PAX9 in Cancer Development
Abstract
:1. Introduction
2. PAX9 in Specific Cancer
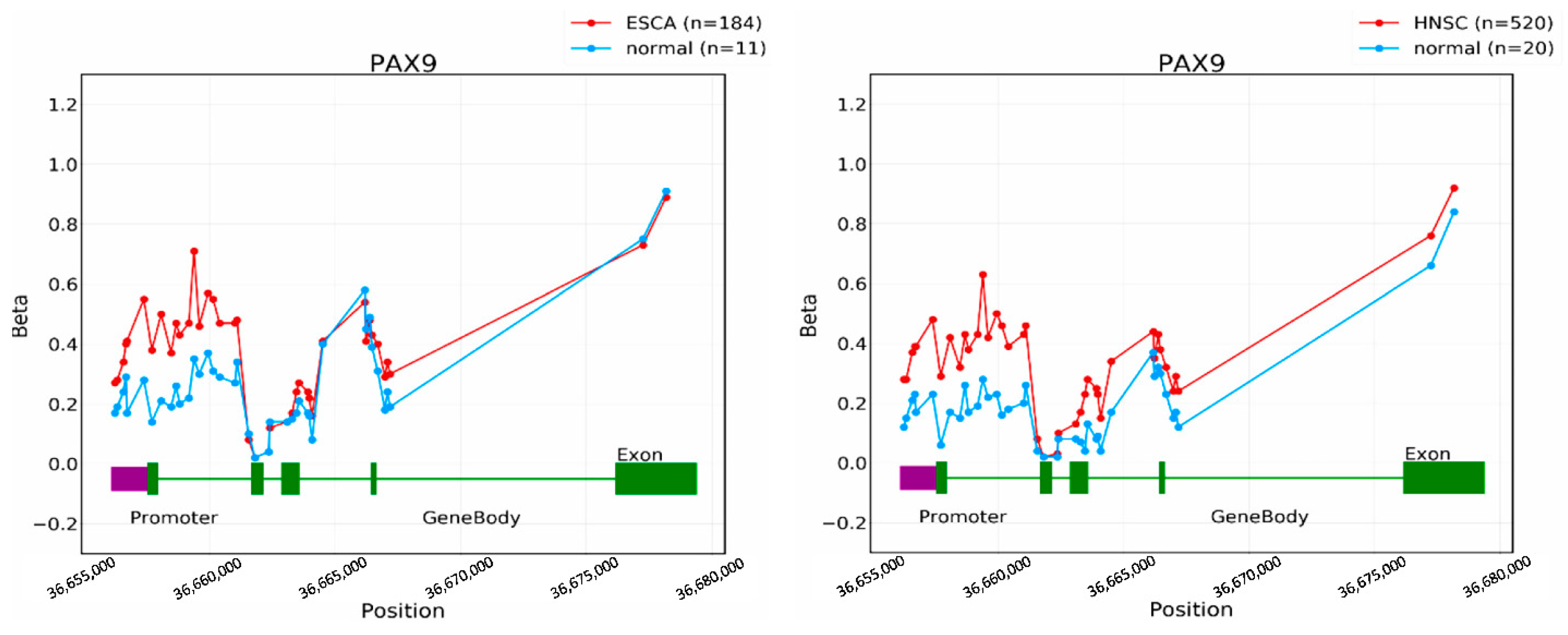
2.1. Head and Neck Squamous Cell Carcinoma (HNSCC)
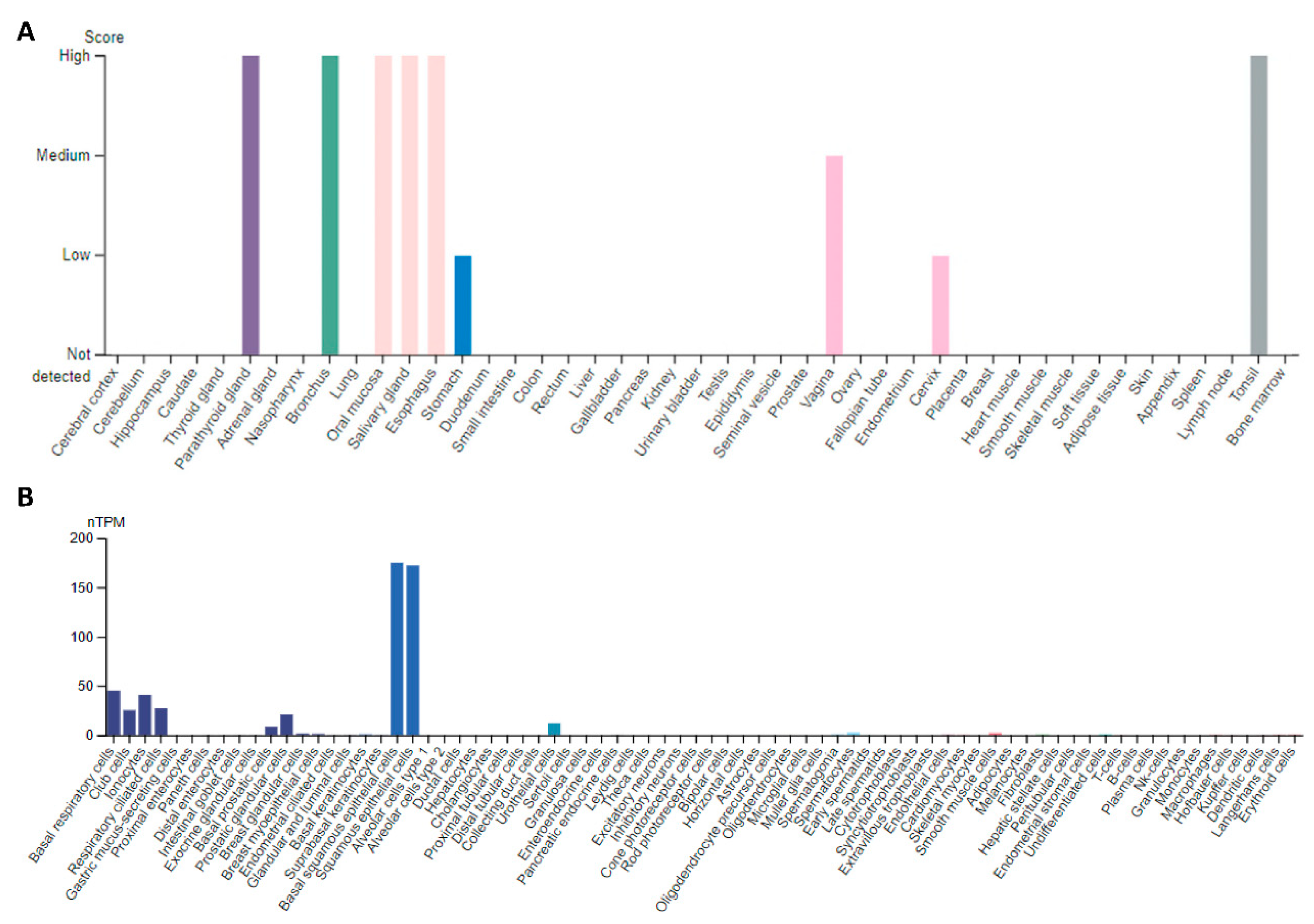
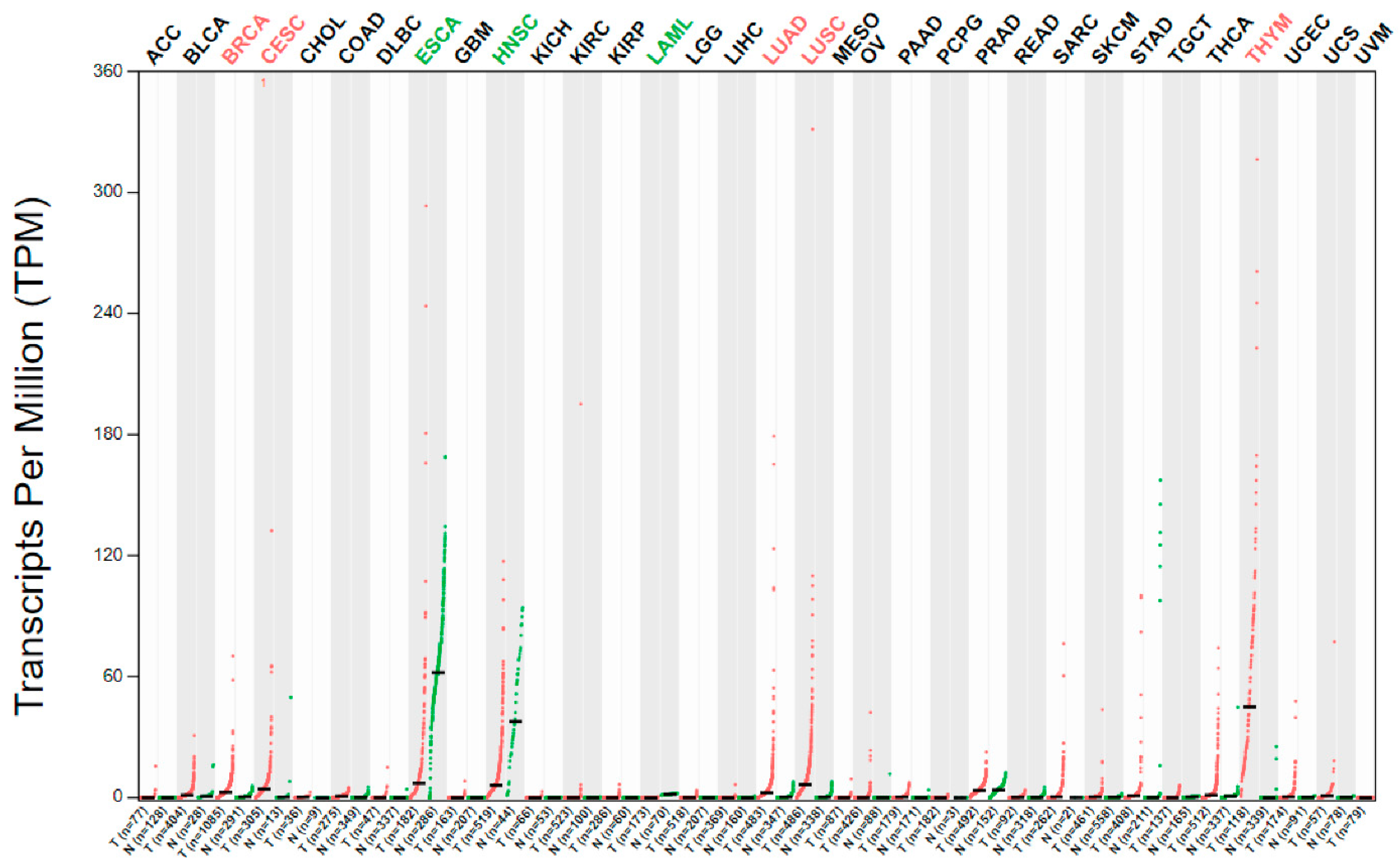
2.2. Esophageal Cancer
2.3. Lung Cancer
2.4. Cervical Cancer
2.5. Ovarian Cancer
2.6. Breast Cancer
2.7. Chronic Lymphocytic Leukemia
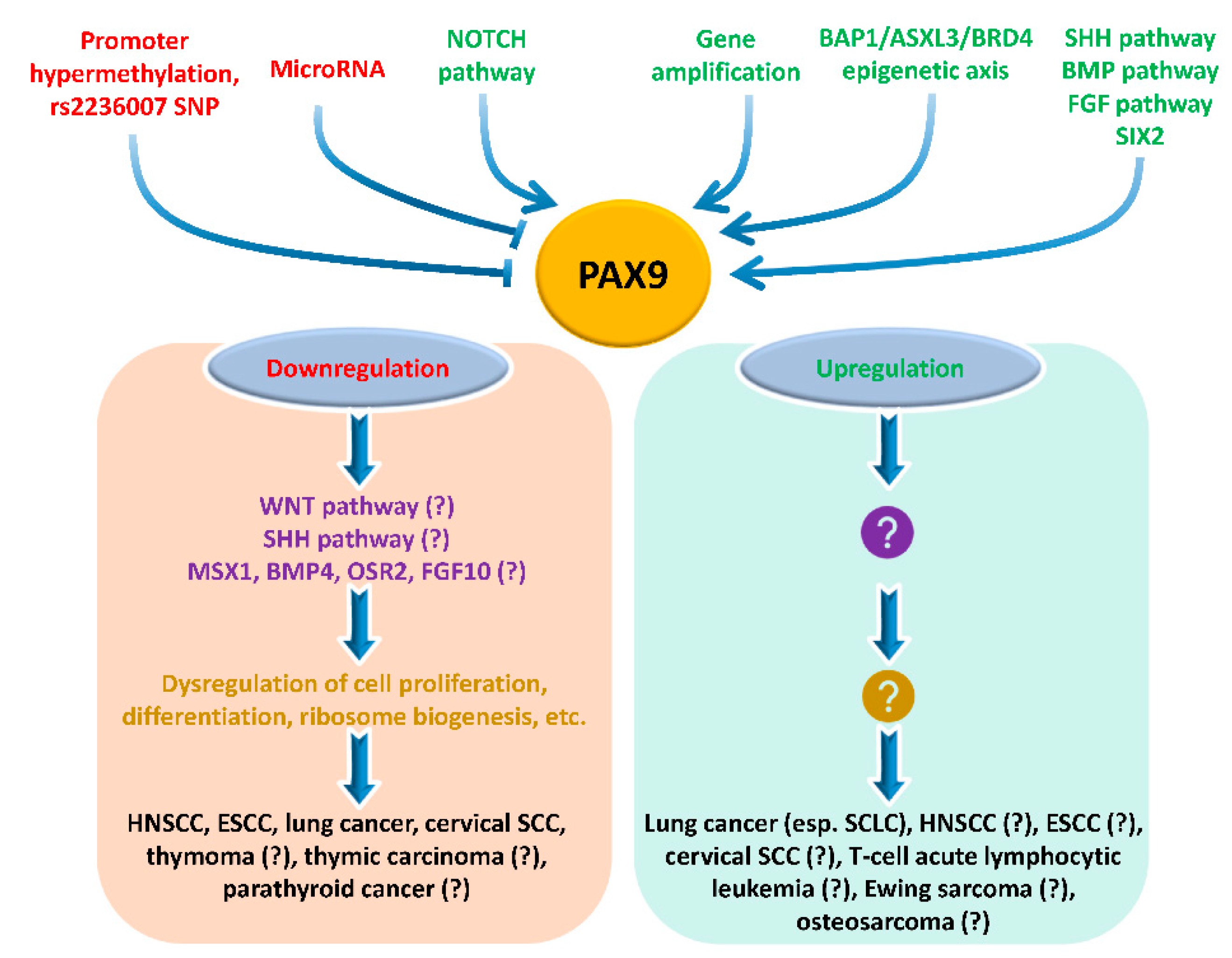
3. Upstream Regulation of PAX9 Expression
4. Downstream Events of PAX9
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Robson, E.J.; He, S.J.; Eccles, M.R. A PANorama of PAX genes in cancer and development. Nat. Rev. Cancer 2006, 6, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Leavey, P.J.; Galindo, R.L. PAX genes in childhood oncogenesis: Developmental biology gone awry? Oncogene 2015, 34, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.; Neubuser, A.; Kratochwil, K.; Balling, R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998, 12, 2735–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonker, L.; Kist, R.; Aw, A.; Wappler, I.; Peters, H. Pax9 is required for filiform papilla development and suppresses skin-specific differentiation of the mammalian tongue epithelium. Mech. Dev. 2004, 121, 1313–1322. [Google Scholar] [CrossRef]
- Kist, R.; Watson, M.; Crosier, M.; Robinson, M.; Fuchs, J.; Reichelt, J.; Peters, H. The formation of endoderm-derived taste sensory organs requires a Pax9-dependent expansion of embryonic taste bud progenitor cells. PLoS Genet. 2014, 10, e1004709. [Google Scholar] [CrossRef] [Green Version]
- Nakatomi, M.; Wang, X.P.; Key, D.; Lund, J.J.; Turbe-Doan, A.; Kist, R.; Aw, A.; Chen, Y.; Maas, R.L.; Peters, H. Genetic interactions between Pax9 and Msx1 regulate lip development and several stages of tooth morphogenesis. Dev. Biol. 2010, 340, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Sivakamasundari, V.; Kraus, P.; Sun, W.; Hu, X.; Lim, S.L.; Prabhakar, S.; Lufkin, T. A developmental transcriptomic analysis of Pax1 and Pax9 in embryonic intervertebral disc development. Biol. Open 2017, 6, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Khasawneh, R.R.; Kist, R.; Queen, R.; Hussain, R.; Coxhead, J.; Schneider, J.E.; Mohun, T.J.; Zaffran, S.; Peters, H.; Phillips, H.M.; et al. Msx1 haploinsufficiency modifies the Pax9-deficient cardiovascular phenotype. BMC Dev. Biol. 2021, 21, 14. [Google Scholar] [CrossRef]
