Potential Actions of Baicalein for Preventing Vascular Calcification of Smooth Muscle Cells In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Effect of Baicalein in the Inhibition of β-GP-Induced VSMC Calcification
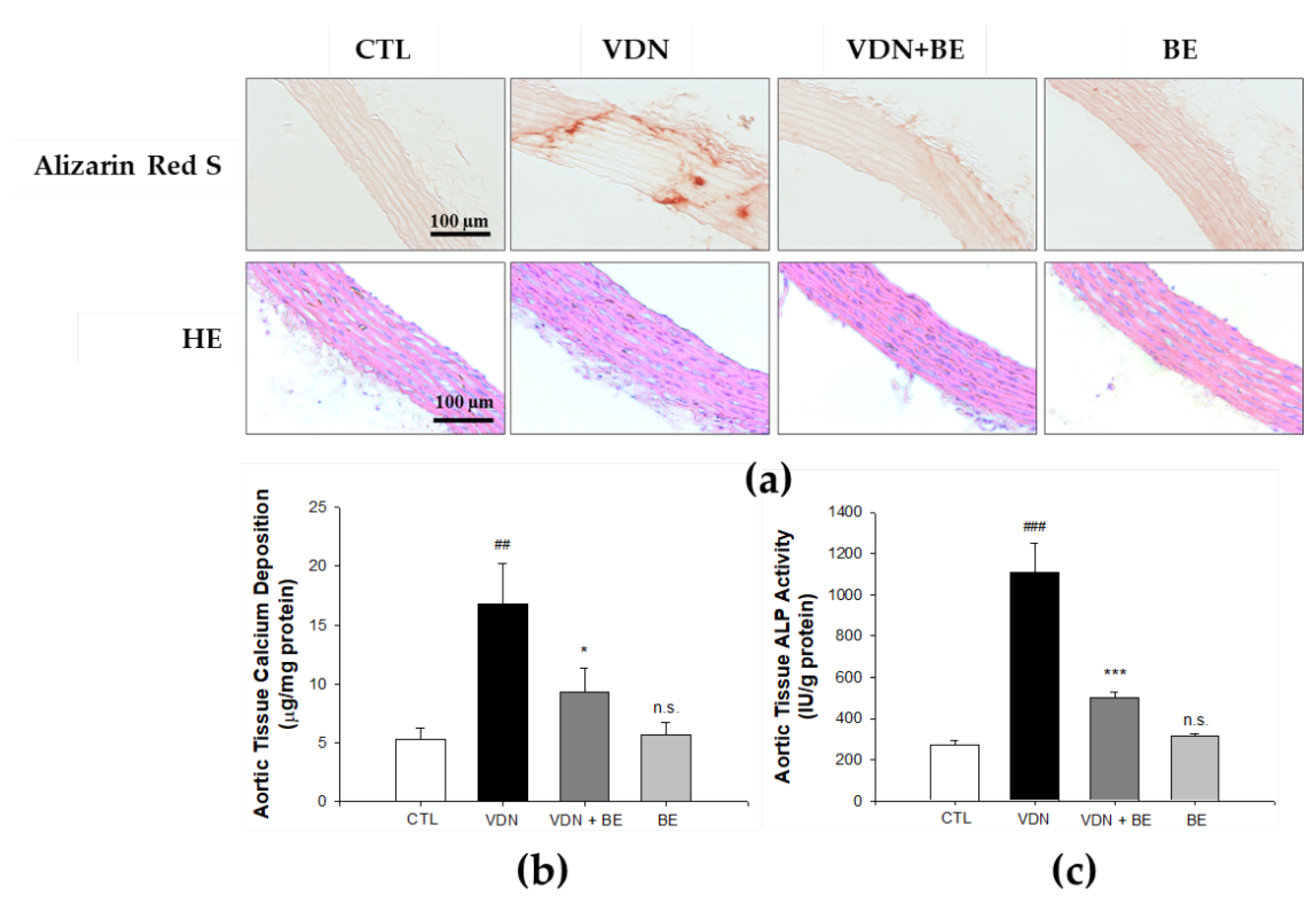
2.2. Effect of Baicalein in Blocking Calcium Deposition and ALP Activity
2.3. Effect of Baicalein in Protecting VSMCs against β-GP-Induced Apoptosis
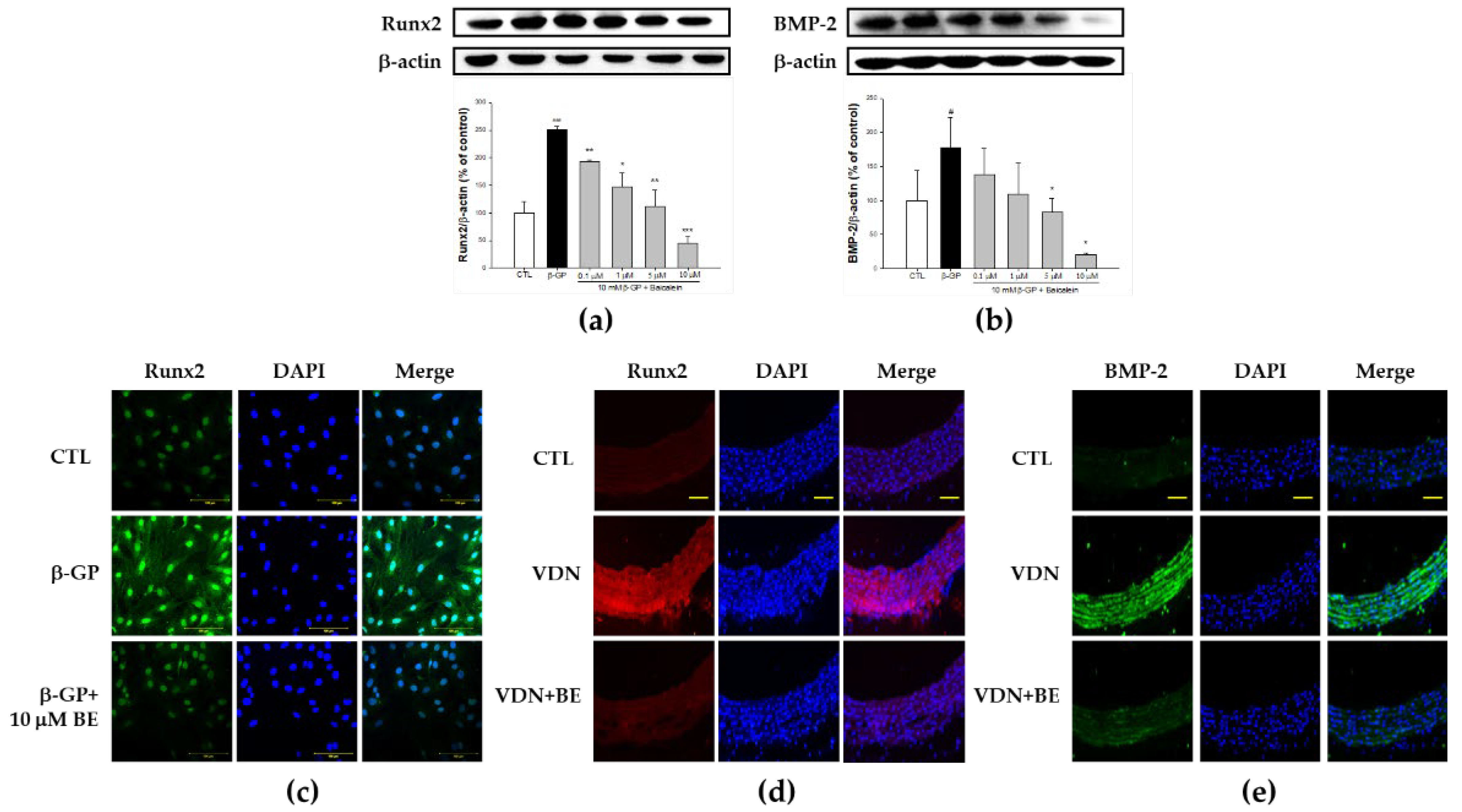
2.4. Effect of Baicalein in the Downregulation of Runx2-BMP-2 Biomarkers in β-GP-Induced VSMCs Calcification
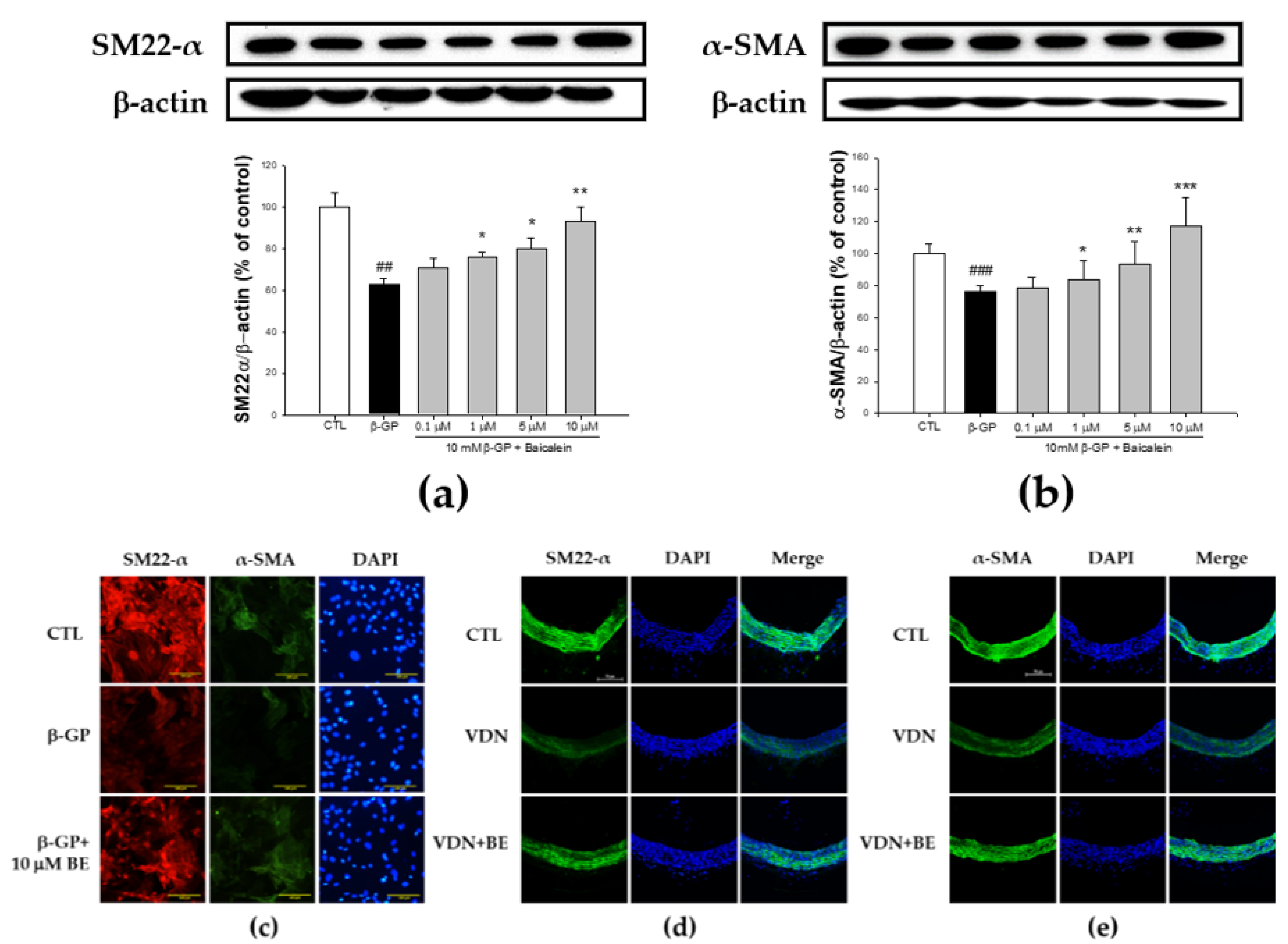
2.5. Effect of Baicalein in the Elevation of SM22-α and α-SMA Expression in β-GP-Induced VSMCs Calcification
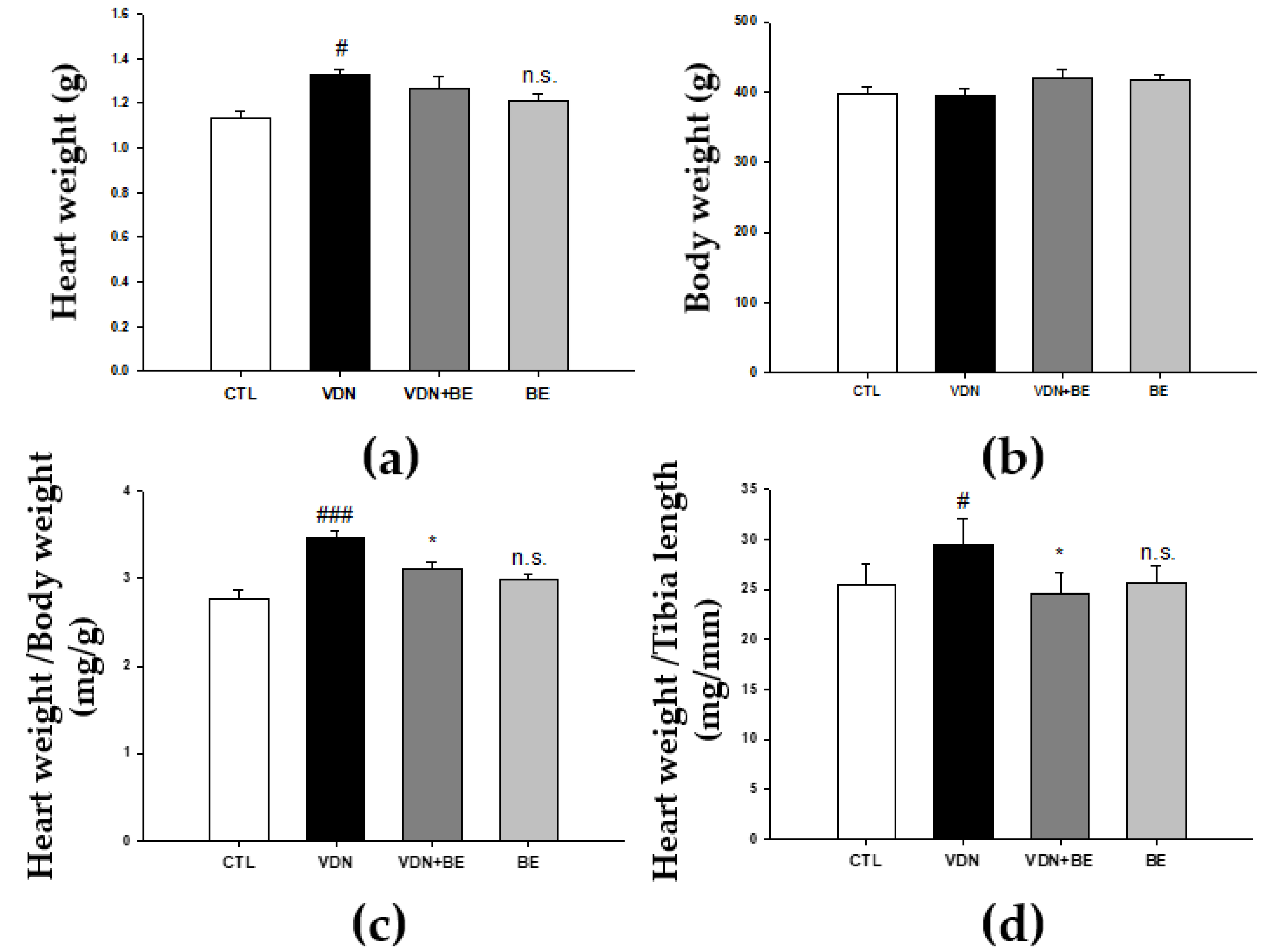
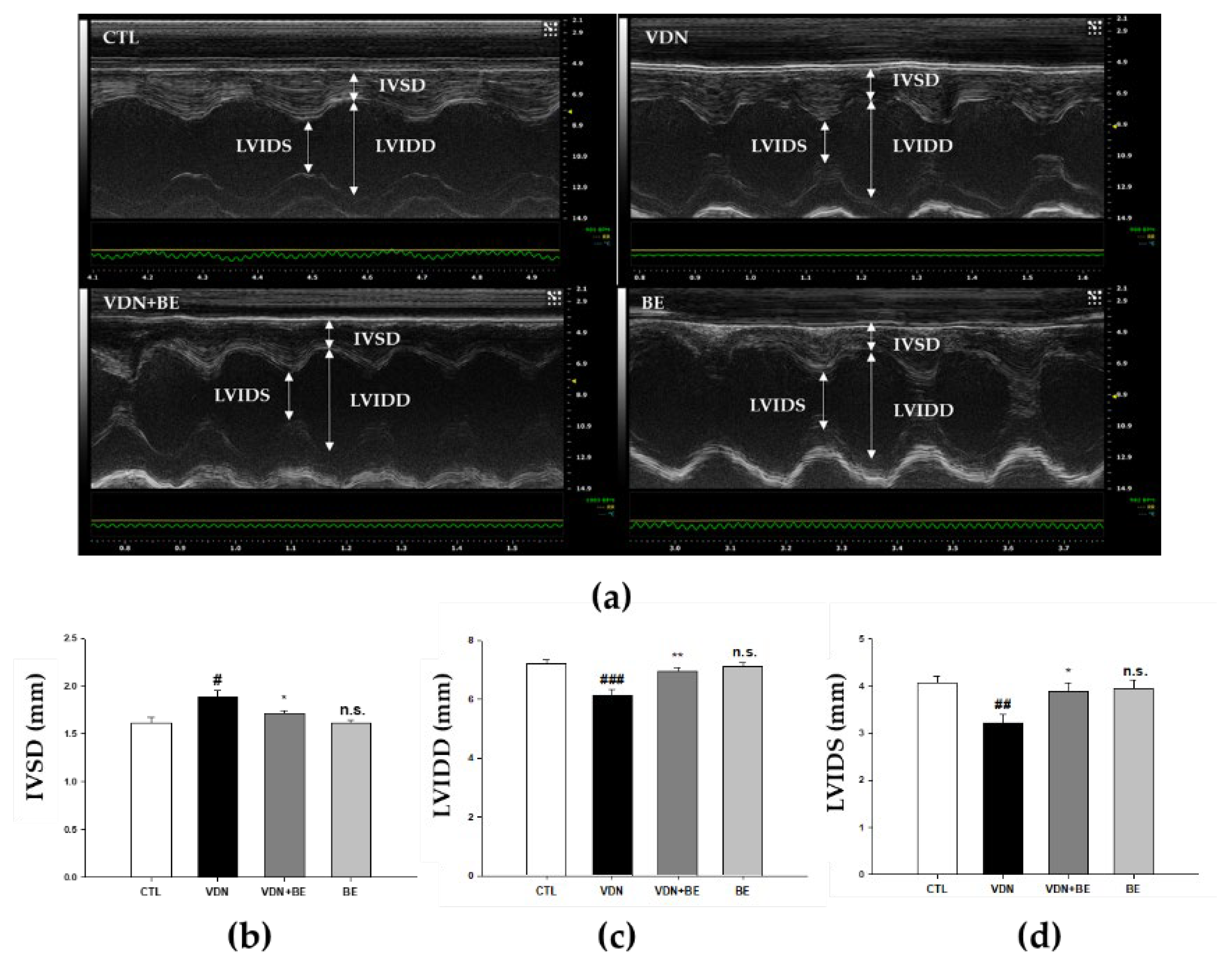
2.6. Effects of Baicalein on Cardiac Morphometry in VDN-Induced VC Rats
2.7. Effects of Baicalein on Left Ventricular Cardiac Performance in VDN-Induced Rats VC
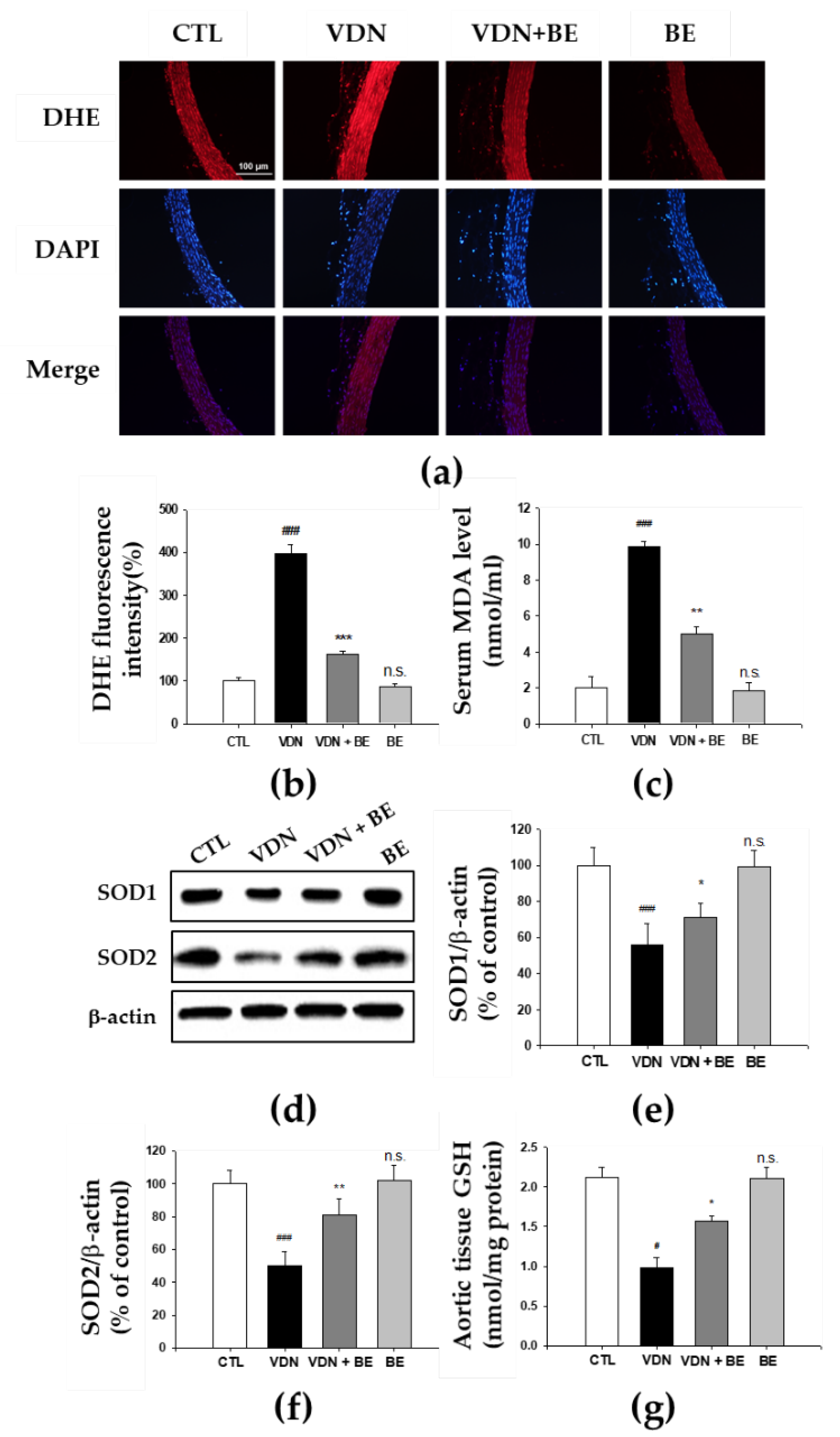
2.8. Effects of Baicalein in the Redox Status of VDN-Induced Rats VC
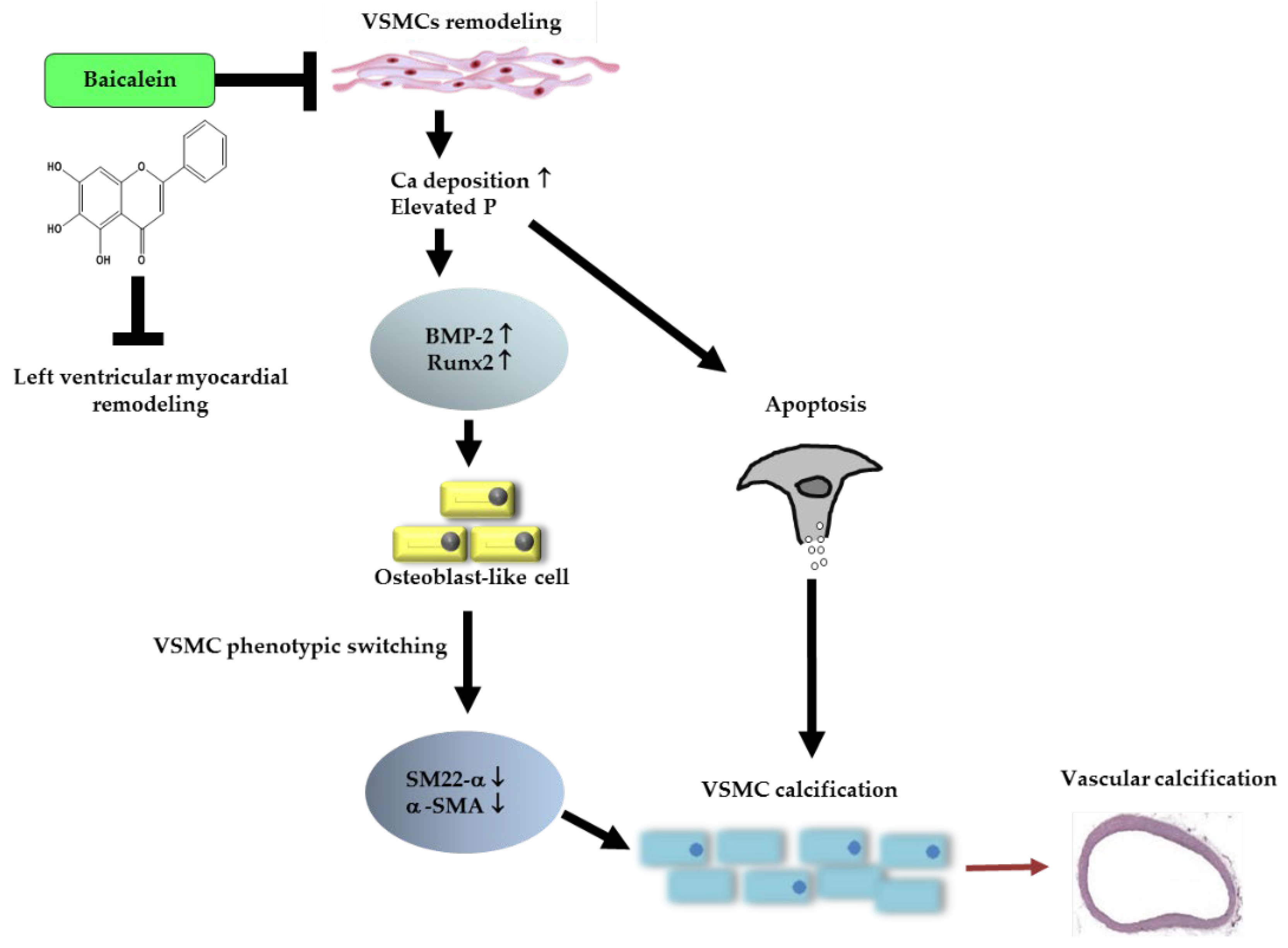
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation and Culture of Primary Rat VSMCs
4.3. In Vitro VSMCs Calcification
4.4. Alkaline Phosphatase (ALP) Activity
4.5. Alizarin Red S Staining and Calcium Content Quantification
4.6. Flow Cytometric Analysis
4.7. Western Blot Analysis
4.8. Immunofluorescence Assay
4.9. Experimental Animals
4.10. Echocardiography
4.11. Determination of In Vivo Redox Status
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gao, J.; Lv, Q.; Cai, H.; Wang, F.; Ye, R.; Liu, X. Calcification in atherosclerotic plaque vulnerability: Friend or foe? Front. Physiol. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Rementer, C.; Giachelli, C.-M. Vascular Calcification: An Update on Mechanisms and Challenges in Treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Sage, A.P.; Tintut, Y.; Demer, L.L. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 2010, 7, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhou, Y.; Teng, X.; Tang, C.; Qi, Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem. Biophys. Res. Commun. 2009, 387, 694–699. [Google Scholar] [CrossRef]
