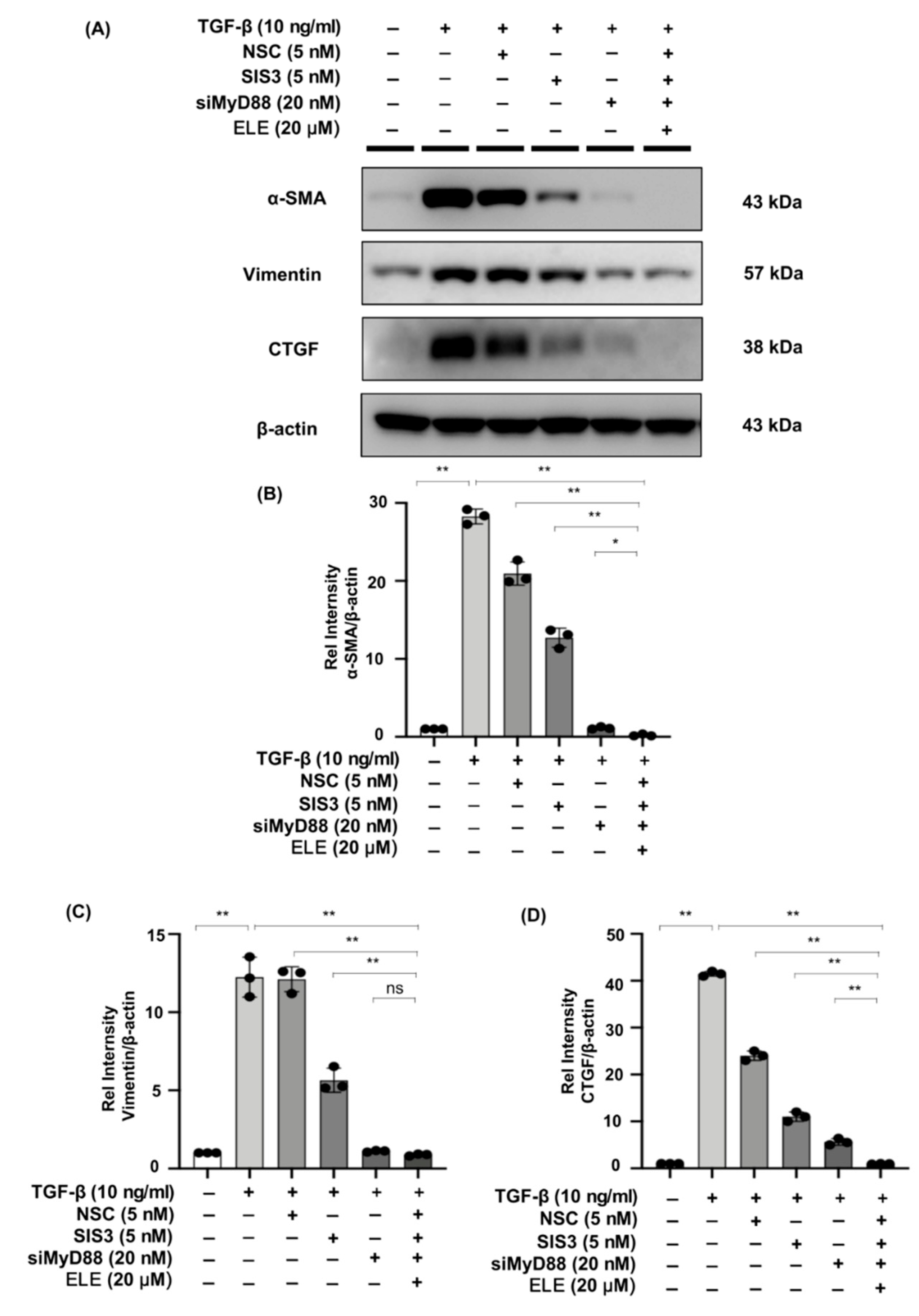
2.8. Co-Treatment of β-Elemene with Specific STAT3 Phosphorylation Inhibitor, Smad3 Phosphorylation Inhibitor, and siMyD88 Effectively Reduces Fibrosis in TGF-β Stimulated NRK49F Cells
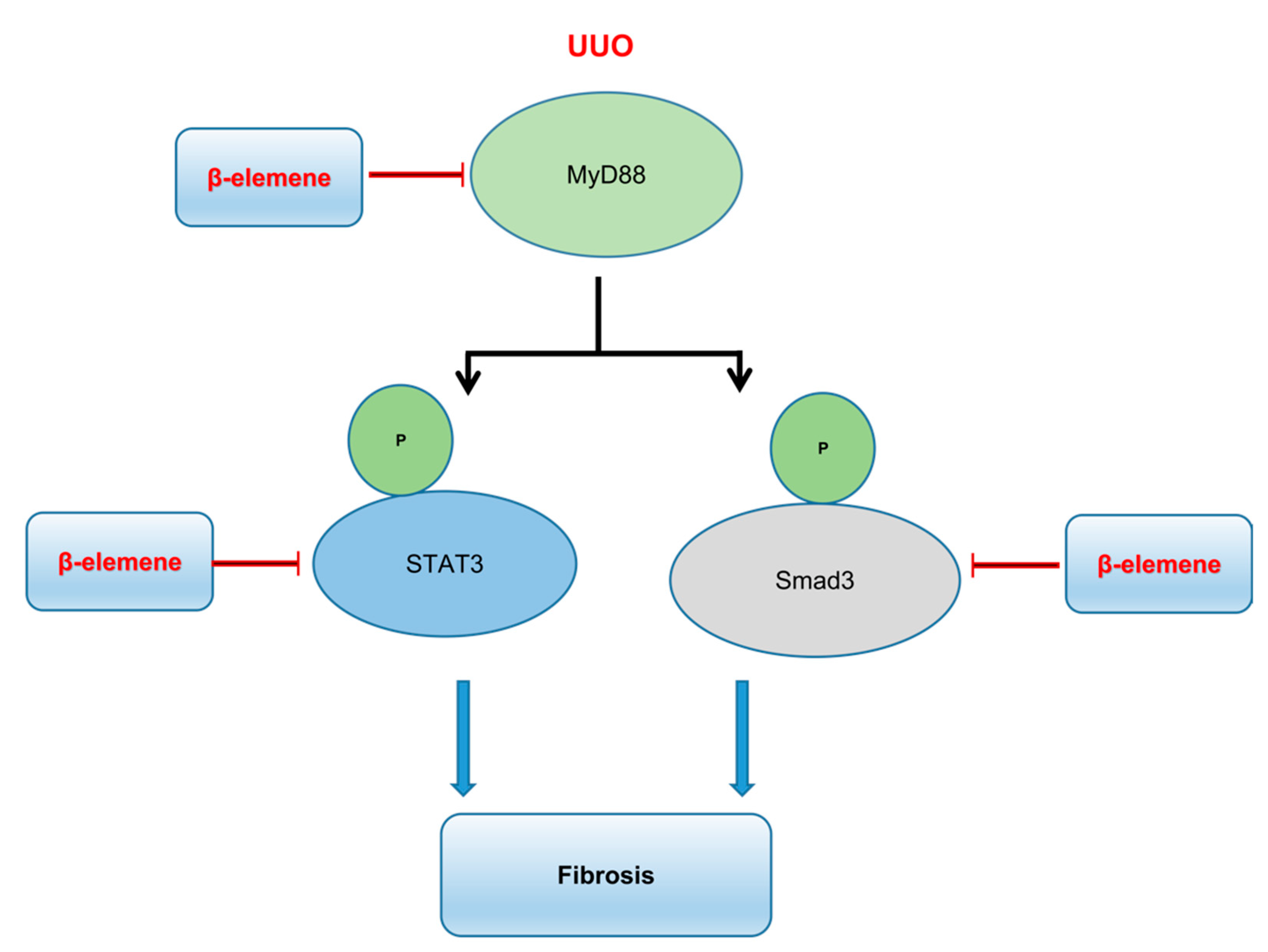
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animal Experiments
4.3. Cell Culture and Treatment
4.4. Histology
4.5. Immunohistochemistry and Immunofluorescence Staining
4.6. Cytotoxicity Assay
4.7. siRNA Knockdown
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-Cause and cause-Specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [Green Version]
- Mohany, M.; Ahmed, M.M.; Al-Rejaie, S.S. Molecular Mechanistic Pathways Targeted by Natural Antioxidants in the Prevention and Treatment of Chronic Kidney Disease. Antioxidants 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Lamas, S.; Ortiz, A. Antifibrotic Agents for the Management of CKD: A Review. Am. J. Kidney Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Campillo, S.; Rodrigues-Diez, R.R.; Tejera-Muñoz, A.; Marquez-Exposito, L.; Goldschmeding, R.; Rodríguez-Puyol, D.; Calleros, L.; Ruiz-Ortega, M. Interplay between extracellular matrix components and cellular and molecular mechanisms in kidney fibrosis. Clin. Sci. 2021, 135, 1999–2029. [Google Scholar] [CrossRef]
- Grande, M.T.; López-Novoa, J.M. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol. 2009, 5, 319–328. [Google Scholar] [CrossRef]
- Kaissling, B.; LeHir, M.; Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.Q.; De Broe, M.E.; Nouwen, E.J. Vimentin expression and distal tubular damage in the rat kidney. Exp. Nephrol. 1996, 4, 172–183. [Google Scholar]
- Yin, Q.; Liu, H. Connective Tissue Growth Factor and Renal Fibrosis. Ren. Fibros. 2019, 1165, 365–380. [Google Scholar] [CrossRef]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, R.L.; Forbes, M.S.; Thornhill, B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009, 75, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- Chaabane, W.; Praddaude, F.; Buleon, M.; Jaafar, A.; Vallet, M.; Rischmann, P.; Galarreta, C.I.; Chevalier, R.L.; Tack, I. Renal functional decline and glomerulotubular injury are arrested but not restored by release of unilateral ureteral obstruction (UUO). Am. J. Physiol. Physiol. 