- Phillips, H.M.; Stothard, C.A.; Shaikh Qureshi, W.M.; Kousa, A.I.; Briones-Leon, J.A.; Khasawneh, R.R.; O’Loughlin, C.; Sanders, R.; Mazzotta, S.; Dodds, R.; et al. Pax9 is required for cardiovascular development and interacts with Tbx1 in the pharyngeal endoderm to control 4th pharyngeal arch artery morphogenesis. Development 2019, 146, dev.177618. [Google Scholar] [CrossRef] [Green Version]
- Fauzi, N.H.; Ardini, Y.D.; Zainuddin, Z.; Lestari, W. A review on non-syndromic tooth agenesis associated with PAX9 mutations. Jpn. Dent. Sci. Rev. 2018, 54, 30–36. [Google Scholar] [CrossRef]
- Kist, R.; Watson, M.; Wang, X.; Cairns, P.; Miles, C.; Reid, D.J.; Peters, H. Reduction of Pax9 gene dosage in an allelic series of mouse mutants causes hypodontia and oligodontia. Hum. Mol. Genet. 2005, 14, 3605–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, H.; Schuster, G.; Neubuser, A.; Richter, T.; Hofler, H.; Balling, R. Isolation of the Pax9 cDNA from adult human esophagus. Mamm. Genome 1997, 8, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Bhol, C.S.; Patil, S.; Sahu, B.B.; Patra, S.K.; Bhutia, S.K. The clinical significance and correlative signaling pathways of paired box gene 9 in development and carcinogenesis. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188561. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Powell, S.K.; Plummer, R.S.; Young, K.P.; Ruggeri, B.A. PAX genes: Roles in development, pathophysiology, and cancer. Biochem. Pharmacol. 2007, 73, 1–14. [Google Scholar] [CrossRef]
- Li, C.G.; Eccles, M.R. PAX Genes in Cancer; Friends or Foes? Front. Genet. 2012, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Stuart, E.T.; Gruss, P. PAX genes: What’s new in developmental biology and cancer? Hum. Mol. Genet. 1995, 4, 1717–1720. [Google Scholar] [CrossRef]
- Tell, G.; Pellizzari, L.; Damante, G. Transcription Factors and Cancer. The Example of Pax Genes. Adv. Clin. Path. 1997, 1, 243–255. [Google Scholar]
- Bai, G.; Song, J.; Yuan, Y.; Chen, Z.; Tian, Y.; Yin, X.; Niu, Y.; Liu, J. Systematic analysis of differentially methylated expressed genes and site-speci fi c methylation as potential prognostic markers in head and neck cancer. J. Cell. Physiol. 2019, 234, 22687–22702. [Google Scholar] [CrossRef] [Green Version]
- Iglesias-Bartolome, R.; Uchiyama, A.; Molinolo, A.A.; Abusleme, L.; Brooks, S.R.; Callejas-Valera, J.L.; Edwards, D.; Doci, C.; Asselin-Labat, M.L.; Onaitis, M.W.; et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci. Transl. Med. 2018, 10, aap8798. [Google Scholar] [CrossRef] [Green Version]
