Extracellular Polymeric Substances and Biocorrosion/Biofouling: Recent Advances and Future Perspectives
Abstract
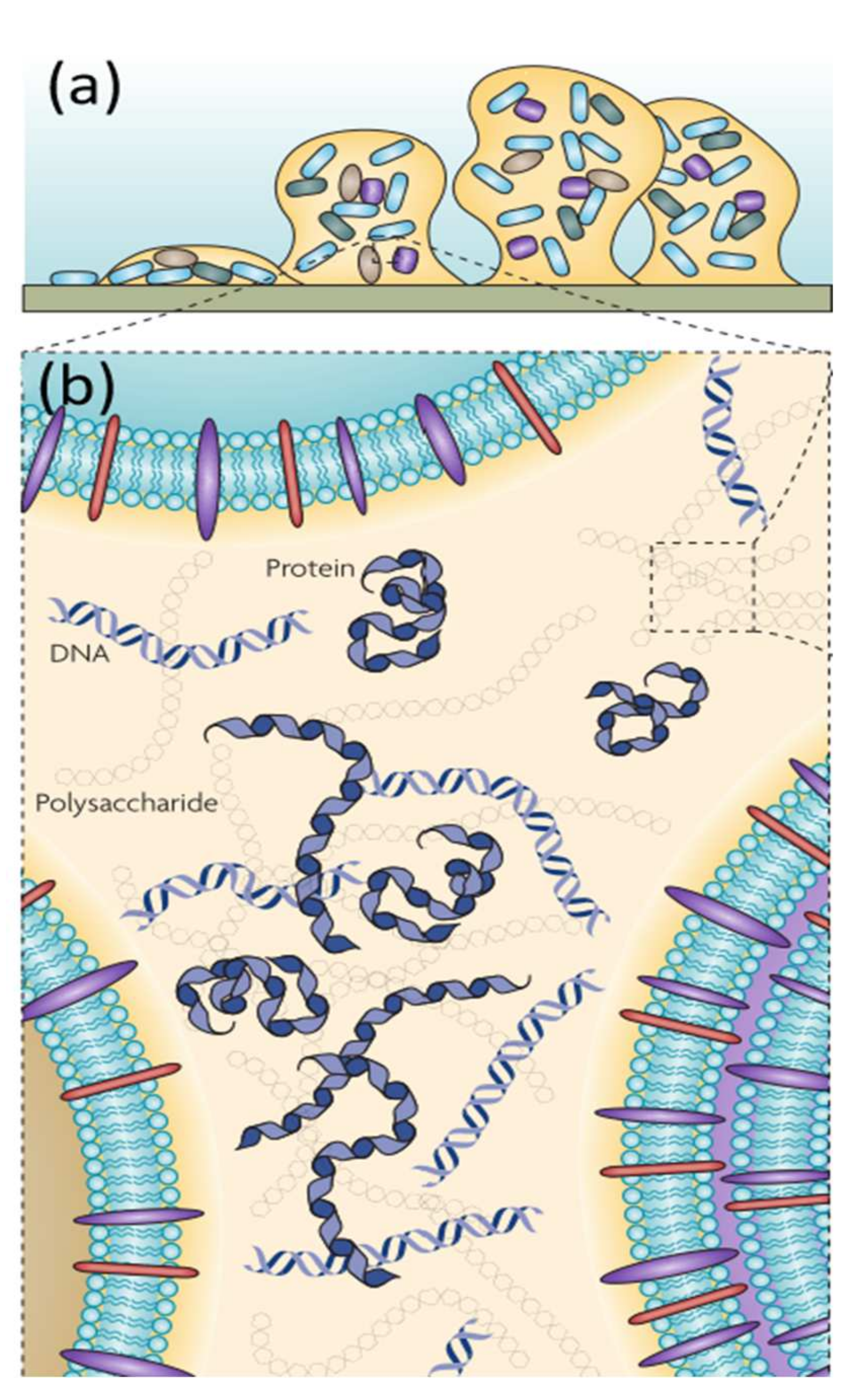
:1. Introduction
| Type | Aerobic/Anaerobic | Corrosion Agents | Mechanism of Corrosion | References |
|---|---|---|---|---|
| Sulfate reducers Desulfovibrio sp. Desulfomonas sp. Desulfotomaculum kuznetsovii Archaeoglobus fulgidus | Anaerobic | H2S and FeS | Cathodic depolarization by hydrogen uptake, anodic depolarization by corrosive iron sulfides, electrons extracted from Fe0 | [6,8,11,27,28,29] |
| Iron oxidizers/manganese oxidizers Gallionella sp. Mariprofundus ferrooxydans Leptothrix sp. Mariprofundus sp. Bacillus sp. | Aerobic | Fe2+ to Fe3+ and Mn2+ to Mn4+: Iron oxide and manganese dioxide formation | Deposition of cathodically reactive ferric and manganic oxides | [14,15,30,31,32,33] |
| Iron reducers Pseudomonas sp. Shewanella sp. Geobacter sulfurreducens | Aerobic | Reduce Fe3+ to Fe2+, Mn4+ to Mn2+ manganese or iron oxide reduction | Reduction of iron and manganese oxides | [15,31,34] |
| Sulfur compound oxidizers Thiobacillus sp. Acidithiobacillus ferrooxidans Acidithiobacillus caldus | Aerobic | H2SO4 | Acids corrode metal | [35,36,37] |
| Acid producing bacteria and fungi Clostridium sp. Fusarium sp. Penicillium sp. Hormoconis sp. Bacillus subtilis Marinobacter sp. | Aerobic and anaerobic | Acids | Dissolve iron, chelate copper, zinc, and iron | [38,39,40,41,42] |
| Slime (EPS) forming bacteria/almost all microorganism Clostridium sp. Bacillus sp. Desulfovibrio sp. Pseudomonas sp. | Aerobic and anaerobic | extracellular polymeric substances (biofilm) or surface compounds/ions | Exopolymers capable of binding metal ions | [43,44,45,46] |
| Methanogens Methanobacterium sp. Methanococcus sp. | Anaerobic | Extracellar hydrogenases, acids, and CO2 | Methane production with direct iron oxidation; syntrophic interaction with fermentative microbes or SRP; deposition of cathodically reactive ferric oxides; consumption of hydrogen generated by CO2 corrosion | [17,47,48,49] |
2. EPS Properties
2.1. Components
2.2. Adhesion
2.3. Redox Active EPS and Role in Electron Transfer
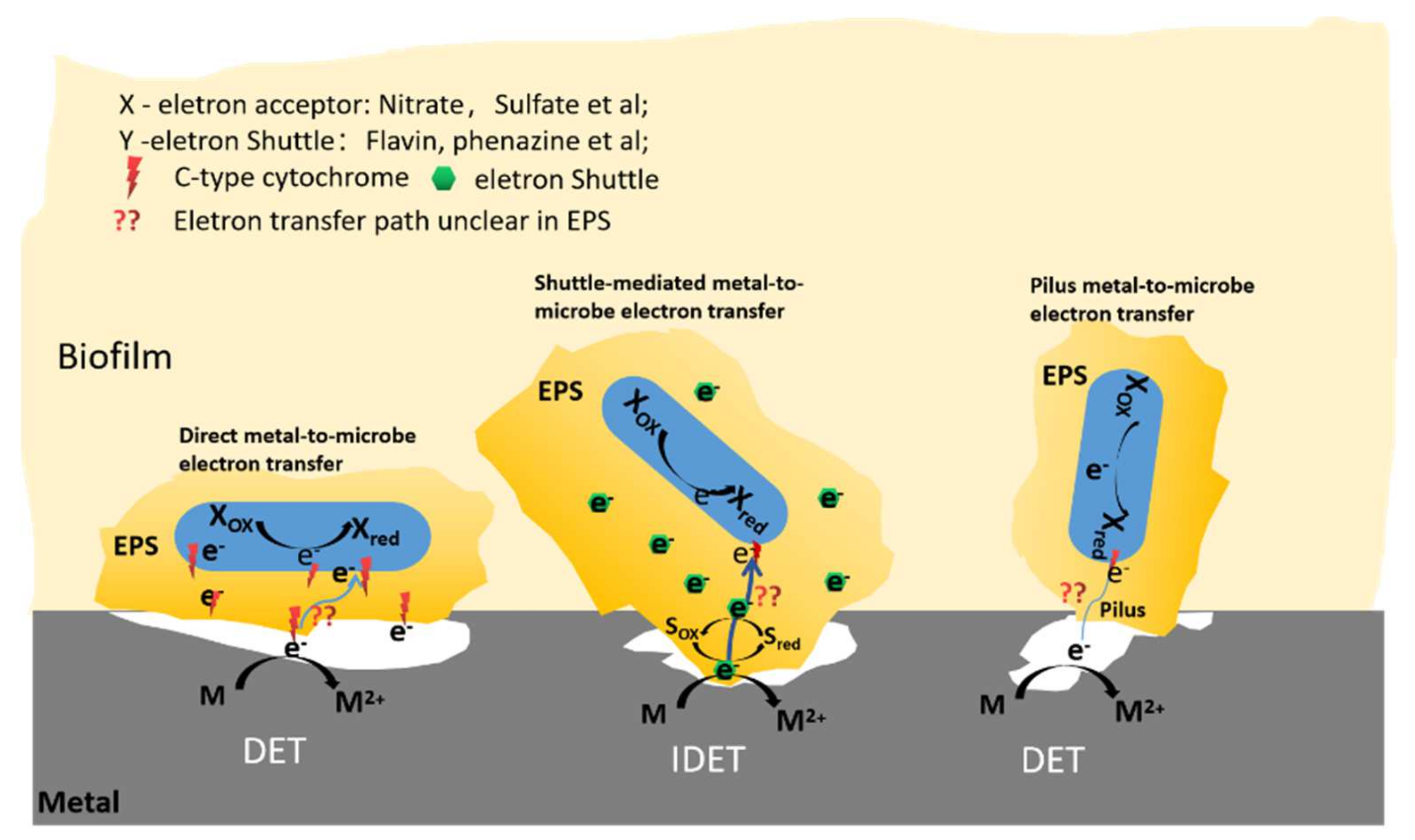
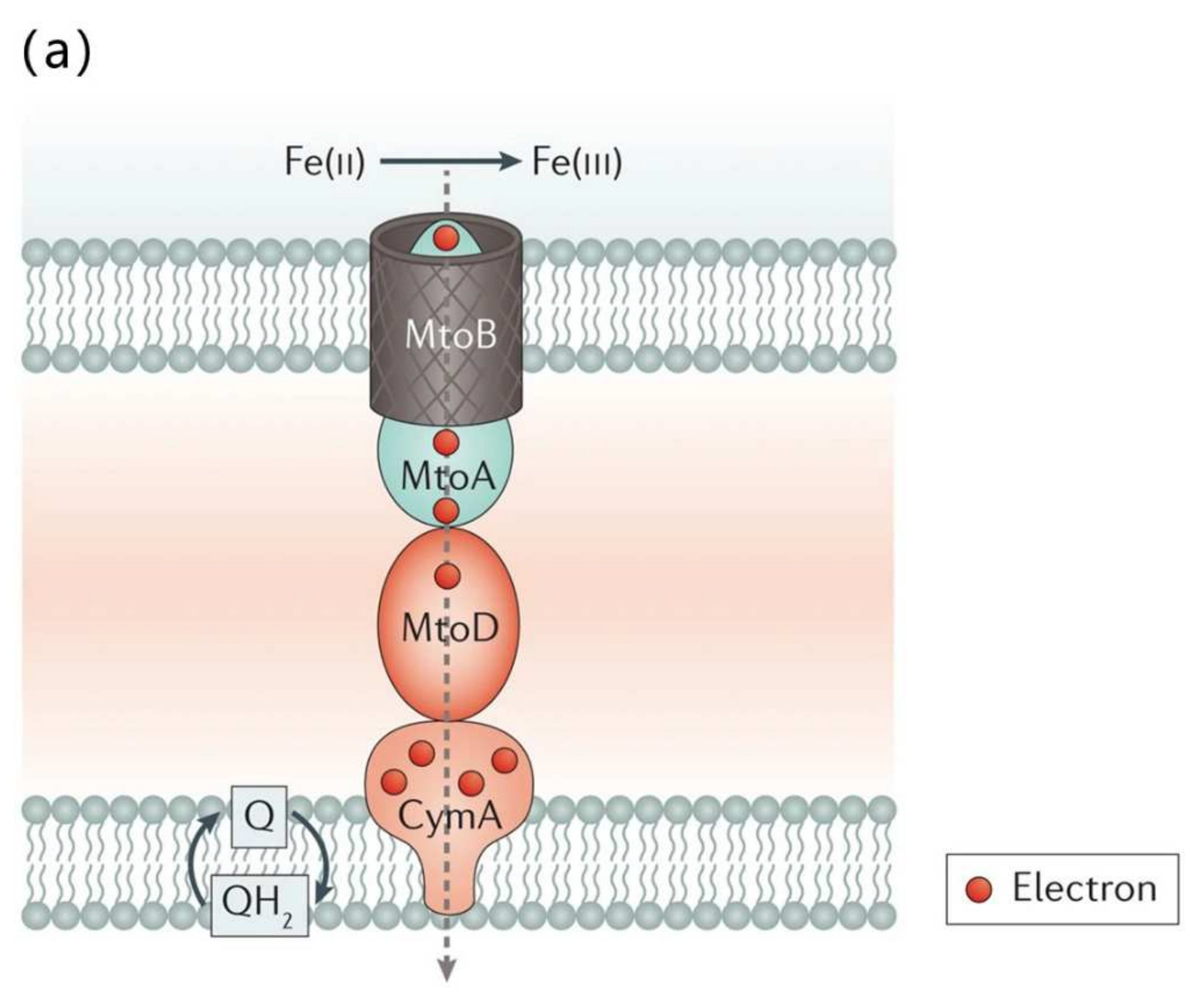


3. EPS Extraction
4. EPS Characterization
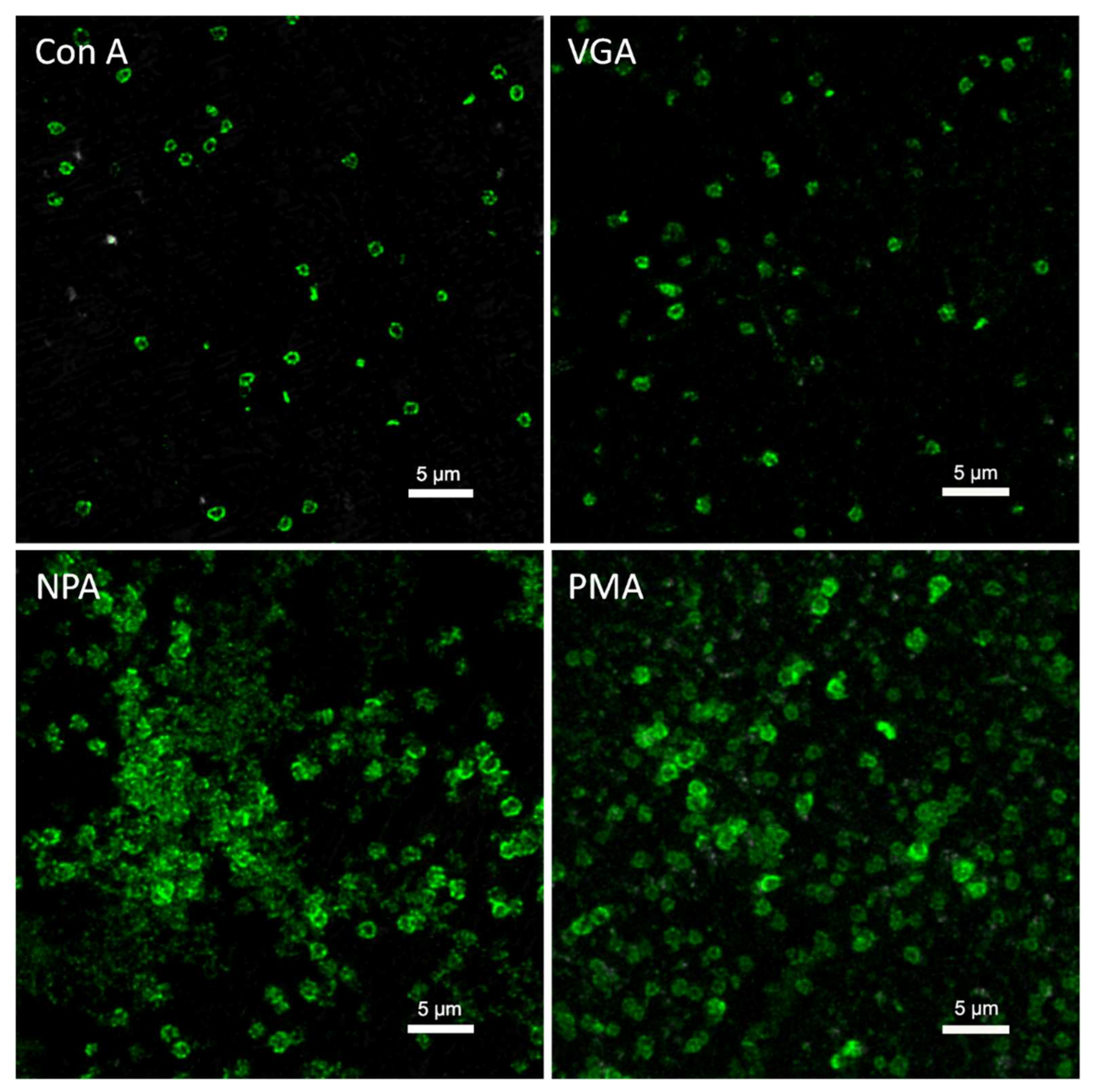
5. Detection of Cell Lysis
6. EPS and Biocorrosion
6.1. EPS Accelerated Corrosion
6.2. EPS Inhibit Corrosion
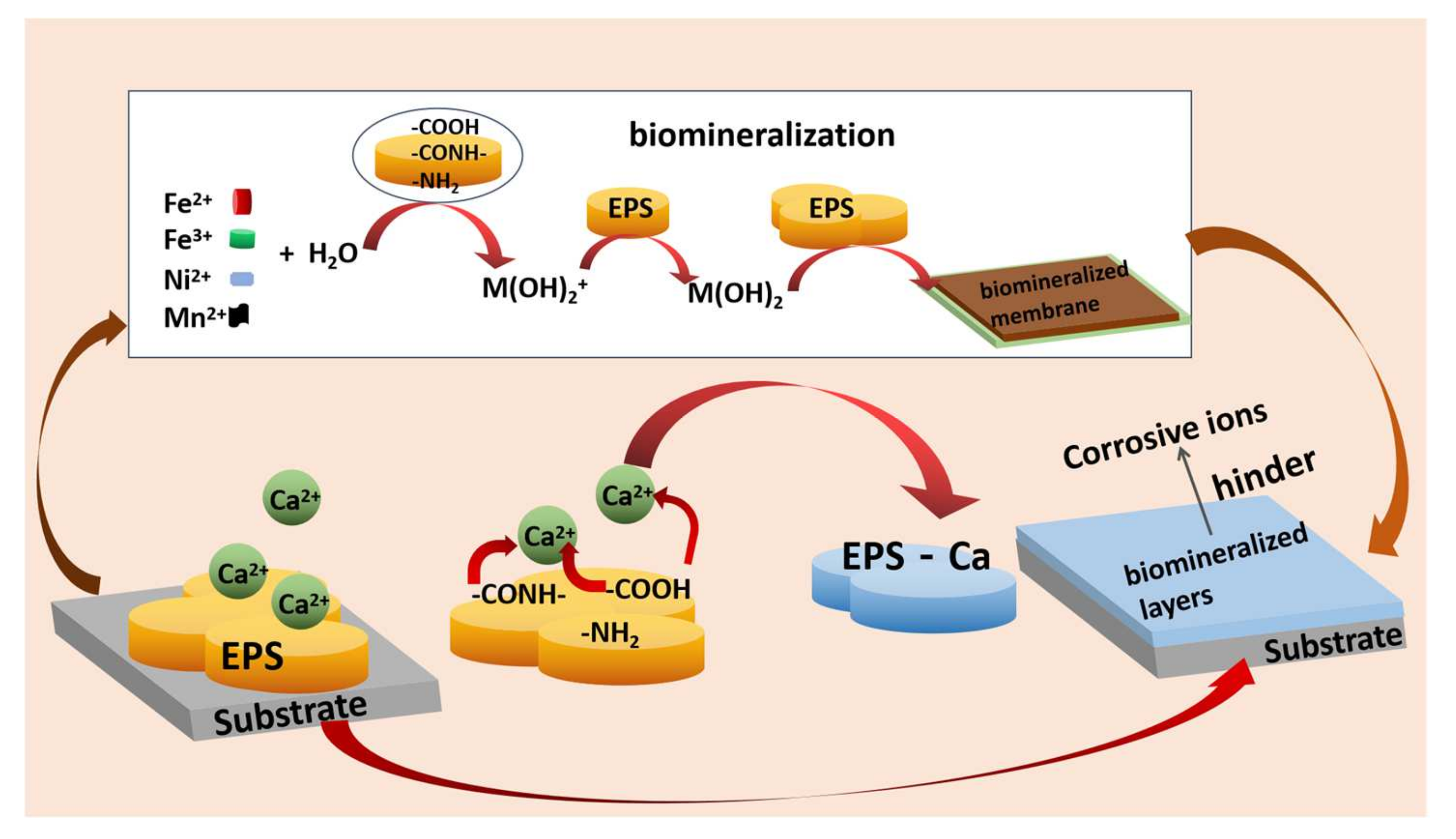
6.3. Application of EPS as a Corrosion Inhibitor
7. Perspective and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The House of Biofilm cells. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Neu, T.R.; Flemming, H.-C. What are Bacterial Extracellular Polymeric Substances? In Microbial Extracellular Polymeric Substances: Characterization, Structure and Function; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. [Google Scholar]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. Npj Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Wikiel, A.J.; Datsenko, I.; Vera, M.; Sand, W. Impact of Desulfovibrio alaskensis biofilms on corrosion behaviour of carbon steel in marine environment. Bioelectrochemistry 2014, 97, 52–60. [Google Scholar] [CrossRef]
- Xu, L.; Guan, F.; Ma, Y.; Zhang, R.; Zhang, Y.; Zhai, X.; Dong, X.; Wang, Y.; Duan, J.; Hou, B. Inadequate dosing of THPS treatment increases microbially influenced corrosion of pipeline steel by inducing biofilm growth of Desulfovibrio hontreensis SY-21. Bioelectrochemistry 2022, 145, 108048. [Google Scholar] [CrossRef]
- Enning, D.; Venzlaff, H.; Garrelfs, J.; Dinh, H.T.; Meyer, V.; Mayrhofer, K.; Hassel, A.W.; Stratmann, M.; Widdel, F. Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ. Microbiol. 2012, 14, 1772–1787. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, Y.; Gu, T. Mechanistic modeling of biocorrosion caused by biofilms of sulfate reducing bacteria and acid producing bacteria. Bioelectrochemistry 2016, 110, 52–58. [Google Scholar] [CrossRef]
- Liu, H.; Meng, G.; Li, W.; Gu, T.; Liu, H. Microbiologically influenced corrosion of carbon steel beneath a deposit in CO2-saturated formation water containing Desulfotomaculum nigrificans. Front. Microbiol. 2019, 10, 1298. [Google Scholar] [CrossRef]