- Evrard, S.; Delanaye, P.; Kamel, S.; Cristol, J.-P.; Cavalier, E.; Arnaud, J.; Zaoui, P.; Carlier, M.; Laville, M.; Fouque, D. Vascular calcification: From pathophysiology to biomarkers. Clin. Chim. Acta 2015, 438, 401–414. [Google Scholar] [CrossRef]
- Viegas, C.S.; Santos, L.; Macedo, A.L.; Matos, A.A.; Silva, A.P.; Neves, P.L.; Staes, A.; Gevaert, K.; Morais, R.; Vermeer, C. Chronic kidney disease circulating calciprotein particles and extracellular vesicles promote vascular calcification: A role for GRP (Gla-Rich Protein). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Voelkl, J.; Lang, F.; Eckardt, K.-U.; Amann, K.; Kuro-o, M.; Pasch, A.; Pieske, B.; Alesutan, I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell. Mol. Life Sci. 2019, 76, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.Y.; Shanahan, C.M. Medial arterial calcification: An overlooked player in peripheral arterial disease. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Zheng, L.; Xu, H.; Tang, D.; Lin, L.; Zhang, J.; Li, C.; Wang, W.; Yuan, Q.; Tao, L. Oxidative stress contributes to vascular calcification in patients with chronic kidney disease. J. Mol. Cell. Cardiol. 2020, 138, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.-M.; Xu, M.-J.; Cai, Y.; Zhao, G.; Guan, Y.; Kong, W.; Tang, C.; Wang, X. Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int. 2011, 79, 1071–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor RUNX2 by Akt signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durik, M.; Kavousi, M.; van der Pluijm, I.; Isaacs, A.; Cheng, C.; Verdonk, K.; Loot, A.E.; Oeseburg, H.; Bhaggoe, U.M.; Leijten, F. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation 2012, 126, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, T.; Jin, G.-B.; Cho, S.; Cyong, J.