- Gerber, J.K.; Richter, T.; Kremmer, E.; Adamski, J.; Hofler, H.; Balling, R.; Peters, H. Progressive loss of PAX9 expression correlates with increasing malignancy of dysplastic and cancerous epithelium of the human oesophagus. J. Pathol. 2002, 197, 293–297. [Google Scholar] [CrossRef]
- Tan, B.; Wang, J.; Song, Q.; Wang, N.; Jia, Y.; Wang, C.; Yao, B.; Liu, Z.; Zhang, X.; Cheng, Y. Prognostic value of PAX9 in patients with esophageal squamous cell carcinoma and its prediction value to radiation sensitivity. Mol. Med. Rep. 2017, 16, 806–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Z.; Ren, S.; Chen, H.; Liu, Y.; Huang, C.; Zhang, Y.L.; Odera, J.O.; Chen, T.; Kist, R.; Peters, H.; et al. PAX9 regulates squamous cell differentiation and carcinogenesis in the oro-oesophageal epithelium. J. Pathol. 2018, 244, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Di, L.; Li, J.; Fan, W.; Liu, Y.; Guo, W.; Liu, W.; Liu, L.; Li, Q.; Chen, L.; et al. A body map of somatic mutagenesis in morphologically normal human tissues. Nature 2021, 597, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Kakiuchi, N.; Yoshizato, T.; Nannya, Y.; Suzuki, H.; Takeuchi, Y.; Shiozawa, Y.; Sato, Y.; Aoki, K.; Kim, S.K.; et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 2019, 565, 312–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colom, B.; Herms, A.; Hall, M.W.J.; Dentro, S.C.; King, C.; Sood, R.K.; Alcolea, M.P.; Piedrafita, G.; Fernandez-Antoran, D.; Ong, S.H.; et al. Mutant clones in normal epithelium outcompete and eliminate emerging tumours. Nature 2021, 598, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, N.; Ogawa, S. Clonal expansion in non-cancer tissues. Nat. Rev. Cancer 2021, 21, 239–256. [Google Scholar] [CrossRef]
- Wang, J.; Qin, R.; Ma, Y.; Wu, H.; Peters, H.; Tyska, M.; Shaheen, N.J.; Chen, X. Differential gene expression in normal esophagus and Barrett’s esophagus. J. Gastroenterol. 2009, 44, 897–911. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, J.; Li, H.; Hu, Y.; Tevebaugh, W.; Yamamoto, M.; Que, J.; Chen, X. Transcript profiling identifies dynamic gene expression patterns and an important role for Nrf2/Keap1 pathway in the developing mouse esophagus. PLoS ONE 2012, 7, e36504. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Li, W.; Chen, X. P63 Deficiency and CDX2 Overexpression Lead to Barrett’s-Like Metaplasia in Mouse Esophageal Epithelium. Dig. Dis. Sci. 2021, 66, 4263–4273. [Google Scholar] [CrossRef]
