Chemosensitization of U-87 MG Glioblastoma Cells by Neobavaisoflavone towards Doxorubicin and Etoposide
Abstract
:1. Introduction
2. Results
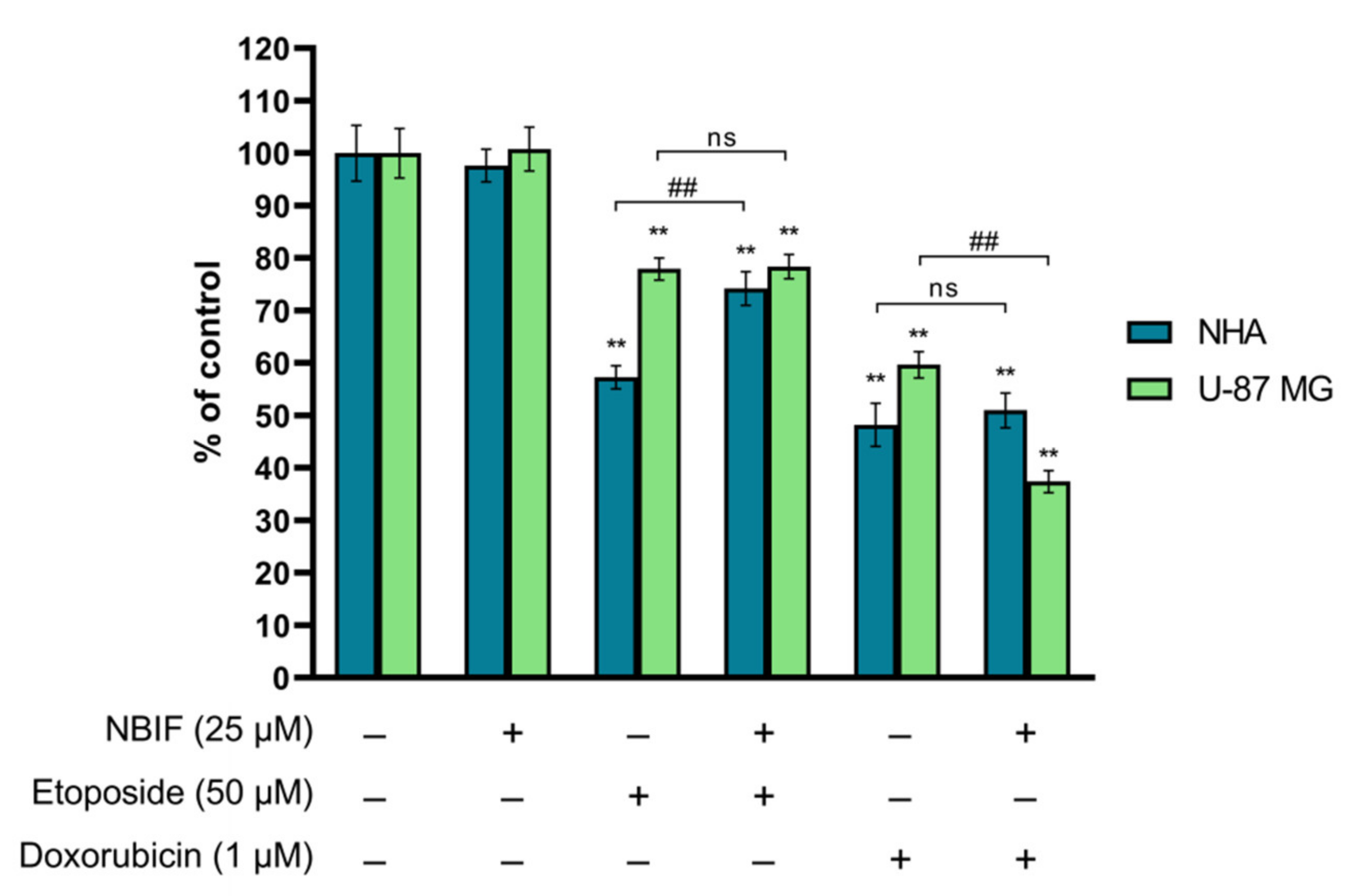
2.1. NBIF in Combined Treatment with Doxorubicin Causes a Reduction in Number of U-87 MG Cells
2.2. Co-Treatment of NBIF with Etoposide or Doxorubicin Intensifies Apoptosis in U-87 MG Cells and Astrocytes
2.3. The Impact of NBIF Combined with Etoposide or Doxorubicin on the Mitochondrial Membrane Potential in NHA and U-87 MG Cells
2.4. Etoposide- or Doxorubicin + NBIF Co-Treatment Prompts Cell Cycle Alterations in U-87 MG Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Cultures
4.3. Cell Treatment
4.4. Cell Count Assay
4.5. Annexin V Assay
4.6. Mitochondrial Potential Assay
4.7. Cell Cycle Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lapointe, T.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, G.; Qi, S. Aggressive treatment in glioblastoma: What determines the survival of patients? J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 112–117. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Birzu, C.; French, P.; Caccese, M.; Cerretti, G.; Idbaih, A.; Zagonel, V.; Lobardi, G. Recurrent glioblastoma: From molecular landscape to new treatment perspectives. Cancers 2020, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- Medikonda, R.; Dunn, G.; Rahman, M.; Fecci, P.; Lim, M. A review of glioblastoma immunotherapy. J. Neurooncol. 2021, 151, 41–53. [Google Scholar] [CrossRef]
- Weller, M.; Van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Lisi, L.; Chiavari, M.; Ciotti, G.M.P.; Lacal, P.M.; Navarra, P.; Graziani, G. DNA inhibitors for the treatment of brain tumors. Expert Opin. Drug. Metab. Toxicol. 2020, 16, 195–207. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Sampson, J.H.; Sathornsumetee, S.; McLendon, R.E.; Herndon, J.E., II; Marcello, J.E.; Norfleet, J.; et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: A phase II study. Br. J. Cancer 2009, 101, 1986–1994. [Google Scholar] [CrossRef]
- Carrillo, J.A.; Hsu, F.P.K.; Delashaw, J.; Bota, D.A. Efficacy and safety of bevacizumab and etoposide combination in patients with recurrent malignant gliomas who have failed bevacizumab. RHC 2014, 5, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Villodre, E.S.; Kipper, F.C.; Silva, A.O.; Lenz, G.; Da Costa Lopez, P.L. Low dose of doxorubicin potentiates the effect of temozolomide in glioblastoma cells. Mol. Neurobiol. 2018, 55, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Horescu, C.; Cioc, C.E.; Tuta, C.; Sevastre, A.S.; Tache, D.E.; Alexandru, O.; Artene, S.A.; Danoiu, S.; Dricu, A.; Oana, P.S. The effect of temozolomide in combination with doxorubicin in glioblastoma cells in vitro. J. Immunoass. Immunochem. 2020, 31, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Castro-Osma, J.A.; Posadas, I.; Alonso-Moreno, C.; Bravo, I.; Garzón, A.; Canales-Vázquez, J.; Ceña, V.; Lara-Sánchez, A.; Albaladejo, J.; et al. Assessment of doxorubicin delivery devices based on tailored bare polycaprolactone against glioblastoma. Int. J. Pharm. 2019, 558, 110–119. [Google Scholar] [CrossRef]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chang, Y.H.; Rajesh, R. Targeted delivery of etoposide, carmustine and doxorubicin to human glioblastoma cells using methoxy poly(ethylene glycol)-poly(ε-caprolactone) nanoparticles conjugated with wheat germ agglutinin and folic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 114–128. [Google Scholar] [CrossRef]