- Amin, O.A.; Aragon, E.; Fahs, A.; Davidson, S.; Ollivier, B.; Hirschler-Rea, A. Iron corrosion induced by the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus at 70 °C. Int. Biodeterior. Biodegrad. 2020, 154, 105056. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Carbon steel biocorrosion at 80 °C by a thermophilic sulfate reducing archaeon biofilm provides evidence for its utilization of elemental iron as electron donor through extracellular electron transfer. Corros. Sci. 2018, 145, 47–54. [Google Scholar] [CrossRef]
- Yue, Y.; Lv, M.; Du, M. The corrosion behavior and mechanism of X65 steel induced by iron-oxidizing bacteria in the seawater environment. Mater. Corros.-Werkst. Korros. 2019, 70, 1852–1861. [Google Scholar] [CrossRef]
- Thyssen, C.; Holuscha, D.; Kuhn, J.; Walter, F.; Fürbeth, W.; Sand, W. Biofilm formation and stainless steel corrosion analysis of Leptothrix discophora. Adv. Mater. Res. 2015, 1130, 79–82. [Google Scholar] [CrossRef]
- Lee, J.S.; McBeth, J.M.; Ray, R.I.; Little, B.J.; Emerson, D. Iron cycling at corroding carbon steel surfaces. Biofouling 2013, 29, 1243–1252. [Google Scholar] [CrossRef]
- Hernandez-Santana, A.; Kokbudak, H.N.; Nanny, M.A. The influence of iron-binding ligands in the corrosion of carbon steel driven by iron-reducing bacteria. Npj Mater. Degrad. 2022, 6, 12. [Google Scholar] [CrossRef]
- Lahme, S.; Mand, J.; Longwell, J.; Smith, R.; Enning, D. Severe corrosion of carbon steel in oil field produced water can be linked to methanogenic archaea containing a special type of [NiFe] hydrogenase. Appl. Environ. Microbiol. 2021, 87, e01819-20. [Google Scholar] [CrossRef]
- Qian, H.; Ju, P.; Zhang, D.; Ma, L.; Hu, Y.; Li, Z.; Huang, L.; Lou, Y.; Du, C. Effect of dissolved oxygen concentration on the microbiologically influenced corrosion of Q235 carbon steel by halophilic archaeon Natronorubrum tibetense. Front. Microbiol. 2019, 10, 844. [Google Scholar] [CrossRef] [Green Version]
- Lahme, S.; Enning, D.; Callbeck, C.M.; Vega, D.M.; Curtis, T.P.; Head, I.M.; Hubert, C.R.J.; Stams, A.J.M. Metabolites of an oil field sulfide-oxidizing, nitrate-reducing Sulfurimonas sp. cause severe corrosion. Appl. Environ. Microbiol. 2019, 85, e01891-18. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Anaerobic corrosion of 304 stainless steel caused by the Pseudomonas aeruginosa biofilm. Front. Microbiol. 2017, 8, 2335. [Google Scholar] [CrossRef]
- Imo, E.; Orji, J.; Nweke, C. Influence of Aspergillus fumigatus on corrosion behaviour of mild steel and aluminium. Int. J. Appl. Microbiol. Biotechnol. Res. 2018, 6, 61–69. [Google Scholar]
- Zhang, Y.; Zhai, X.; Guan, F.; Dong, X.; Sun, J.; Zhang, R.; Duan, J.; Zhang, B.; Hou, B. Microbiologically influenced corrosion of steel in coastal surface seawater contaminated by crude oil. Npj Mater. Degrad. 2022, 6, 35. [Google Scholar] [CrossRef]
- Tuck, B.; Watkin, E.; Somers, A.; Machuca, L.L. A critical review of marine biofilms on metallic materials. Npj Mater. Degrad. 2022, 6, 25. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef]
- Kip, N.; van Veen, J.A. The dual role of microbes in corrosion. ISME J. 2015, 9, 542–551. [Google Scholar] [CrossRef]
- Enning, D.; Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: New views of an old problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef] [Green Version]
- Lan, X.; Zhang, J.; Wang, Z.; Zhang, R.; Sand, W.; Zhang, L.; Duan, J.; Zhu, Q.; Hou, B. Corrosion of an AZ31B magnesium alloy by sulfate-reducing prokaryotes in a mudflat environment. Microorganisms 2022, 10, 839. [Google Scholar] [CrossRef]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Anandkumar, B.; Choi, J.H.; Venkatachari, G.; Maruthamuthu, S. Molecular characterization and corrosion behavior of thermophilic (55 degrees C) SRB Desulfotomaculum kuznetsovii isolated from cooling tower in petroleum refinery. Mater. Corros.-Werkst. Und Korros. 2009, 60, 730–737. [Google Scholar] [CrossRef]