2013, 304, F432–F439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Shao, X.; Tian, L.; Gu, L.; Zhang, M.; Wang, Q.; Wu, B.; Wang, L.; Yao, J.; Xu, X.; et al. Astragaloside IV Ameliorates Renal Fibrosis via the Inhibition of Mitogen-Activated Protein Kinases and Antiapoptosis In Vivo and In Vitro. J. Pharmacol. Exp. Ther. 2014, 350, 552–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ucero, A.C.; Benito-Martin, A.; Izquierdo, M.C.; Sanchez-Niño, M.D.; Sanz, A.B.; Ramos, A.M.; Berzal, S.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Unilateral ureteral obstruction: Beyond obstruction. Int. Urol. Nephrol. 2013, 46, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Humeres, C.; Frangogiannis, N.G. The role of Smad signaling cascades in cardiac fibrosis. Cell. Signal. 2020, 77, 109826. [Google Scholar] [CrossRef]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signal-ing in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar]
- Verrecchia, F.; Vindevoghel, L.; Lechleider, R.J.; Uitto, J.; Roberts, A.B.; Mauviel, A. Smad3/AP-1 interactions control transcriptional responses to TGF-β in a promoter-specific manner. Oncogene 2001, 20, 3332–3340. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wu, Z.; Phan, S. Smad3 Mediates Transforming Growth Factor-β–Induced α-Smooth Muscle Actin Expression. Am. J. Respir. Cell Mol. Biol. 2003, 29, 397–404. [Google Scholar] [CrossRef]
- Yang, F.; Chung, A.C.; Huang, X.R.; Lan, H.Y. Angiotensin II Induces Connective Tissue Growth Factor and Collagen I Expression via Transforming Growth Factor–β–Dependent and –Independent Smad Pathways. Hypertension 2009, 54, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. New Insights into Epithelial-Mesenchymal Transition in Kidney Fibrosis. J. Am. Soc. Nephrol. 2009, 21, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Kunnumakkara, A.B.; Harikumar, K.B.; Gupta, S.R.; Tharakan, S.T.; Koca, C.; Dey, S.; Sung, B. Signal Transducer and Activator of Transcription-3, Inflammation, and Cancer: How Intimate Is the Relationship? Ann. N. Y. Acad. Sci. 2009, 1171, 59–76. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Peng, D.; Shi, W.; Guo, J.; Huo, S.; Men, L.; Zhang, C.; Li, S.; Lv, J.; Lin, L. IL-6/STAT3 Signaling Promotes Cardiac Dysfunction by Upregulating FUNDC1-Dependent Mitochondria-Associated Endoplasmic Reticulum Membranes Formation in Sepsis Mice. Front. Cardiovasc. Med. 2022, 8, 790612. [Google Scholar] [CrossRef]
- El-Tanani, M.; Al Khatib, A.O.; Aladwan, S.M.; Abuelhana, A.; McCarron, P.A.; Tambuwala, M.M. Importance of STAT3 signalling in cancer, metastasis and therapeutic interventions. Cell. Signal. 2022, 92, 110275. [Google Scholar] [CrossRef]