-C. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002, 68, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Zhang, R.; Piao, M.J.; Chae, S.; Kim, H.S.; Park, J.H.; Jung, K.S.; Hyun, J.W. Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicol. Ind. Health 2012, 28, 412–421. [Google Scholar] [CrossRef]
- Choi, E.-O.; Park, C.; Hwang, H.-J.; Hong, S.H.; Kim, G.-Y.; Cho, E.-J.; Kim, W.-J.; Choi, Y.H. Baicalein induces apoptosis via ROS-dependent activation of caspases in human bladder cancer 5637 cells. Int. J. Oncol. 2016, 49, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Sun, W.; Yu, N.; Sun, J.; Yu, X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.; Xiu, Z. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods. 2018, 46, 256–267. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Hassan, N.A.; Mahmoud, M.F.; Fahmy, A. Baicalein protects against hypertension associated with diabetes: Effect on vascular reactivity and stiffness. Phytomedicine 2014, 21, 1742–1745. [Google Scholar] [CrossRef]
- Cui, G.; Luk, S.C.W.; Li, R.A.; Chan, K.K.K.; Lei, S.W.; Wang, L.; Shen, H.; Leung, G.P.H.; Lee, S.M.Y. Cytoprotection of baicalein against oxidative stress-induced cardiomyocytes injury through the Nrf2/Keap1 pathway. J. Cardiovasc. Pharmacol. Ther. 2015, 65, 39–46. [Google Scholar] [CrossRef]
- Lee, S.-I.; Kim, S.-Y.; Park, K.-R.; Kim, E.-C. Baicalein promotes angiogenesis and odontoblastic differentiation via the BMP and Wnt pathways in human dental pulp cells. Am. J. Chin. Med. 2016, 44, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, R.-l.; Croce, C.M.; Stein, J.L.; Lian, J.B.; Van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Oyama, T.; Asada, M.; Harada, D.; Ito, Y.; Inagawa, M.; Suzuki, Y.; Sugano, S.; Katsube, K.-I.; Karsenty, G. MAML1 enhances the transcriptional activity of Runx2 and plays a role in bone development. PLoS Genet. 2013, 9, e1003132. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Morohashi, K.; Yilmaz, A.; Kuramochi, K.; Parihar, A.; Brahimaj, B.; Grotewold, E.; Doseff, A.I. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc. Natl. Acad. Sci. USA 2013, 110, E2153–E2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Probst, Y.C.; Guan, V.X.; Kent, K. Dietary phytochemical intake from foods and health outcomes: A systematic review protocol and preliminary scoping. BMJ Open 2017, 7, e013337. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Fedirko, V.; De Magistris, M.S.; Ericson, U.; Amiano, P.; Trichopoulou, A. Estimated dietary intakes of flavonols, flavanones and flavones in the european prospective investigation into cancer and nutrition (epic) 24 hour dietary recall cohort. Br. J. Nutr. 2011, 106, 1915–1925. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Huang, T.; Rice, M.S.; Rimm, E.B.; Tworoger, S.S. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am. J. Clin. Nutr. 2014, 100, 1344–1351. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wang, H.; Wang, D.; Chen, Y.; Zhao, Y.; Xia, W. Using an FFQ to assess intakes of dietary flavonols and flavones among female adolescents in the suihua area of northern china. Public Health Nutr. 2015, 18, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Neven, E.; d’Haese, P.C. Vascular calcification in chronic renal failure: What have we learned from animal studies? Circ. Res. 2011, 108, 249–264. [Google Scholar] [CrossRef]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B.; Schurgers, L. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation: Involvement of vitamin K-dependent processes. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef]
- Proudfoot, D. Molecular mechanisms of arterial calcification. Artery Res. 2009, 3, 128–131. [Google Scholar] [CrossRef]