- McBeth, J.M.; Little, B.J.; Ray, R.I.; Farrar, K.M.; Emerson, D. Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments. Appl. Environ. Microbiol. 2011, 77, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Rao, T.S.; Sairam, T.N.; Viswanathan, B.; Nair, K.V.K. Carbon steel corrosion by iron oxidising and sulphate reducing bacteria in a freshwater cooling system. Corros. Sci. 2000, 42, 1417–1431. [Google Scholar] [CrossRef]
- Chen, S.; Deng, H.; Liu, G.; Zhang, D. Corrosion of Q235 carbon steel in seawater containing Mariprofundus ferrooxydans and Thalassospira sp. Front. Microbiol. 2019, 10, 936. [Google Scholar] [CrossRef] [PubMed]
- Linhardt, P. Twenty years of experience with corrosion failures caused by manganese oxidizing microorganisms. Mater. Corros.-Werkst. Korros. 2010, 61, 1034–1039. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Holmes, D.E.; Ueki, T.; Palacios, P.A.; Lovley, D.R. Iron corrosion via direct metal-microbe electron transfer. Mbio 2019, 10, e00303-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-M.; Zhang, Y.-Y.; Liu, J.-H.; Yu, M. Corrosion behavior of steel A3 influenced by Thiobacillus ferrooxidans. Acta Phys.-Chim. Sin. 2008, 24, 1553–1557. [Google Scholar] [CrossRef]
- Inaba, Y.; West, A.C.; Banta, S. Enhanced microbial corrosion of stainless steel by Acidithiobacillus ferrooxidans through the manipulation of substrate oxidation and over expression of rus. Biotechnol. Bioeng. 2020, 117, 3475–3485. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, B.; Xu, D.; Jiang, C.; Li, Q.; Gu, T. Severe microbiologically influenced corrosion of S32654 super austenitic stainless steel by acid producing bacterium Acidithiobacillus caldus SM-1. Bioelectrochemistry 2018, 123, 34–44. [Google Scholar] [CrossRef]
- Ramos Monroy, O.A.; Ruiz Ordaz, N.; Hernandez Gayosso, M.J.; Juarez Ramirez, C.; Galindez Mayer, J. The corrosion process caused by the activity of the anaerobic sporulated bacterium Clostridium celerecrescens on API XL 52 steel. Environ. Sci. Pollut. Res. 2019, 26, 29991–30002. [Google Scholar] [CrossRef]
- Juzeliunas, E.; Ramanauskas, R.; Lugauskas, A.; Leinartas, K.; Samuleviciene, M.; Sudavicius, A.; Juskenas, R. Microbially influenced corrosion of zinc and aluminium—Two-year subjection to influence of Aspergillus niger. Corros. Sci. 2007, 49, 4098–4112. [Google Scholar] [CrossRef]
- Little, B.; Staehle, R.; Davis, R. Fungal influenced corrosion of post-tensioned cables. Int. Biodeterior. Biodegrad. 2001, 47, 71–77. [Google Scholar] [CrossRef]
- Usher, K.M.; Kaksonen, A.H.; MacLeod, I.D. Marine rust tubercles harbour iron corroding archaea and sulphate reducing bacteria. Corros. Sci. 2014, 83, 189–197. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, L.; Fu, Q.; Sun, J.; An, Z.Y.; Ding, R.; Li, Y.; Zhao, X.D. Effect of Bacillus subtilis on corrosion behavior of 10MnNiCrCu steel in marine environment. Sci. Rep. 2020, 10, 5744. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Yang, D.; Xu, J.; Xu, D.; Gu, T. Microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm under organic carbon starvation. Corros. Sci. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Stadler, R.; Fuerbeth, W.; Harneit, K.; Grooters, M.; Woellbrink, M.; Sand, W. First evaluation of the applicability of microbial extracellular polymeric substances for corrosion protection of metal substrates. Electrochim. Acta 2008, 54, 91–99. [Google Scholar] [CrossRef]
- San, N.O.; Nazir, H.; Donmez, G. Microbial corrosion of Ni-Cu alloys by Aeromonas eucrenophila bacterium. Corros. Sci. 2011, 53, 2216–2221. [Google Scholar] [CrossRef]
- Stadler, R.; Wei, L.; Fuerbeth, W.; Grooters, M.; Kuklinski, A. Influence of bacterial exopolymers on cell adhesion of Desulfovibrio vulgaris on high alloyed steel: Corrosion inhibition by extracellular polymeric substances (EPS). Mater. Corros.-Werkst. Korros. 2010, 61, 1008–1016. [Google Scholar] [CrossRef]
- Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 2016, 9, 141–148. [Google Scholar] [CrossRef]
