MTP8 from Triticum urartu Is Primarily Responsible for Manganese Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. RNA Isolation, Reverse Transcription, and qRT-PCR
2.3. Expression of TuMTP8 in Yeast
2.4. Yeast Growth Assay
2.5. Metal Transport in Yeast Cells
2.6. Transient Expression of TuMTP8 in Tobacco
2.7. Plant Transformation
2.8. Stress Tolerance of Arabidopsis Overexpressing TuMTP8
2.9. Statistical Analysis
3. Results
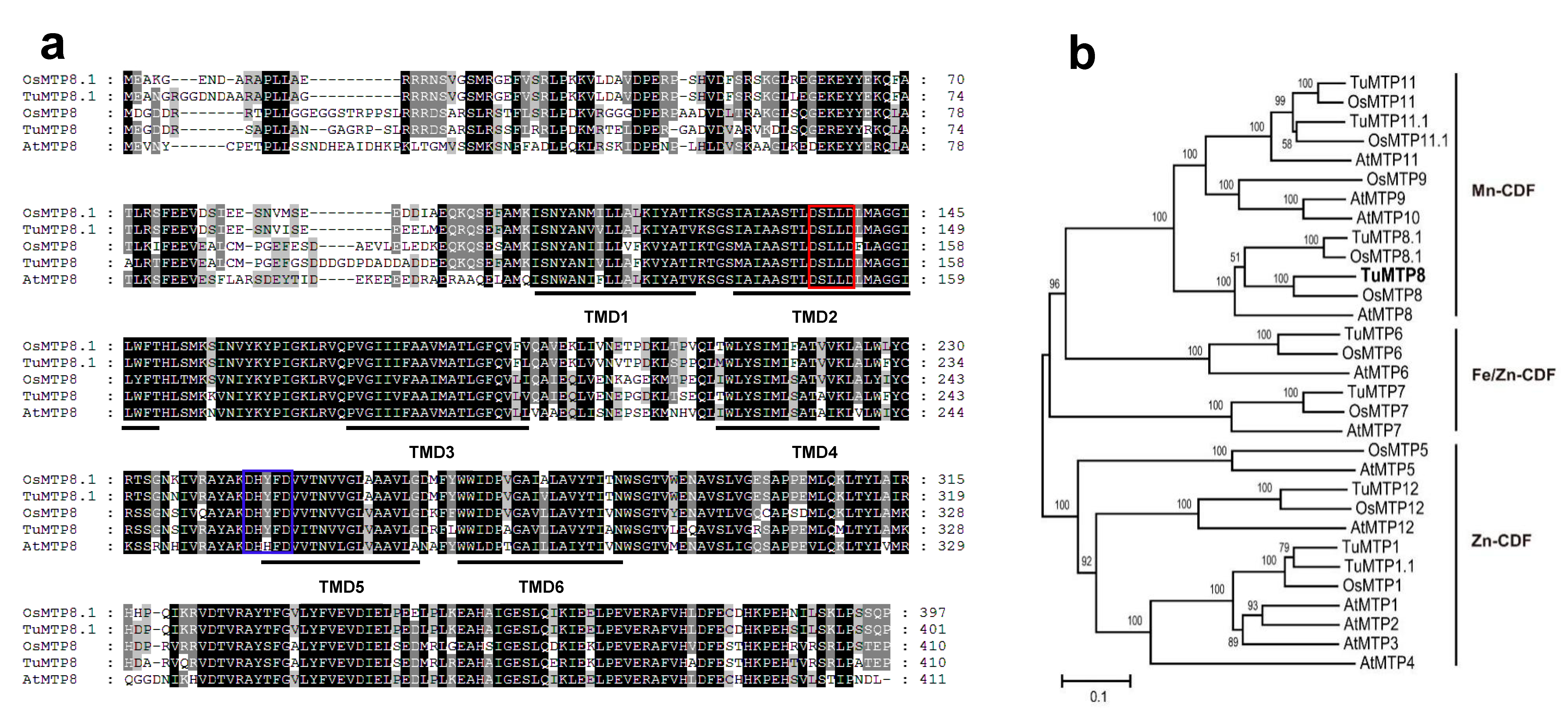
3.1. Molecular Features of TuMTP8
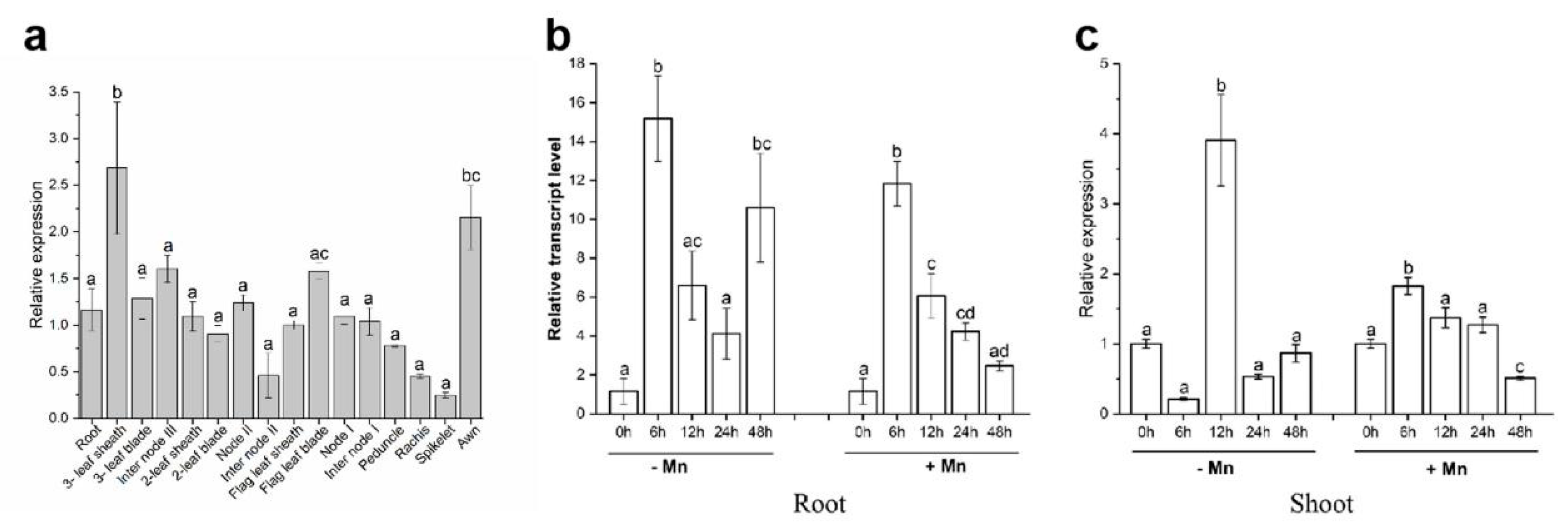
3.2. Expression Pattern of TuMTP8
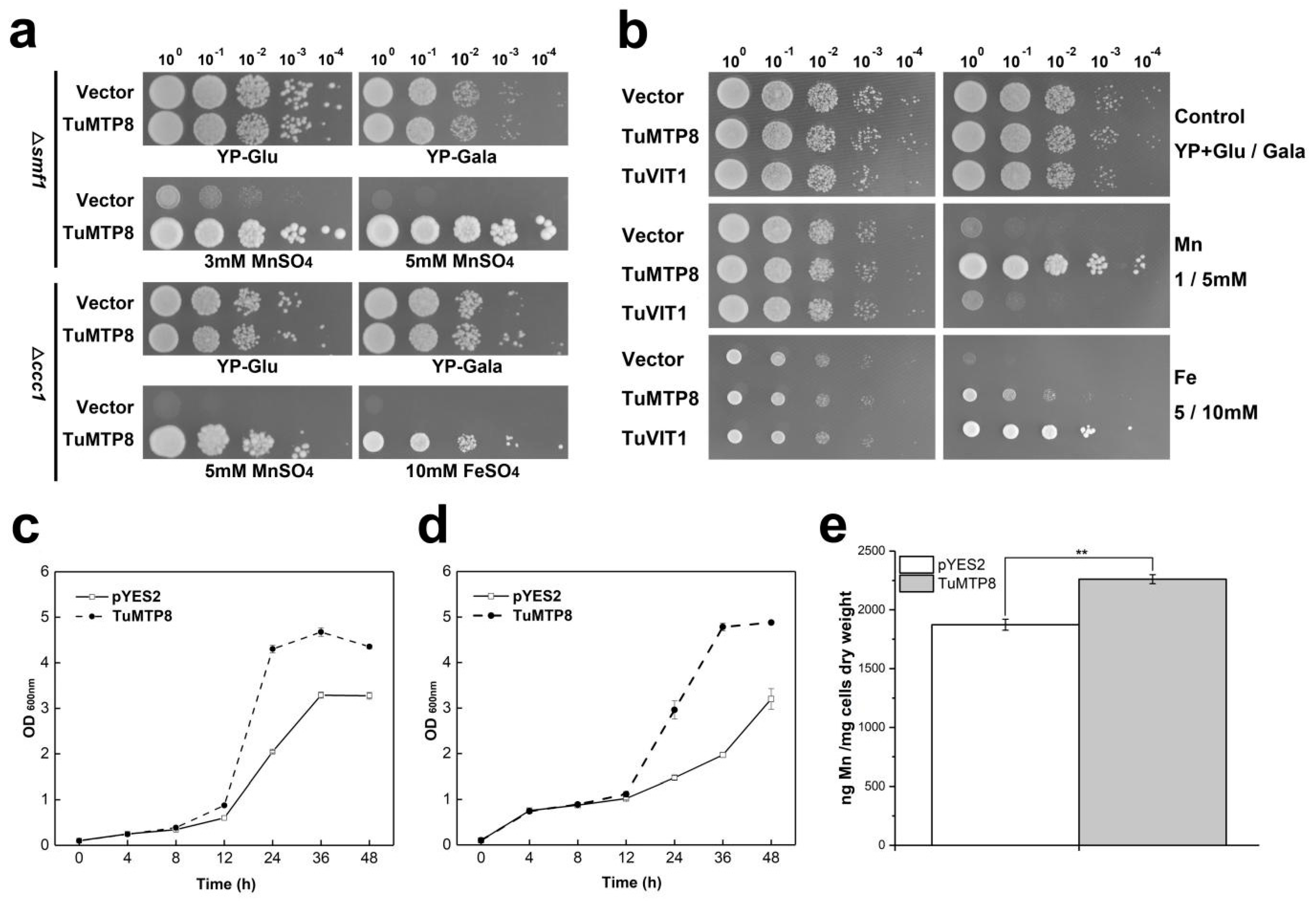
3.3. TuMTP8 Conferred Mn and Fe Tolerance in Yeast
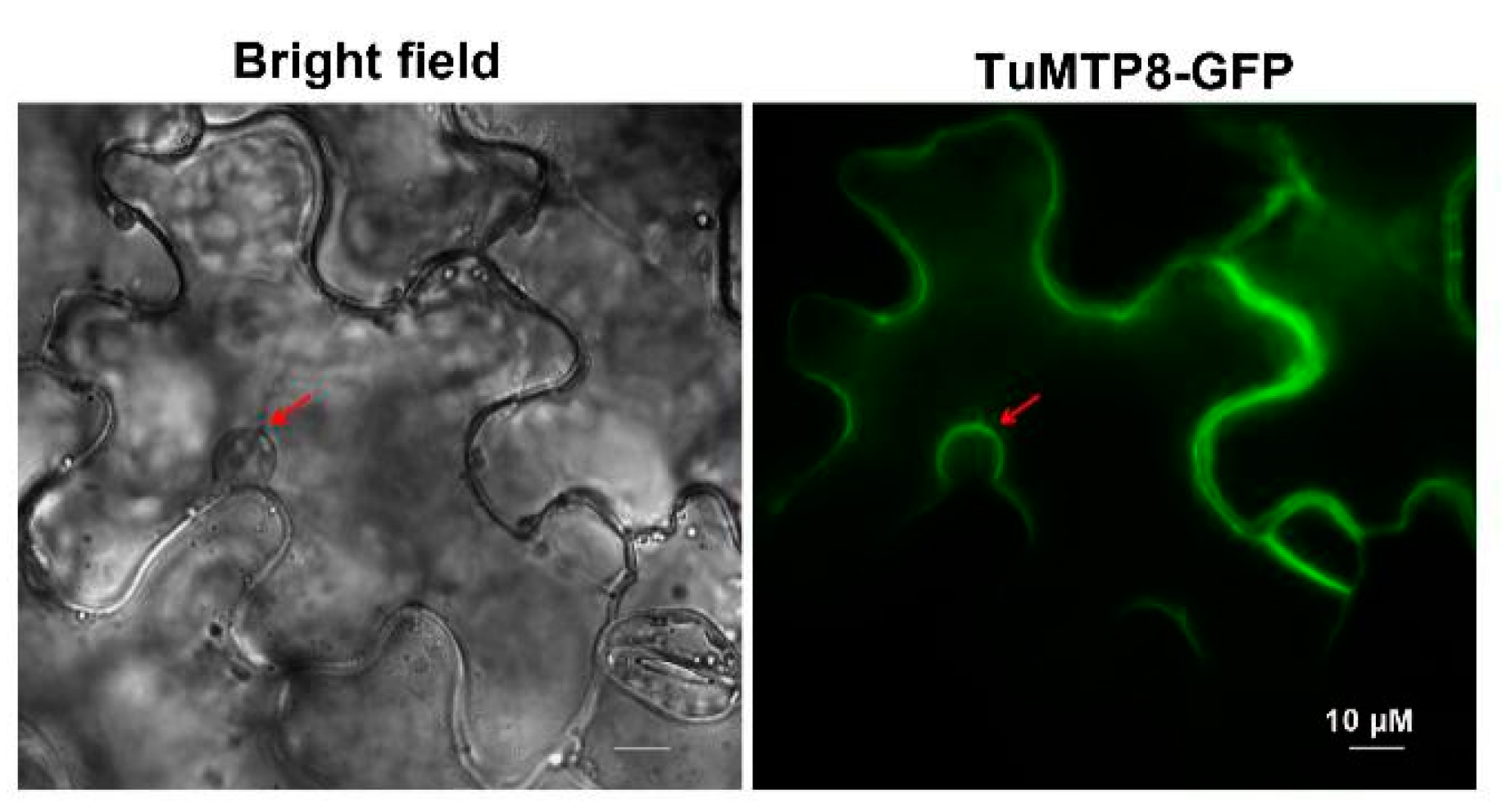
3.4. TuMTP8 Localized to the Vacuolar Membrane of Plant Cells
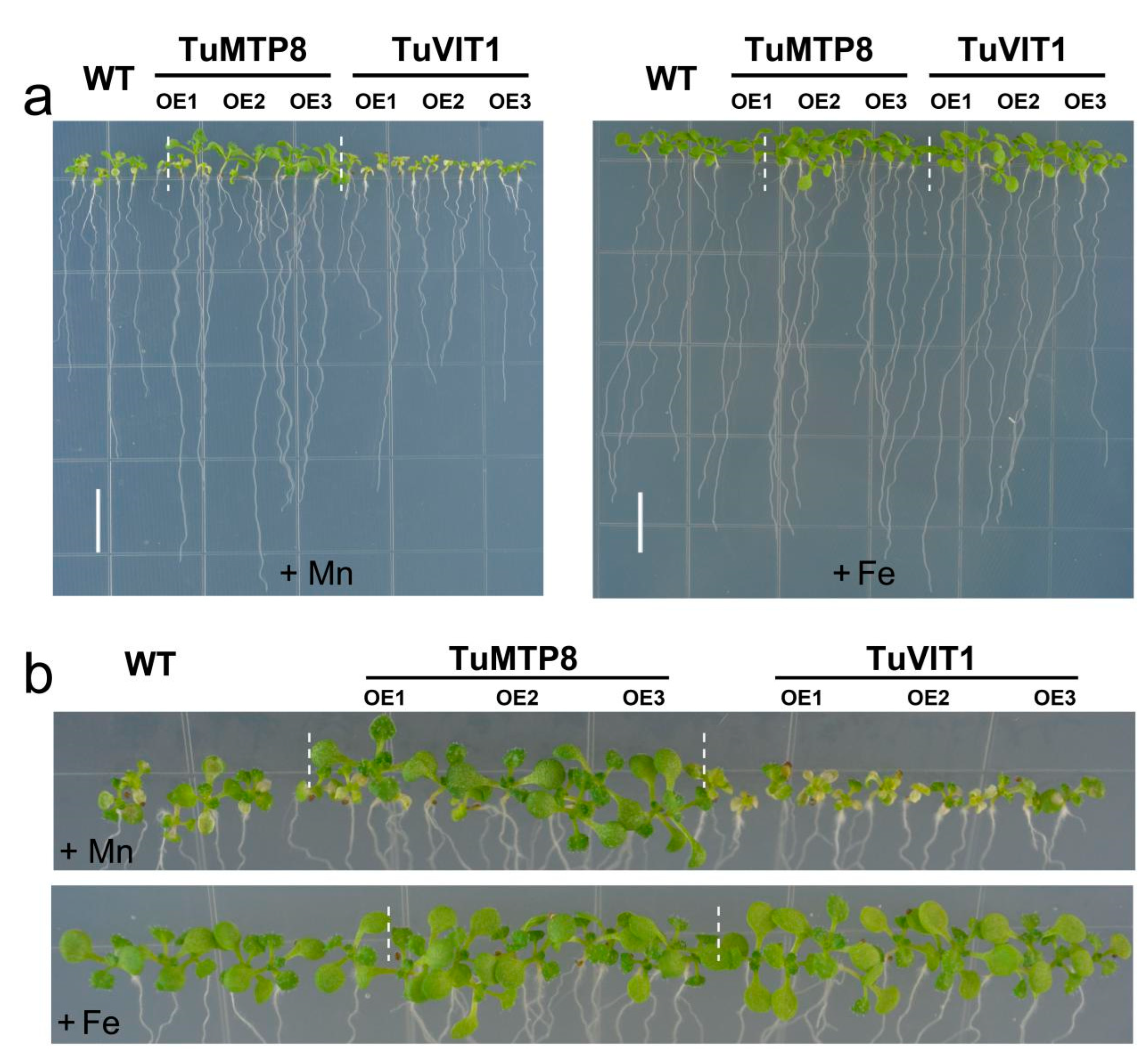
3.5. Expression of TuMTP8 in Arabidopsis Conferred Mn Tolerance
4. Discussion
4.1. Tonoplast-Localized TuMTP8 Was a Mn and Fe-Specific Transporter
4.2. Corresponding Solutions of TuMTP8 to Mn Toxicity
4.3. Possible Selection Mechanism of TuMTP8 on Mn and Fe