- Huang, G.; Yan, H.; Ye, S.; Tong, C.; Ying, Q. STAT3 Phosphorylation at Tyrosine 705 and Serine 727 Differentially Regulates Mouse ESC Fates. Stem Cells 2013, 32, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal. Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Kasembeli, M.M.; Bharadwaj, U.; Robinson, P.; Tweardy, D.J. Contribution of STAT3 to Inflammatory and Fibrotic Diseases and Prospects for its Targeting for Treatment. Int. J. Mol. Sci. 2018, 19, 2299. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, D.; Šumová, B.; Mallano, T.; Chen, C.-W.; Distler, A.; Bergmann, C.; Ludolph, I.; Horch, R.E.; Gelse, K.; Ramming, A.; et al. Activation of STAT3 integrates common pro-fibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat. Commun. 2017, 8, 1130. [Google Scholar] [CrossRef] [Green Version]
- Kuratsune, M.; Masaki, T.; Hirai, T.; Kiribayashi, K.; Yokoyama, Y.; Arakawa, T.; Yorioka, N.; Kohno, N. Signal transducer and activator of transcription 3 involvement in the development of renal interstitial fibrosis after unilateral ureteral obstruction. Nephrology 2007, 12, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Shen, Y.; Wang, Z.; Shao, D.-C.; Liu, J.; Fu, L.-J.; Kong, Y.-L.; Zhou, L.; Xue, H.; Huang, Y.; et al. Inhibition of STAT3 acetylation is associated with attenuated renal fibrosis in the obstructed kidney. Acta Pharmacol. Sin. 2014, 35, 1045–1054. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Jang, E.; Lee, J.H. Preclinical Evidence of Curcuma longa and Its Noncurcuminoid Constituents against Hepatobiliary Diseases: A Review. Evid. Based Complement. Altern. Med. 2020, 2020, 8761435. [Google Scholar] [CrossRef]
- Bai, Z.; Yao, C.; Zhu, J.; Xie, Y.; Ye, X.-Y.; Bai, R.; Xie, T. Anti-Tumor Drug Discovery Based on Natural Product β-Elemene: Anti-Tumor Mechanisms and Structural Modification. Molecules 2021, 26, 1499. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Z.; Chen, J.; Zheng, Y.; Limsila, B.; Lu, M.; Gao, T.; Yang, Q.; Fu, C.; Liao, W. Terpenoids from Curcumae Rhizoma: Their anti-cancer effects and clinical uses on combination and versus drug therapies. Biomed. Pharmacother. 2021, 138, 111350. [Google Scholar] [CrossRef]