- Deutzmann, J.S.; Sahin, M.; Spormann, A.M. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. Mbio 2015, 6, e00496-15. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Wang, L.-Y.; Mbadinga, S.M.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation. Amb Express 2015, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Trueman, B.F.; Stoddart, A.K.; Gagnon, G.A. Understanding the impact of extracellular polymeric substances on lead release in drinking water systems. Acs Omega 2018, 3, 14824–14832. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhang, D. Study of corrosion behavior of copper in 3.5 wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 2018, 136, 275–284. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Asif, M.; Zhang, G.; Liu, H. The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria. Corros. Sci. 2017, 114, 102–111. [Google Scholar] [CrossRef]
- Dong, Y.H.; Guo, N.; Liu, T.; Dong, L.H.; Yin, Y.S. Effect of extracellular polymeric substances isolated from Vibrio natriegens on corrosion of carbon steel in seawater. Corros. Eng. Sci. Technol. 2016, 51, 455–462. [Google Scholar] [CrossRef]
- Jin, J.; Guan, Y. The mutual co-regulation of extracellular polymeric substances and iron ions in biocorrosion of cast iron pipes. Bioresour. Technol. 2014, 169, 387–394. [Google Scholar] [CrossRef]
- Jin, J.; Wu, G.; Zhang, Z.; Guan, Y. Effect of extracellular polymeric substances on corrosion of cast iron in the reclaimed wastewater. Bioresour. Technol. 2014, 165, 162–165. [Google Scholar] [CrossRef]
- Li, S.; Qu, Q.; Li, L.; Xia, K.; Li, Y.; Zhu, T. Bacillus cereus s-EPS as a dual bio-functional corrosion and scale inhibitor in artificial seawater. Water Res. 2019, 166, 115094. [Google Scholar] [CrossRef]
- Moradi, M.; Song, Z.; Xiao, T. Exopolysaccharide produced by Vibrio neocaledonicus sp as a green corrosion inhibitor: Production and structural characterization. J. Mater. Sci. Technol. 2018, 34, 2447–2457. [Google Scholar] [CrossRef]
- Bautista, B.E.T.; Wikiel, A.J.; Datsenko, I.; Vera, M.; Sand, W.; Seyeux, A.; Zanna, S.; Frateur, I.; Marcus, P. Influence of extracellular polymeric substances (EPS) from Pseudomonas NCIMB 2021 on the corrosion behaviour of 70Cu-30Ni alloy in seawater. J. Electroanal. Chem. 2015, 737, 184–197. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.-C.; Neu, T.; Wingender, J. The Perfect Slime-and the ‘Dark Matter’ of Biofilms; IWA Publishers: London, UK, 2016; pp. 1–14. [Google Scholar]
- Chan, K.Y.; Xu, L.C.; Fang, H.H.P. Anaerobic electrochemical corrosion rug of mild steel in the presence of extracellular polymeric substances produced by a culture enriched in sulfate-reducing bacteria. Environ. Sci. Technol. 2002, 36, 1720–1727. [Google Scholar] [CrossRef]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar] [CrossRef]
- Fagerlund, A.; Langsrud, S.; Heir, E.; Mikkelsen, M.I.; Moretro, T. Biofilm matrix composition affects the susceptibility of food associated Staphylococci to cleaning and disinfection agents. Front. Microbiol. 2016, 7, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, Z.U.; Rehm, B.H.A. Dual roles of Pseudomonas aeruginosa AlgE in secretion of the virulence factor alginate and formation of the secretion complex. Appl. Environ. Microbiol. 2013, 79, 2002–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atalah, J.; Blamey, L.; Gelineo-Albersheim, I.; Blamey, J.M. Characterization of the EPS from a thermophilic corrosive consortium. Biofouling 2019, 35, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.H.; Liu, T.; Liu, H.F. Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling 2011, 27, 487–495. [Google Scholar] [CrossRef]
- Booker, A.E.; Hoyt, D.W.; Meulia, T.; Eder, E.; Nicora, C.D.; Purvine, S.O.; Daly, R.A.; Moore, J.D.; Wunch, K.; Pfiffner, S.M.; et al. Deep-subsurface pressure stimulates metabolic plasticity in shale-colonizing Halanaerobium spp. Appl. Environ. Microbiol. 2019, 85, e00018-19. [Google Scholar] [CrossRef] [Green Version]
- Geesey, G.G.; Jang, L.; Jolley, J.G.; Hankins, M.R.; Iwaoka, T.; Griffiths, P.R. Binding of metal-ions by extracellular polymers of biofilm bacteria. Water Sci. Technol. 1988, 20, 161–165. [Google Scholar] [CrossRef]
- Cheng, S.; Lau, K.-T.; Chen, S.; Chang, X.; Liu, T.; Yin, Y. Microscopical observation of the marine bacterium Vibrio natriegeus growth on metallic corrosion. Mater. Manuf. Processes 2010, 25, 293–297. [Google Scholar] [CrossRef]