- Chen, H.; Beasley, A.; Hu, Y.; Chen, X. A Zebrafish Model for Studies on Esophageal Epithelial Biology. PLoS ONE 2015, 10, e0143878. [Google Scholar] [CrossRef]
- Kendall, J.; Liu, Q.; Bakleh, A.; Krasnitz, A.; Nguyen, K.C.; Lakshmi, B.; Gerald, W.L.; Powers, S.; Mu, D. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 16663–16668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tata, P.R.; Chow, R.D.; Saladi, S.V.; Tata, A.; Konkimalla, A.; Bara, A.; Montoro, D.; Hariri, L.P.; Shih, A.R.; Mino-Kenudson, M.; et al. Developmental History Provides a Roadmap for the Emergence of Tumor Plasticity. Dev. Cell 2018, 44, 679–693.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Szczepanski, A.P.; Tsuboyama, N.; Abdala-Valencia, H.; Goo, Y.A.; Singer, B.D.; Bartom, E.T.; Yue, F.; Wang, L. PAX9 Determines Epigenetic State Transition and Cell Fate in Cancer. Cancer Res. 2021, 81, 4696–4708. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.Q.; Niu, H.B.; Zhang, C.X. PAX9 functions as a tumor suppressor gene for cervical cancer via modulating cell proliferation and apoptosis. Kaohsiung J. Med. Sci. 2022, 38, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Sharma, M.; Lee, Y.H.; Lee, N.H.; Kim, S.Y.; Yun, J.S.; Nam, S.Y.; Hwang, P.H.; Jhee, E.C.; Yi, H.K. Pax9 mediated cell survival in oral squamous carcinoma cell enhanced by c-myb. Cell Biochem. Funct. 2008, 26, 892–899. [Google Scholar] [CrossRef]
- Soto, J.A.; Rodriguez-Antolin, C.; Vera, O.; Pernia, O.; Esteban-Rodriguez, I.; Dolores Diestro, M.; Benitez, J.; Sanchez-Cabo, F.; Alvarez, R.; De Castro, J.; et al. Transcriptional epigenetic regulation of Fkbp1/Pax9 genes is associated with impaired sensitivity to platinum treatment in ovarian cancer. Clin. Epigenetics 2021, 13, 167. [Google Scholar] [CrossRef]
- Michailidou, K.; Hall, P.; Gonzalez-Neira, A.; Ghoussaini, M.; Dennis, J.; Milne, R.L.; Schmidt, M.K.; Chang-Claude, J.; Bojesen, S.E.; Bolla, M.K.; et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat. Genet. 2013, 45, 353–361. [Google Scholar] [CrossRef]
- Guo, X.; Lin, W.; Bao, J.; Cai, Q.; Pan, X.; Bai, M.; Yuan, Y.; Shi, J.; Sun, Y.; Han, M.R.; et al. A Comprehensive cis-eQTL Analysis Revealed Target Genes in Breast Cancer Susceptibility Loci Identified in Genome-wide Association Studies. Am. J. Hum. Genet. 2018, 102, 890–903. [Google Scholar] [CrossRef] [Green Version]