- Dai, Z.-J.; Tang, W.; Lu, W.-F.; Gao, J.; Kang, H.-F.; Ma, X.-B.; Min, W.-L.; Wang, X.-J.; Wu, W.-Y. Antiproliferative and apoptotic effects of β-elemene on human hepatoma HepG2 cells. Cancer Cell Int. 2013, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, Z.; Gao, J.; Xie, J.; Yang, L.; Hu, S. Downregulation effects of beta-elemene on the levels of plasma en-dotoxin, serum TNF-alpha, and hepatic CD14 expression in rats with liver fibrosis. Front. Med. 2011, 5, 101–105. [Google Scholar] [CrossRef]
- George, C.; Mogueo, A.; Okpechi, I.; Echouffo-Tcheugui, J.B.; Kengne, A.P. Chronic kidney disease in low-income to middle-income countries: The case for increased screening. BMJ Glob. Health 2017, 2, e000256. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.; Cooper, M.E.; Jandeleit-Dahm, K.A. Cardiovascular Disease and Diabetic Kidney Disease. Semin. Nephrol. 2018, 38, 217–232. [Google Scholar] [CrossRef]
- Devarajan, P.; Jefferies, J.L. Progression of chronic kidney disease after acute kidney injury. Prog. Pediatr. Cardiol. 2015, 41, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Klinkhammer, B.M.; Goldschmeding, R.; Floege, J.; Boor, P. Treatment of Renal Fibrosis—Turning Challenges into Opportunities. Adv. Chronic Kidney Dis. 2017, 24, 117–129. [Google Scholar] [CrossRef]
- Tampe, D.; Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 226–237. [Google Scholar] [CrossRef]
- Hong, C.; Zhuang, H.; Cai, B.; Chen, J.; Huang, S.; Fang, T. β-Elemene Attenuates Fibrosis after Esophageal Endoscopic Submucosal Dissection via Modulating the HIF-1α/HK2/p38-MAPK Signaling Axis. ACS Biomater. Sci. Eng. 2021, 7, 3399–3408. [Google Scholar] [CrossRef]
- Tang, P.M.K.; Zhang, Y.-Y.; Mak, T.S.-K.; Tang, P.C.-T.; Huang, X.-R.; Lan, H.-Y. Transforming growth factor-β signalling in renal fibrosis: From Smads to non-coding RNAs. J. Physiol. 2018, 596, 3493–3503. [Google Scholar] [CrossRef]
- Lan, H.Y.; Chung, A.C.-K. TGF-β/Smad Signaling in Kidney Disease. Semin. Nephrol. 2012, 32, 236–243. [Google Scholar] [CrossRef]
- Lan, H.Y. Diverse Roles of TGF-β/Smads in Renal Fibrosis and Inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, Y.; Zhang, Y.; Song, J.; Wang, Q.; Zheng, L.; Liu, D. Beta-Elemene Blocks Epithelial-Mesenchymal Transition in Human Breast Cancer Cell Line MCF-7 through Smad3-Mediated Down-Regulation of Nuclear Transcription Factors. PLoS ONE 2013, 8, e58719. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Muragaki, Y.; Saika, S.; Roberts, A.B.; Ooshima, A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Investig. 2003, 112, 1486–1494. [Google Scholar] [CrossRef]