- Wang, J.; Tian, B.; Bao, Y.; Qian, C.; Yang, Y.; Niu, T.; Xin, B. Functional exploration of extracellular polymeric substances (EPS) in the bioleaching of obsolete electric vehicle LiNi(x)Co(y)Mn1-O-x-y(2) Li-ion batteries. J. Hazard. Mater. 2018, 354, 250–257. [Google Scholar] [CrossRef]
- Tielen, P.; Strathmann, M.; Jaeger, K.E.; Flemming, H.C.; Wingender, J. Alginate acetylation influences initial surface colonization by mucoid Pseudomonas aeruginosa. Microbiol. Res. 2005, 160, 165–176. [Google Scholar] [CrossRef]
- Mayer, C.; Moritz, R.; Kirschner, C.; Borchard, W.; Maibaum, R.; Wingender, J.; Flemming, H.C. The role of intermolecular interactions: Studies on model systems for bacterial biofilms. Int. J. Biol. Macromol. 1999, 26, 3–16. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Ye, J.; Hu, A.; Ren, G.; Chen, M.; Tang, J.; Zhang, P.; Zhou, S.; He, Z. Enhancing sludge methanogenesis with improved redox activity of extracellular polymeric substances by hematite in red mud. Water Res. 2018, 134, 54–62. [Google Scholar] [CrossRef]
- Gralnick, J.A.; Newman, D.K. Extracellular respiration. Mol. Microbiol. 2007, 65, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R. Extracellular electron transfer: Wires, capacitors, iron lungs, and more. Geobiology 2008, 6, 225–231. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Yang, C.; Ueki, T.; Pittman, C.C.; Xu, D.; Woodard, T.L.; Holmes, D.E.; Gu, T.; Wang, F.; Lovley, D.R. Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species. ISME J. 2021, 15, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Lekbach, Y.; Liu, T.; Li, Y.; Moradi, M.; Dou, W.; Xu, D.; Smith, J.A.; Lovley, D.R. Microbial corrosion of metals: The corrosion microbiome. Adv. Microb. Physiol. 2021, 78, 317–390. [Google Scholar] [PubMed]
- Pirbadian, S.; Barchinger, S.E.; Leung, K.M.; Byun, H.S.; Jangir, Y.; Bouhenni, R.A.; Reed, S.B.; Romine, M.F.; Saffarini, D.A.; Shi, L.; et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl. Acad. Sci. USA 2014, 111, 12883–12888. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Shi, L.; Brown, R.N.; Xiong, Y.; Fredrickson, J.K.; Romine, M.F.; Marshall, M.J.; Lipton, M.S.; Beyenal, H. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ. Microbiol. 2011, 13, 1018–1031. [Google Scholar] [CrossRef]
- Lovley, D.R.; Coates, J.D.; BluntHarris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Stams, A.J.M.; de Bok, F.A.M.; Plugge, C.M.; van Eekert, M.H.A.; Dolfing, J.; Schraa, G. Exocellular electron transfer in anaerobic microbial communities. Environ. Microbiol. 2006, 8, 371–382. [Google Scholar] [CrossRef]
- Beech, W.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.H.; Tse, E.C.M.; Yates, M.D.; Otero, F.J.; Trammell, S.A.; Stemp, E.D.A.; Barton, J.K.; Tender, L.M.; Newman, D.K. Extracellular DNA promotes eficient extracellular electron transfer by pyocyanin in Pseudomonas aeruginosa biofilms. Cell 2020, 182, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.M.; Ulstrup, J.; Zhao, F. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 2017, 3, e1700623. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Lu, X.; Liu, H.; Li, J.; Li, W.; Song, R.; Wang, R.; Zhang, D.; Zhu, J. Mediation of extracellular polymeric substances in microbial reduction of hematite by Shewanella oneidensis MR-1. Front. Microbiol. 2019, 10, 575. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Jia, X.S. Extraction of extracellular polymer from anaerobic sludges. Biotechnol. Tech. 1996, 10, 803–808. [Google Scholar] [CrossRef]
- Dignac, M.F.; Urbain, V.; Rybacki, D.; Bruchet, A.; Snidaro, D.; Scribe, P. Chemical description of extracellular polymers: Implication on activated sludge floc structure. Water Sci. Technol. 1998, 38, 45–53. [Google Scholar] [CrossRef]
- Dai, Y.-F.; Xiao, Y.; Zhang, E.-H.; Liu, L.-D.; Qiu, L.; You, L.-X.; Mahadevan, G.D.; Chen, B.-L.; Zhao, F. Effective methods for extracting extracellular polymeric substances from Shewanella oneidensis MR-1. Water Sci. Technol. 2016, 74, 2987–2996. [Google Scholar] [CrossRef]