- Ren, N.; Li, Y.; Xiong, Y.; Li, P.; Ren, Y.; Huang, Q. Functional Screenings Identify Regulatory Variants Associated with Breast Cancer Susceptibility. Curr. Issues Mol. Biol. 2021, 43, 1756–1777. [Google Scholar] [CrossRef]
- Rani, L.; Mathur, N.; Gupta, R.; Gogia, A.; Kaur, G.; Dhanjal, J.K.; Sundar, D.; Kumar, L.; Sharma, A. Genome-wide DNA methylation profiling integrated with gene expression profiling identifies PAX9 as a novel prognostic marker in chronic lymphocytic leukemia. Clin. Epigenetics 2017, 9, 57. [Google Scholar] [CrossRef]
- Charbord, P.; Pouget, C.; Binder, H.; Dumont, F.; Stik, G.; Levy, P.; Allain, F.; Marchal, C.; Richter, J.; Uzan, B.; et al. A systems biology approach for defining the molecular framework of the hematopoietic stem cell niche. Cell Stem Cell 2014, 15, 376–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGlinn, E.; van Bueren, K.L.; Fiorenza, S.; Mo, R.; Poh, A.M.; Forrest, A.; Soares, M.B.; Bonaldo Mde, F.; Grimmond, S.; Hui, C.C.; et al. Pax9 and Jagged1 act downstream of Gli3 in vertebrate limb development. Mech. Dev. 2005, 122, 1218–1233. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jing, J.; Li, J.; Zhao, H.; Punj, V.; Zhang, T.; Xu, J.; Chai, Y. BMP signaling orchestrates a transcriptional network to control the fate of mesenchymal stem cells in mice. Development 2017, 144, 2560–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweat, Y.Y.; Sweat, M.; Mansaray, M.; Cao, H.; Eliason, S.; Adeyemo, W.L.; Gowans, L.J.J.; Eshete, M.A.; Anand, D.; Chalkley, C.; et al. Six2 regulates Pax9 expression, palatogenesis and craniofacial bone formation. Dev. Biol. 2020, 458, 246–256. [Google Scholar] [CrossRef]
- Mandler, M.; Neubuser, A. FGF signaling is necessary for the specification of the odontogenic mesenchyme. Dev. Biol. 2001, 240, 548–559. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhou, R.; Deng, M.; Xue, N.; Li, T.; Guo, Y.; Gao, L.; Fan, R.; Zhao, D. Long non-coding RNA DIO3OS binds to microRNA-130b to restore radiosensitivity in esophageal squamous cell carcinoma by upregulating PAX9. Cancer Gene Ther. 2021. [Google Scholar] [CrossRef]