4.4. Comparative Analysis of TuMTP8 and TaMTP8
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Doncheva, S.; Georgieva, K.; Vassileva, V.; Stoyanova, Z.; Popov, N.; Ignatov, G. Effects of Succinate on Manganese Toxicity in Pea Plants. J. Plant Nutr. 2005, 28, 47–62. [Google Scholar] [CrossRef]
- Mora, M.d.l.L.; Rosas, A.; Ribera, A.; Rengel, Z. Differential tolerance to Mn toxicity in perennial ryegrass genotypes: In-volvement of antioxidative enzymes and root exudation of carboxylates. Plant Soil 2009, 320, 79–89. [Google Scholar] [CrossRef]
- Castaings, L.; Caquot, A.; Loubet, S.; Curie, C. The high-affinity metal Transporters NRAMP1 and IRT1 Team up to Take up Iron under Sufficient Metal Provision. Sci. Rep. 2016, 6, 37222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cailliatte, R.; Schikora, A.; Briat, J.-F.; Mari, S.; Curie, C. High-Affinity Manganese Uptake by the Metal Transporter NRAMP1 Is Essential for Arabidopsis Growth in Low Manganese Conditions. Plant Cell 2010, 22, 904–917. [Google Scholar] [CrossRef] [Green Version]
- Korshunova, Y.O.; Eide, D.; Clark, W.G.; Guerinot, M.L.; Pakrasi, H.B. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol. Biol. 1999, 40, 37–44. [Google Scholar] [CrossRef]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Lanquar, V.; Ramos, M.S.; Lelièvre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Kraemer, U.; Thomine, S. Export of Vacuolar Manganese by AtNRAMP3 and AtNRAMP4 Is Required for Optimal Photosynthesis and Growth under Manganese Deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef] [Green Version]
- Hirschi, K.D. Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased man-ganese tolerance. Plant Physiol. 2000, 124, 125. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.-H.; Pittman, J.K.; Shigaki, T.; Hirschi, K.D. Characterization of CAX4, an Arabidopsis H+/Cation Antiporter. Plant Physiol. 2002, 128, 1245–1254. [Google Scholar] [CrossRef] [Green Version]
- Pittman, J.K.; Shigaki, T.; Marshall, J.L.; Morris, J.L.; Cheng, N.-H.; Hirschi, K.D. Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol. Biol. 2004, 56, 959–971. [Google Scholar] [CrossRef]