- Brown, M.J.; Lester, J.N. Comparison of bacterial extracellular polymer extraction methods. Appl. Environ. Microbiol. 1980, 40, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Fang, H.H.P. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Christensen, B.E.; Kjosbakken, J.; Smidsrod, O. Partial chemical and physical characterization of two extracellular polysaccharides produced by marine, periphytic Pseudomonas sp strain NCMB-2021. Appl. Environ. Microbiol. 1985, 50, 837–845. [Google Scholar] [CrossRef] [Green Version]
- Forster, C.F.; Clarke, A.R. The production of polymer from activated-sludge by ethanolic extraction and its relation to treatment-plant operation. Water Pollut. Control 1983, 82, 430–433. [Google Scholar]
- Wuertz, S.; Spaeth, R.; Hinderberger, A.; Grieba, T.; Flemming, H.C.; Wilderer, P.A. A new method for extraction of extracellular polymeric substances from biofilms and activated sludge suitable for direct quantification of sorbed metals. Water Sci. Technol. 2001, 43, 25–31. [Google Scholar] [CrossRef]
- Frolund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Jahn, A.; Nielsen, P.H. Extraction of extracellular polymeric substances (EPS) from biofilms using a cation exchange resin. Water Sci. Technol. 1995, 32, 157–164. [Google Scholar] [CrossRef]
- Tapia, J.; Munoz, J.; Gonzalez, F.; Blázquez, M.; Malki, M.; Ballester, A. Extraction of extracellular polymeric substances from the acidophilic bacterium Acidiphilium 3.2 Sup (5). Water Sci. Technol. 2009, 59, 1959–1967. [Google Scholar] [CrossRef] [Green Version]
- Pronk, M.; Neu, T.R.; van Loosdrecht, M.C.M.; Lin, Y.M. The acid soluble extracellular polymeric substance of aerobic granular sludge dominated by Defluviicoccus sp. Water Res. 2017, 122, 148–158. [Google Scholar] [CrossRef]
- Neu, T.R.; Lawrence, J.R. Advanced techniques for in situ analysis of the biofilm matrix (structure, composition, dynamics) by means of laser scanning microscopy. In Microbial Biofilms; Donelli, G., Ed.; Springer: New York, NY, USA, 2014; pp. 43–64. [Google Scholar]
- Neu, T.R.; Lawrence, J.R. Lectin-binding analysis in biofilm systems. In Methods in Enzymology; Abelson, J., Simon, M., Eds.; Academic Press: Cambridge, MA, USA, 1999; Volume 310, pp. 145–152. [Google Scholar]
- Strathmann, M.; Wingender, J.; Flemming, H.C. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J. Microbiol. Methods 2002, 50, 237–248. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Swerhone, G.D.W.; Kuhlicke, U.; Neu, T.R. In Situ evidence for microdomains in the polymer matrix of bacterial microcolonies. Can. J. Microbiol. 2007, 53, 450–458. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Neu, T.R.; Bellenberg, S.; Kuhlicke, U.; Sand, W.; Vera, M. Use of lectins to in situ visualize glycoconjugates of extracellular polymeric substances in acidophilic archaeal biofilms. Microb. Biotechnol. 2015, 8, 448–461. [Google Scholar] [CrossRef]
- Zhang, R.; Neu, T.R.; Zhang, Y.; Bellenberg, S.; Kuhlicke, U.; Li, Q.; Sand, W.; Vera, M. Visualization and analysis of EPS glycoconjugates of the thermoacidophilic archaeon Sulfolobus metallicus. Appl. Microbiol. Biotechnol. 2015, 99, 7343–7356. [Google Scholar] [CrossRef] [PubMed]
- Zippel, B.; Neu, T.R. Characterization of glycoconjugates of extracellular polymeric substances in Tufa-associated biofilms by using fluorescence lectin-binding analysis. Appl. Environ. Microbiol. 2011, 77, 505–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqbool, T.; Cho, J.; Shin, K.H.; Hur, J. Using stable isotope labeling approach and two dimensional correlation spectroscopy to explore the turnover cycles of different carbon structures in extracellular polymeric substances. Water Res. 2020, 170, 115355. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, C.; Zhao, H.; Chen, G.; Chen, X. A subcellular level study of copper speciation reveals the synergistic mechanism of microbial cells and EPS involved in copper binding in bacterial biofilms. Environ. Pollut. 2020, 263, 114485. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Cao, Y.; Huang, Q.; Walker, S.L.; Cai, P. Reactions between bacterial exopolymers and goethite: A combined macroscopic and spectroscopic investigation. Water Res. 2012, 46, 5613–5620. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Lv, J.; Li, G.; Zhang, Z.; Zhang, J.; et al. Chromium immobilization by extra- and intraradical fungal structures of arbuscular mycorrhizal symbioses. J. Hazard. Mater. 2016, 316, 34–42. [Google Scholar] [CrossRef]