- Iyemere, V.; Proudfoot, D.; Weissberg, P.; Shanahan, C. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J. Intern. Med. 2006, 260, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-J.; Hu, B.-B.; Shi, X.-L.; Ren, M.-M.; Yu, W.-B.; Cen, S.-D.; Hu, R.-D.; Deng, H. Baicalein enhances the osteogenic differentiation of human periodontal ligament cells by activating the Wnt/β-catenin signaling pathway. Arch. Oral. Biol. 2017, 78, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Chen, Y.; Li, C.; Lu, L. Quercetin attenuates Ox-LDL-induced calcification in vascular smooth muscle cells by regulating ROS-TLR4 signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2018, 38, 980–985. [Google Scholar] [CrossRef]
- Vargas, F.; Romecín, P.; García-Guillén, A.I.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Flavonoids in kidney health and disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867. [Google Scholar] [CrossRef] [Green Version]
- Kapustin, A.N.; Davies, J.D.; Reynolds, J.L.; McNair, R.; Jones, G.T.; Sidibe, A.L.; Schurgers, J.; Skepper, J.N.; Proudfoot, D.; Mayr, M. Calcium regulates key components of vascular smooth muscle cell–derived matrix vesicles to enhance mineralization. Circ. Res. 2011, 109, e1–e12. [Google Scholar] [CrossRef] [Green Version]
- Giachelli, C.M. Vascular calcification mechanisms. J. Am. Soc. Nephrol. 2004, 15, 2959–2964. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.L.; Pai, A.; Moe, S.M.; Giachelli, C.M. Direct effects of phosphate on vascular cell function. Adv. Chronic. Kidney Dis. 2011, 18, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, M.; Ciceri, P.; Galassi, A.; Mangano, M.; Carugo, S.; Capelli, I.; Cianciolo, G. The key role of phosphate on vascular calcification. Toxins 2019, 11, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manivannan, J.; Prashanth, M.; Kumar, V.S.; Shairam, M.; Subburaj, J. Systems biological understanding of the regulatory network and the possible therapeutic strategies for vascular calcification. Mol. Biosyst. 2016, 12, 3683–3694. [Google Scholar] [CrossRef] [PubMed]