- Inazaki, K.; Kanamaru, Y.; Kojima, Y.; Sueyoshi, N.; Okumura, K.; Kaneko, K.; Yamashiro, Y.; Ogawa, H.; Nakao, A. Smad3 deficiency attenuates renal fibrosis, inflammation, and apoptosis after unilateral ureteral obstruction. Kidney Int. 2004, 66, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Félix, J.M.; González-Núñez, M.; Martínez-Salgado, C.; López-Novoa, J.M. TGF-β/BMP proteins as therapeutic targets in renal fibrosis. Where have we arrived after 25years of trials and tribulations? Pharmacol. Ther. 2015, 156, 44–58. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, Y.; Liu, G.; Zhang, X.; Xiaobai, Z.; Huang, S.; Liu, H.; He, L. Role of Stat3 Signaling in Control of EMT of Tubular Epithelial Cells During Renal Fibrosis. Cell. Physiol. Biochem. 2017, 42, 2552–2558. [Google Scholar] [CrossRef]
- Pang, M.; Ma, L.; Gong, R.; Tolbert, E.; Mao, H.; Ponnusamy, M.; Chin, Y.E.; Yan, H.; Dworkin, L.D.; Zhuang, S. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010, 78, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Skuginna, V.; Lech, M.; Allam, R.; Ryu, M.; Clauss, S.; Susanti, H.E.; Römmele, C.; Garlanda, C.; Mantovani, A.; Anders, H.-J. Toll-Like Receptor Signaling and SIGIRR in Renal Fibrosis upon Unilateral Ureteral Obstruction. PLoS ONE 2011, 6, e19204. [Google Scholar] [CrossRef] [Green Version]
- Braga, T.T.; Correa-Costa, M.; Guise, Y.F.S.; Castoldi, A.; De Oliveira, C.D.; Hyane, M.I.; Cenedeze, M.A.; Teixeira, S.A.; Muscara, M.N.; Perez, K.R.; et al. MyD88 Signaling Pathway Is Involved in Renal Fibrosis by Favoring a TH2 Immune Response and Activating Alternative M2 Macrophages. Mol. Med. 2012, 18, 1231–1239. [Google Scholar] [CrossRef]
- Sun, W.; Byon, C.H.; Kim, D.H.; Choi, H.I.; Park, J.S.; Joo, S.Y.; Kim, I.J.; Jung, I.; Bae, E.H.; Ma, S.K.; et al. Renoprotective Effects of Maslinic Acid on Experimental Renal Fibrosis in Unilateral Ureteral Obstruction Model via Targeting MyD88. Front. Pharmacol. 2021, 12, 708575. [Google Scholar] [CrossRef]
- Fu, X.-Q.; Liu, B.; Wang, Y.-P.; Li, J.-K.; Zhu, P.-L.; Li, T.; Tse, K.-W.; Chou, J.-Y.; Yin, C.-L.; Bai, J.-X.; et al. Activation of STAT3 is a key event in TLR4 signaling-mediated melanoma progression. Cell Death Dis. 2020, 11, 246. [Google Scholar] [CrossRef]