- Liu, H.-C.; Xia, J.-L.; Nie, Z.-Y.; Zhen, X.-J.; Zhang, L.-J. Differential expression of extracellular thiol groups of moderately thermophilic Sulfobacillus thermosulfidooxidans and extremely thermophilic Acidianus manzaensis grown on S0 and Fe2+. Arch. Microbiol. 2015, 197, 823–831. [Google Scholar] [CrossRef]
- Mitsunobu, S.; Zhu, M.; Takeichi, Y.; Ohigashi, T.; Suga, H.; Jinno, M.; Makita, H.; Sakata, M.; Ono, K.; Mase, K.; et al. Direct detection of Fe(II) in extracellular polymeric substances (EPS) at the mineral-microbe interface in bacterial pyrite leaching. Microbes Environ. 2016, 31, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Wingender, J.; Strathmann, M.; Rode, A.; Leis, A.; Flemming, H.C. Isolation and biochemical characterization of extracellular polymeric substances from Pseudomonas aeruginosa. In Microbial Growth in Biofilms, Pt A: Developmental and Molecular Biological Aspects; Doyle, R.J., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2001; Volume 336, pp. 302–314. [Google Scholar]
- Nagata, T.; Meon, B.; Kirchman, D.L. Microbial degradation of peptidoglycan in seawater. Limnol. Oceanogr. 2003, 48, 745–754. [Google Scholar] [CrossRef]
- Ng, F.M.W.; Dawes, E.A. Chemostat studies on regulation of glucose-metabolism in Pseudomonas-aeruginosa by citrate. Biochem. J. 1973, 132, 129–140. [Google Scholar] [CrossRef]
- Wang, S.; Redmile-Gordon, M.; Mortimer, M.; Cai, P.; Wu, Y.; Peacock, C.L.; Gao, C.; Huang, Q. Extraction of extracellular polymeric substances (EPS) from red soils (Ultisols). Soil Biol. Biochem. 2019, 135, 283–285. [Google Scholar] [CrossRef]
- Boulos, L.; Prevost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD (R) BacLight (TM): Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 1999, 37, 77–86. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q. Formation of extracellular polymeric substances from acidogenic sludge in H2-producing process. Appl. Microbiol. Biotechnol. 2007, 74, 208–214. [Google Scholar] [CrossRef]
- Arkan, S.; Ilhan-Sungur, E.; Cansever, N. Corrosive metabolic activity of Desulfovibrio sp. on 316L stainless steel. J. Mater. Eng. Perform. 2016, 25, 5352–5362. [Google Scholar] [CrossRef]
- Li, X.L.; Narenkumar, J.; Rajasekar, A.; Ting, Y.-P. Biocorrosion of mild steel and copper used in cooling tower water and its control. 3 Biotech 2018, 8, 178. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Zhang, R.; Zhang, B.; Zhai, X.; Li, W.; Xu, L.; Jiang, Q.; Duan, J.; Hou, B. Metagenomic resolution of functional diversity in copper surface-associated marine biofilms. Front. Microbiol. 2019, 10, 2863. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.H.P.; Xu, L.C.; Chan, K.Y. Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Res. 2002, 36, 4709–4716. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically influenced corrosion: An update. Int. Mater. Rev. 2014, 59, 384–393. [Google Scholar] [CrossRef]
- Little, B.J.; Hinks, J.; Blackwood, D.J. Microbially influenced corrosion: Towards an interdisciplinary perspective on mechanisms. Int. Biodeterior. Biodegrad. 2020, 154, 105062. [Google Scholar] [CrossRef]
- Go, L.C.; Holmes, W.; Depan, D.; Hernandez, R. Evaluation of extracellular polymeric substances extracted from waste activated sludge as a renewable corrosion inhibitor. Peerj 2019, 7, e7193. [Google Scholar] [CrossRef]
- Khan, M.S.; Yang, C.; Zhao, Y.; Pan, H.; Zhao, J.; Shahzad, M.B.; Kolawole, S.K.; Ullah, I.; Yang, K. An induced corrosion inhibition of X80 steel by using marine bacterium Marinobacter salsuginis. Colloids Surf. B Biointerfaces 2020, 189, 110858. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Yuan, X.; Xu, Y.; Xu, D.; Zhang, D.; Feng, D.; Wang, F. Marine biofilms with significant corrosion inhibition performance by secreting extracellular polymeric substances. ACS Appl. Mater. Interfaces 2021, 13, 47272–47282. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Fan, Y.; Li, Z.; Cristiani, P.; Chen, X.; Xu, D.; Wang, F.; Gu, T. Marine Vibrio spp. protect carbon steel against corrosion through secreting extracellular polymeric substances. Npj Mater. Degrad. 2022, 6, 6. [Google Scholar] [CrossRef]