- Cao, W.Q.; Li, Y.; Hou, Y.J.; Yang, M.X.; Fu, X.Q.; Zhao, B.S.; Jiang, H.M.; Fu, X.Y. Enhanced anticancer efficiency of doxorubicin against human glioma by natural borneol through triggering ROS-mediated signal. Biomed. Pharm. 2019, 118, 109261. [Google Scholar] [CrossRef]
- Leary, M.; Heerboth, S.; Lapinska, K.; Sarkar, S. Sensitization of drug resistant cancer cells: A matter of combination therapy. Cancers 2018, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Cháirez-Ramírez, M.H.; De la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as antitumor agents targeting key players in cancer-driving signaling pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Blackburn, G.L.; Zhou, J.R. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol. Carcinog. 2007, 46, 534–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, L.A.; Ali, S.; Banerjee, S.; Munkarah, A.R.; Morris, R.T.; Sarkar, F.H. Sensitization of ovarian cancer cells to cisplatin by genistein: The role of NF-kappaB. J. Ovarian Res. 2008, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Adhikary, A.; Bhattacharyya, P.; Das, T.; Sa, G. Death by design: Where curcumin sensitizes drug-resistant tumours. Anticancer Res. 2012, 32, 2567–2584. [Google Scholar]
- Sahin, K.; Tuzcu, M.; Basak, N.; Caglayan, B.; Kilic, U.; Sahin, F.; Kucuk, O. Sensitization of cervical cancer cells to cisplatin by genistein: The role of NFκB and Akt/mTOR signaling pathways. J. Oncol. 2012, 2012, 461562. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Kannappan, R.; Reuter, S.; Kim, J.H.; Aggarwal, B.B. Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.M.; Bayoumi, H.M.; Al-Harthi, S.E.; Damanhouri, Z.A.; Elshal, M.F. Modulation of doxorubicin cytotoxicity by resveratrol in a human breast cancer cell line. Cancer Cell Int. 2012, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.P.; Wang, G.; Zhao, Z.B.; Wang, Q.; Shi, Y. Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef] [Green Version]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.L.; Cross, N.A.; Jordan-Mahy, N. Polyphenols act synergistically with doxorubicin and etoposide in leukaemia cell lines. Cell Death Discov. 2015, 1, 15043. [Google Scholar] [CrossRef]
- Jia, H.; Yang, Q.; Wang, T.; Cao, Y.; Jiang, Q.Y.; Ma, H.D.; Sun, H.W.; Hou, M.X.; Yang, Y.P.; Feng, F. Rhamnetin induces sensitization of hepatocellular carcinoma cells to a small molecular kinase inhibitor or chemotherapeutic agents. Biochim. Biophys. Acta 2016, 1860, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wei, Y.; Liu, Y.; Lu, X.; Ding, F.; Wang, J.; Yang, S. Resveratrol promotes sensitization to doxorubicin by inhibiting epithelial-mesenchymal transition and modulating SIRT1/β-catenin signaling pathway in breast cancer. Cancer Med. 2019, 8, 1246–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, J.E.; Puga, R.; Lenz, G.; Trindade, C.; Filippi-Chiela, E. Cellular mechanisms triggered by the cotreatment of resveratrol and doxorubicin in breast cancer: A translational in vitro-in silico model. Oxid. Med. Cell Longev. 2020, 2020, 5432651. [Google Scholar] [CrossRef] [PubMed]