- Lomashvili, K.A.; Cobbs, S.; Hennigar, R.A.; Hardcastle, K.I.; O’Neill, W.C. Phosphate-induced vascular calcification: Role of pyrophosphate and osteopontin. J. Am. Soc. Nephrol. 2004, 15, 1392–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proudfoot, D.; Skepper, J.N.; Hegyi, L.; Bennett, M.R.; Shanahan, C.M.; Weissberg, P.L. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 2000, 87, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Zheng, H.; Tao, H.; Yu, W.; Jiang, X.; Li, A.; Jin, H.; Lv, A.; Li, H. Vitamin K2 inhibits rat vascular smooth muscle cell calcification by restoring the Gas6/Axl/Akt anti-apoptotic pathway. Mol. Cell. Biochem. 2017, 433, 149–159. [Google Scholar] [CrossRef]
- Clarke, M.C.; Littlewood, T.D.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Bennett, M.R. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ. Res. 2008, 102, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Ponnusamy, A.; Sinha, S.; Hyde, G.D.; Borland, S.J.; Taylor, R.F.; Pond, E.; Eyre, H.J.; Inkson, C.A.; Gilmore, A.; Ashton, N. FTI-277 inhibits smooth muscle cell calcification by up-regulating PI3K/Akt signaling and inhibiting apoptosis. PLoS ONE 2018, 13, e0196232. [Google Scholar] [CrossRef] [Green Version]
- Lowery, J.W.; Pazin, D.; Intini, G.S.; Kokabu, V.; Chappuis, L.P.; Capelo, V.R. The role of BMP2 signaling in the skeleton. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 177–185. [Google Scholar] [CrossRef]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The role of vascular smooth muscle cells in arterial remodeling: Focus on calcification-related processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef] [Green Version]
- Kono, K.; Fujii, H.; Nakai, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Shinohara, M.; Hirata, M.; Fukagawa, M.; Nishi, S. Anti-oxidative effect of vitamin D analog on incipient vascular lesion in non-obese type 2 diabetic rats. Am. J. Nephrol. 