- Talukdar, F.R.; Soares Lima, S.C.; Khoueiry, R.; Laskar, R.S.; Cuenin, C.; Sorroche, B.P.; Boisson, A.C.; Abedi-Ardekani, B.; Carreira, C.; Menya, D.; et al. Genome-Wide DNA Methylation Profiling of Esophageal Squamous Cell Carcinoma from Global High-Incidence Regions Identifies Crucial Genes and Potential Cancer Markers. Cancer Res. 2021, 81, 2612–2624. [Google Scholar] [CrossRef]
- Rauch, T.; Li, H.; Wu, X.; Pfeifer, G.P. MIRA-assisted microarray analysis, a new technology for the determination of DNA methylation patterns, identifies frequent methylation of homeodomain-containing genes in lung cancer cells. Cancer Res. 2006, 66, 7939–7947. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Ren, S.; Chen, H.; Li, J.; Huang, C.; Li, Y.; Han, Y.; Li, Y.; Sun, Z.; Chen, X.; et al. Alcohol drinking inhibits NOTCH-PAX9 signaling in esophageal squamous epithelial cells. J. Pathol. 2021, 253, 384–395. [Google Scholar] [CrossRef]
- Li, Y.; Hibbs, M.A.; Gard, A.L.; Shylo, N.A.; Yun, K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells 2012, 30, 741–752. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, K.A.; Rones, M.S.; Mercola, M. Notch regulates cell fate in the developing pronephros. Dev. Biol. 2000, 227, 567–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidi, H.; Gustafason, D.; Pellegrini, M.; Gasson, J. Identification of novel targets of CSL-dependent Notch signaling in hematopoiesis. PLoS ONE 2011, 6, e20022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onuma, Y.; Takahashi, S.; Asashima, M.; Kurata, S.; Gehring, W.J. Conservation of Pax 6 function and upstream activation by Notch signaling in eye development of frogs and flies. Proc. Natl. Acad. Sci. USA 2002, 99, 2020–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olguin, H.C.; Pisconti, A. Marking the tempo for myogenesis: Pax7 and the regulation of muscle stem cell fate decisions. J. Cell. Mol. Med. 2012, 16, 1013–1025. [Google Scholar] [CrossRef]
- Wen, Y.; Bi, P.; Liu, W.; Asakura, A.; Keller, C.; Kuang, S. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 2012, 32, 2300–2311. [Google Scholar] [CrossRef] [Green Version]
- Jayasena, C.S.; Ohyama, T.; Segil, N.; Groves, A.K. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 2008, 135, 2251–2261. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.M.; Jaskula-Sztul, R.; Ahmed, K.; Harrison, A.D.; Kunnimalaiyaan, M.; Chen, H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol. Cancer Ther. 2013, 12, 1276–1287. [Google Scholar] [CrossRef] [Green Version]