- Maszczyk, M.; Rzepka, Z.; Rok, J.; Beberok, A.; Wrześniok, D. Neobavaisoflavone may modulate the activity of topoisomerase inhibitors towards U-87 MG Cells: An in vitro study. Molecules 2021, 26, 4516. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Júnior, R.G.; Christiane Adrielly, A.F.; Da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Sędek, Ł.; Paradysz, A.; Król, W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol. Rep. 2011, 63, 139–148. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, W.I.; Ko, H.; So, Y.; Kang, K.S.; Kim, I.; Kim, K.; Yoon, H.G.; Kim, T.J.; Choi, K.C. Neobavaisoflavone sensitizes apoptosis via the inhibition of metastasis in TRAIL-resistant human glioma U373MG cells. Life Sci. 2014, 95, 101–107. [Google Scholar] [CrossRef]
- Ye, H.; He, X.; Feng, X. Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharm. 2020, 129, 110369. [Google Scholar] [CrossRef]
- Cai, X.; Zhou, F.; Xie, X.; Zheng, D.; Yao, Y.; Zhao, C.; Huang, X.; Hu, K. Neobavaisoflavone demonstrates valid anti-tumor effects in non-small- cell lung cancer by inhibiting STAT3. Comb. Chem. High Throughput Screen. 2022, 25, 29–37. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameyanda Poonacha, S.; Harishkumar, M.; Radha, M.; Varadarajan, R.; Nalilu, S.K.; Shetty, S.S.; Shetty, P.K.; Chandrashekharappa, R.B.; Sreenivas, M.G.; Bhandary Bavabeedu, S.K. Insight into oroxylinA-7-O-β-D-glucuronide-enriched oroxylum indicum bark extract in oral cancer HSC-3 cell apoptotic mechanism: Role of mitochondrial microenvironment. Molecules 2021, 26, 7430. [Google Scholar] [CrossRef] [PubMed]
- Leal-Esteban, L.C.; Fajas, L. Cell cycle regulators in cancer cell metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165715. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting cell-cycle machinery in cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, B.; Cosmai, L.; Porta, C. Conventional chemotherapy. In Onco-Nephrology, 1st ed.; Finkel, K., Perazella, M., Cohen, E., Eds.; Elsevier: Philadelphia, PA, USA, 2019; Volume 4, pp. 128–151. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maszczyk, M.; Banach, K.; Karkoszka, M.; Rzepka, Z.; Rok, J.; Beberok, A.; Wrześniok, D. Chemosensitization of U-87 MG Glioblastoma Cells by Neobavaisoflavone towards Doxorubicin and Etoposide. Int. J. Mol. Sci. 2022, 23, 5621. https://doi.org/10.3390/ijms23105621
Maszczyk M, Banach K, Karkoszka M, Rzepka Z, Rok J, Beberok A, Wrześniok D. Chemosensitization of U-87 MG Glioblastoma Cells by Neobavaisoflavone towards Doxorubicin and Etoposide. International Journal of Molecular Sciences. 2022; 23(10):5621. https://doi.org/10.3390/ijms23105621
Chicago/Turabian StyleMaszczyk, Mateusz, Klaudia Banach, Marta Karkoszka, Zuzanna Rzepka, Jakub Rok, Artur Beberok, and Dorota Wrześniok. 2022. "Chemosensitization of U-87 MG Glioblastoma Cells by Neobavaisoflavone towards Doxorubicin and Etoposide" International Journal of Molecular Sciences 23, no. 10: 5621. https://doi.org/10.3390/ijms23105621
APA StyleMaszczyk, M., Banach, K., Karkoszka, M., Rzepka, Z., Rok, J., Beberok, A., & Wrześniok, D. (2022). Chemosensitization of U-87 MG Glioblastoma Cells by Neobavaisoflavone towards Doxorubicin and Etoposide. International Journal of Molecular Sciences, 23(10), 5621. https://doi.org/10.3390/ijms23105621