2013, 37, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Nakai, K.; Yonekura, Y.; Kono, K.; Goto, S.; Hirata, M.; Shinohara, M.; Nishi, S.; Fukagawa, M. The vitamin D receptor activator maxacalcitol provides cardioprotective effects in diabetes mellitus. Cardiovasc. Drugs Ther. 2015, 29, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, S.; Fujii, H.; Kono, K.; Nakai, K.; Hamada, Y.; Yamato, H.; Shinohara, M.; Kitazawa, R.; Kitazawa, S.; Nishi, S. Carvedilol ameliorates low-turnover bone disease in non-obese type 2 diabetes. Am. J. Nephrol. 2011, 34, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-T.; Shao, Y.-D.; Liu, Y.-Z.; Xiao, X.; Cheng, Z.-B.; Qu, S.-L.; Huang, L.; Zhang, C. Oxidative stress in vascular calcification. Clin. Chim. Acta 2021, 519, 101–110. [Google Scholar] [CrossRef]
- Liberman, M.; Bassi, E.; Martinatti, M.-K.; Lario, F.C.; Wosniak, J., Jr.; Pomerantzeff, P.-M.; Laurindo, F.-R. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Derwall, M.; Malhotra, R.; Lai, C.-S.; Beppu, Y.; Aikawa, E.; Seehra, J.-S.; Zapol, W.-M.; Bloch, K.-D.; Yu, P.B. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Virdis, A.; Duranti, E.; Taddei, S. Oxidative stress and vascular damage in hypertension: Role of angiotensin II. Int. J. Hypertens. 2011, 22, 535–542. [Google Scholar] [CrossRef]
- Yamada, S.; Taniguchi, M.; Tokumoto, M.; Toyonaga, J.; Fujisaki, K.; Suehiro, T.; Noguchi, H.; Iida, M.; Tsuruya, K.; Kitazono, T. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: Important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J. Bone Miner. Res. 2012, 27, 474–485. [Google Scholar] [CrossRef]
- Wu, J.-R.; Liou, S.-F.; Lin, S.-W.; Chai, C.-Y.; Dai, Z.-K.; Liang, J.-C.; Chen, J.; Yeh, J.-L. Lercanidipine inhibits vascular smooth muscle cell proliferation and neointimal formation via reducing intracellular reactive oxygen species and inactivating Ras-ERK1/2 signaling. Pharmacol. Res. 2009, 59, 48–56. [Google Scholar] [CrossRef]