- Samba-Mondonga, M.; Calvé, A.; Mallette, F.A.; Santos, M.M. MyD88 Regulates the Expression of SMAD4 and the Iron Regulatory Hormone Hepcidin. Front. Cell Dev. Biol. 2018, 6, 105. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, J.S.; Kim, J.H.; Jung, S.M.; Lee, J.Y.; Kim, S.-J.; Park, S.H. Smad6-specific recruitment of Smurf E3 ligases mediates TGF-β1-induced degradation of MyD88 in TLR4 signalling. Nat. Commun. 2011, 2, 460. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Du, D.; Zhang, L.; Yang, Y.; Zou, Z.; Li, Z.; Zhou, L.; Shang, R.; Zhou, P. TJ-M2010-5, A self-developed MyD88 inhibitor, attenuates liver fibrosis by inhibiting the NF-κB pathway. Chem. Interact. 2022, 354, 109839. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, I.J.; Bae, E.H.; Ma, S.K.; Lee, J.; Kim, S.W. Angiotensin-(1-7) Attenuates Kidney Injury Due to Obstructive Nephropathy in Rats. PLoS ONE 2015, 10, e0142664. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.-T.; Bai, Y.; Li, J.; Qiao, Y.; Liu, Y.; Zheng, J. Elemene Emulsion Injection Administration Reduces Neuropathic Pain by Inhibiting Astrocytic NDRG2 Expression within Spinal Dorsal Horn. Chin. J. Integr. Med. 2021, 27, 912–918. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Kim, D.H.; Byon, C.H.; Choi, H.I.; Park, J.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. β-Elemene Attenuates Renal Fibrosis in the Unilateral Ureteral Obstruction Model by Inhibition of STAT3 and Smad3 Signaling via Suppressing MyD88 Expression. Int. J. Mol. Sci. 2022, 23, 5553. https://doi.org/10.3390/ijms23105553
Sun W, Kim DH, Byon CH, Choi HI, Park JS, Bae EH, Ma SK, Kim SW. β-Elemene Attenuates Renal Fibrosis in the Unilateral Ureteral Obstruction Model by Inhibition of STAT3 and Smad3 Signaling via Suppressing MyD88 Expression. International Journal of Molecular Sciences. 2022; 23(10):5553. https://doi.org/10.3390/ijms23105553
Chicago/Turabian StyleSun, Wenjuan, Dong Hyun Kim, Chang Hyun Byon, Hoon In Choi, Jung Sun Park, Eun Hui Bae, Seong Kwon Ma, and Soo Wan Kim. 2022. "β-Elemene Attenuates Renal Fibrosis in the Unilateral Ureteral Obstruction Model by Inhibition of STAT3 and Smad3 Signaling via Suppressing MyD88 Expression" International Journal of Molecular Sciences 23, no. 10: 5553. https://doi.org/10.3390/ijms23105553
APA StyleSun, W., Kim, D. H., Byon, C. H., Choi, H. I., Park, J. S., Bae, E. H., Ma, S. K., & Kim, S. W. (2022). β-Elemene Attenuates Renal Fibrosis in the Unilateral Ureteral Obstruction Model by Inhibition of STAT3 and Smad3 Signaling via Suppressing MyD88 Expression. International Journal of Molecular Sciences, 23(10), 5553. https://doi.org/10.3390/ijms23105553