- Bulut-Karslioglu, A.; Perrera, V.; Scaranaro, M.; de la Rosa-Velazquez, I.A.; van de Nobelen, S.; Shukeir, N.; Popow, J.; Gerle, B.; Opravil, S.; Pagani, M.; et al. A transcription factor-based mechanism for mouse heterochromatin formation. Nat. Struct. Mol. Biol. 2012, 19, 1023–1030. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Yu, Q.; Weng, M.; Chen, Z. The Function and Regulatory Network of Pax9 Gene in Palate Development. J. Dent. Res. 2019, 98, 277–287. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, Y.; Lan, Y.; Jia, S.; Jiang, R. Pax9 regulates a molecular network involving Bmp4, Fgf10, Shh signaling and the Osr2 transcription factor to control palate morphogenesis. Development 2013, 140, 4709–4718. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.; Zhou, J.; D’Souza, R.N. Pax9’s dual roles in modulating Wnt signaling during murine palatogenesis. Dev. Dyn. 2020, 249, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Zhou, J.; Fanelli, C.; Wee, Y.; Bonds, J.; Schneider, P.; Mues, G.; D’Souza, R.N. Small-molecule Wnt agonists correct cleft palates in Pax9 mutant mice in utero. Development 2017, 144, 3819–3828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farley-Barnes, K.I.; Deniz, E.; Overton, M.M.; Khokha, M.K.; Baserga, S.J. Paired Box 9 (PAX9), the RNA polymerase II transcription factor, regulates human ribosome biogenesis and craniofacial development. PLoS Genet. 2020, 16, e1008967. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Thomas, G.; Volarevic, S. Ribosome biogenesis in cancer: New players and therapeutic avenues. Nat. Rev. Cancer 2018, 18, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sheng, T.; Zhang, Y.; Zhang, X.; He, J.; Huang, S.; Chen, K.; Sultz, J.; Adegboyega, P.A.; Zhang, H.; et al. Hedgehog signaling is activated in subsets of esophageal cancers. Int. J. Cancer 2006, 118, 139–148. [Google Scholar] [CrossRef]
- Van Dop, W.A.; Rosekrans, S.L.; Uhmann, A.; Jaks, V.; Offerhaus, G.J.; van den Bergh Weerman, M.A.; Kasper, M.; Heijmans, J.; Hardwick, J.C.; Verspaget, H.W.; et al. Hedgehog signalling stimulates precursor cell accumulation and impairs epithelial maturation in the murine oesophagus. Gut 2013, 62, 348–357. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, C.; Zhang, L.Y.; Dong, S.S.; Lu, L.H.; Chen, J.; Dai, Y.; Li, Y.; Kong, K.L.; Kwong, D.L.; et al. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/beta-catenin signalling pathway. Gut 2011, 60, 1635–1643. [Google Scholar] [CrossRef] [Green Version]