- Liou, S.-F.; Nguyen, T.T.N.; Hsu, J.-H.; Sulistyowati, E.; Huang, S.-E.; Wu, B.-N.; Lin, M.-C.; Yeh, J.-L. The preventive effects of xanthohumol on vascular calcification induced by vitamin d3 plus nicotine. Antioxidants 2020, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.J.; Carvalho, D.O.; Cruz, J.M.; Guido, L.F.; Barros, A.A. Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Nat. Prod. Commun. 2009, 4, 591–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, B.-D.; Kumar, J.-M.; Sistla, R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS ONE 2015, 10, e0134139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulistyowati, E.; Hsu, J.-H.; Cheng, Y.-B.; Chang, F.-R.; Chen, Y.-F.; Yeh, J.-L. Indonesian herbal medicine prevents hypertension-induced left ventricular hypertrophy by diminishing NADPH oxidase-dependent oxidative stress. Oncotarget 2017, 8, 86784. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulistyowati, E.; Hsu, J.-H.; Lee, S.-J.; Huang, S.-E.; Sihotang, W.Y.; Wu, B.-N.; Dai, Z.-K.; Lin, M.-C.; Yeh, J.-L. Potential Actions of Baicalein for Preventing Vascular Calcification of Smooth Muscle Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 5673. https://doi.org/10.3390/ijms23105673
Sulistyowati E, Hsu J-H, Lee S-J, Huang S-E, Sihotang WY, Wu B-N, Dai Z-K, Lin M-C, Yeh J-L. Potential Actions of Baicalein for Preventing Vascular Calcification of Smooth Muscle Cells In Vitro and In Vivo. International Journal of Molecular Sciences. 2022; 23(10):5673. https://doi.org/10.3390/ijms23105673
Chicago/Turabian StyleSulistyowati, Erna, Jong-Hau Hsu, Szu-Jung Lee, Shang-En Huang, Widya Yanti Sihotang, Bin-Nan Wu, Zen-Kong Dai, Ming-Chung Lin, and Jwu-Lai Yeh. 2022. "Potential Actions of Baicalein for Preventing Vascular Calcification of Smooth Muscle Cells In Vitro and In Vivo" International Journal of Molecular Sciences 23, no. 10: 5673. https://doi.org/10.3390/ijms23105673
APA StyleSulistyowati, E., Hsu, J.-H., Lee, S.-J., Huang, S.-E., Sihotang, W. Y., Wu, B.-N., Dai, Z.-K., Lin, M.-C., & Yeh, J.-L. (2022). Potential Actions of Baicalein for Preventing Vascular Calcification of Smooth Muscle Cells In Vitro and In Vivo. International Journal of Molecular Sciences, 23(10), 5673. https://doi.org/10.3390/ijms23105673