- Guo, Z.; Pan, S.; Liu, T.; Zhao, Q.; Wang, Y.; Guo, N.; Chang, X.; Liu, T.; Dong, Y.; Yin, Y. Bacillus subtilis inhibits Vibrio natriegens-induced corrosion via biomineralization in seawater. Front. Microbiol. 2019, 10, 1111. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, W.; Cui, T.; Qian, H.; Huang, L.; Ma, L.; Hao, X.; Zhang, D. Microbiologically influenced corrosion inhibition of carbon steel via biomineralization induced by Shewanella putrefaciens. Npj Mater. Degrad. 2021, 5, 59. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Z.; Zeng, Z.; Guo, N.; Lei, Y.; Liu, T.; Sun, S.; Chang, X.; Yin, Y.; Wang, X. Marine bacteria provide lasting anticorrosion activity for steel via biofilm-induced mineralization. Acs Appl. Mater. Interfaces 2018, 10, 40317–40327. [Google Scholar] [CrossRef]
- Ghafari, M.D.; Bahrami, A.; Rasooli, I.; Arabian, D.; Ghafari, F. Bacterial exopolymeric inhibition of carbon steel corrosion. Int. Biodeterior. Biodegrad. 2013, 80, 29–33. [Google Scholar] [CrossRef]
- Finkenstadt, V.L.; Cote, G.L.; Willett, J.L. Corrosion protection of low-carbon steel using exopolysaccharide coatings from Leuconostoc mesenteroides. Biotechnol. Lett. 2011, 33, 1093–1100. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Xing, X.; Hu, W. Corrosion behavior of X65 steel in seawater containing sulfate reducing bacteria under aerobic conditions. Bioelectrochemistry 2018, 122, 40–50. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Tan, Y.; Meng, G.; Liu, H.; Cheng, Y.; Liu, H. Characterizations of the biomineralization film caused by marine Pseudomonas stutzeri and its mechanistic effects on X80 pipeline steel corrosion. J. Mater. Sci. Technol. 2022, 125, 15–28. [Google Scholar] [CrossRef]
- Roux, S.; Bur, N.; Ferrari, G.; Tribollet, B.; Feugeas, F. Influence of a biopolymer admixture on corrosion behaviour of steel rebars in concrete. Mater. Corros.-Werkst. Korros. 2010, 61, 1026–1033. [Google Scholar] [CrossRef]
- Kuklinski, A. Development of Extracellular Polymeric Substance-Derived Protective Films Against Microbiologically Influenced Corrosion by Desulfovibrio vulgaris. Ph.D. Thesis, Faculty of Chemistry, Univesity of Duiburg-Essen, Duisburg, Germany, 2017. [Google Scholar]
- Wu, X.; Wu, X.; Zhou, X.; Gu, Y.; Zhou, H.; Shen, L.; Zeng, W. The roles of extracellular polymeric substances of Pandoraea sp. XY-2 in the removal of tetracycline. Bioprocess Biosyst. Eng. 2020, 43, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Li, F.; Zhang, D.; Xu, D.; Li, Z.; Jia, R.; Jin, Y.; Song, H.; Li, H.; Wang, Q.; et al. Direct microbial electron uptake as a mechanism for stainless steel corrosion in aerobic environments. Water Res. 2022, 219, 118553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Neu, T.R.; Blanchard, V.; Vera, M.; Sand, W. Biofilm dynamics and EPS production of a thermoacidophilic bioleaching archaeon. New Biotechnol. 2019, 51, 21–30. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, R.; Duan, J.; Shi, X.; Zhang, Y.; Guan, F.; Sand, W.; Hou, B. Extracellular Polymeric Substances and Biocorrosion/Biofouling: Recent Advances and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 5566. https://doi.org/10.3390/ijms23105566
Wang Y, Zhang R, Duan J, Shi X, Zhang Y, Guan F, Sand W, Hou B. Extracellular Polymeric Substances and Biocorrosion/Biofouling: Recent Advances and Future Perspectives. International Journal of Molecular Sciences. 2022; 23(10):5566. https://doi.org/10.3390/ijms23105566
Chicago/Turabian StyleWang, Yanan, Ruiyong Zhang, Jizhou Duan, Xin Shi, Yimeng Zhang, Fang Guan, Wolfgang Sand, and Baorong Hou. 2022. "Extracellular Polymeric Substances and Biocorrosion/Biofouling: Recent Advances and Future Perspectives" International Journal of Molecular Sciences 23, no. 10: 5566. https://doi.org/10.3390/ijms23105566
APA StyleWang, Y., Zhang, R., Duan, J., Shi, X., Zhang, Y., Guan, F., Sand, W., & Hou, B. (2022). Extracellular Polymeric Substances and Biocorrosion/Biofouling: Recent Advances and Future Perspectives. International Journal of Molecular Sciences, 23(10), 5566. https://doi.org/10.3390/ijms23105566