- Long, A.; Giroux, V.; Whelan, K.A.; Hamilton, K.E.; Tetreault, M.P.; Tanaka, K.; Lee, J.S.; Klein-Szanto, A.J.; Nakagawa, H.; Rustgi, A.K. WNT10A promotes an invasive and self-renewing phenotype in esophageal squamous cell carcinoma. Carcinogenesis 2015, 36, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Y.; Chen, X. NOTCH and Esophageal Squamous Cell Carcinoma. Adv. Exp. Med. Biol. 2021, 1287, 59–68. [Google Scholar] [CrossRef]
- Kist, R.; Greally, E.; Peters, H. Derivation of a mouse model for conditional inactivation of Pax9. Genesis 2007, 45, 460–464. [Google Scholar] [CrossRef]
- Feng, J.; Jing, J.; Sanchez-Lara, P.A.; Bootwalla, M.S.; Buckley, J.; Wu, N.; Yan, Y.; Chai, Y. Generation and characterization of tamoxifen-inducible Pax9-CreER knock-in mice using CrispR/Cas9. Genesis 2016, 54, 490–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, B.; Schmitt, C.E.; Oh, S.; Kim, J.D.; Choi, W.; Han, O.; Kim, M.; Kim, M.J.; Ham, H.J.; Kim, S.; et al. Pax9 is essential for granulopoiesis but dispensable for erythropoiesis in zebrafish. Biochem. Biophys. Res. Commun. 2021, 534, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, H.; Wang, H.; Guo, F.; Du, X.; Ma, L.; Zhao, L.; Pan, Z.; Gui, H.; Yuan, T.; et al. Characterization of zebrafish Pax1b and Pax9 in fin bud development. Biomed. Res. Int. 2014, 2014, 309385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, W.R.; Draper, B.W.; Stock, D.W. Fgf signaling is required for zebrafish tooth development. Dev. Biol. 2004, 274, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Swartz, M.E.; Sheehan-Rooney, K.; Dixon, M.J.; Eberhart, J.K. Examination of a palatogenic gene program in zebrafish. Dev. Dyn. 2011, 240, 2204–2220. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, Y.; Paiboonrungruang, C.; Li, Y.; Peters, H.; Kist, R.; Xiong, Z. PAX9 in Cancer Development. Int. J. Mol. Sci. 2022, 23, 5589. https://doi.org/10.3390/ijms23105589
Chen X, Li Y, Paiboonrungruang C, Li Y, Peters H, Kist R, Xiong Z. PAX9 in Cancer Development. International Journal of Molecular Sciences. 2022; 23(10):5589. https://doi.org/10.3390/ijms23105589
Chicago/Turabian StyleChen, Xiaoxin, Yahui Li, Chorlada Paiboonrungruang, Yong Li, Heiko Peters, Ralf Kist, and Zhaohui Xiong. 2022. "PAX9 in Cancer Development" International Journal of Molecular Sciences 23, no. 10: 5589. https://doi.org/10.3390/ijms23105589