- Edmond, C.; Shigaki, T.; Ewert, S.; Nelson, M.D.; Connorton, J.M.; Chalova, V.; Noordally, Z.; Pittman, J.K. Comparative analysis of CAX2-like cation transporters indicates functional and regulatory diversity. Biochem. J. 2009, 418, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-H.; Car, S.; Socha, A.L.; Hindt, M.N.; Punshon, T.; Guerinot, M.L. The Arabidopsis MTP8 transporter determines the localization of manganese and iron in seeds. Sci. Rep. 2017, 7, 11024. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xie, W.; Yang, C.; Xu, J.; Li, J.; Wang, H.; Chen, X.; Huang, C.-F. NRAMP2, a trans-Golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New Phytol. 2017, 217, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chanroj, S.; Wu, Z.; Romanowsky, S.M.; Harper, J.F.; Sze, H. A distinct endosomal Ca2+/Mn2+ pump affects root growth through the secretory process. Plant Physiol. 2008, 147, 1675–1689. [Google Scholar] [CrossRef] [Green Version]
- Delhaize, E.; Gruber, B.D.; Pittman, J.K.; White, R.G.; Leung, H.; Miao, Y.; Jiang, L.; Ryan, P.R.; Richardson, A.E. A role for theAtMTP11gene of Arabidopsis in manganese transport and tolerance. Plant J. 2007, 51, 198–210. [Google Scholar] [CrossRef]
- Milner, M.J.; Jesse, S.; Eric, C.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 Is a Major Transporter Responsible for Manganese and Cadmium Uptake in Rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [Green Version]
- Ueno, D.; Sasaki, A.; Yamaji, N.; Miyaji, T.; Fujii, Y.; Takemoto, Y.; Moriyama, S.; Che, J.; Moriyama, Y.; Iwasaki, K.; et al. A polarly localized transporter for efficient manganese uptake in rice. Nat. Plants 2015, 1, 15170. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef]
- Chen, Z.; Fujii, Y.; Yamaji, N.; Masuda, S.; Takemoto, Y.; Kamiya, T.; Yusuyin, Y.; Iwasaki, K.; Kato, S.-I.; Maeshima, M.; et al. Mn tolerance in rice is mediated by MTP8.1, a member of the cation diffusion facilitator family. J. Exp. Bot. 2013, 64, 4375–4387. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, Y.; Tsunemitsu, Y.; Fujii-Kashino, M.; Mitani-Ueno, N.; Yamaji, N.; Ma, J.F.; Kato, S.-I.; Iwasaki, K.; Ueno, D. The Tonoplast-Localized Transporter MTP8.2 Contributes to Manganese Detoxification in the Shoots and Roots of Oryza sativa L. Plant Cell Physiol. 2017, 58, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Menguer, P.K.; Farthing, E.; Peaston, K.A.; Ricachenevsky, F.K.; Fett, J.P.; Williams, L.E. Functional analysis of the rice vacuolar zinc transporter OsMTP1. J. Exp. Bot. 2013, 64, 2871–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eroglu, S.; Meier, B.; Von Wirén, N.; Peiter, E.; Eroglu, S.; Meier, B.; Von Wirén, N.; Peiter, E. The Vacuolar Manganese Transporter MTP8 Determines Tolerance to Iron Deficiency-Induced Chlorosis in Arabidopsis. Plant Physiol. 2015, 170, 1030–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanini, B.; Blaudez, D.; Jeandroz, S.; Sanders, D.; Chalot, M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: Improved signature and prediction of substrate specificity. BMC Genom. 2007, 8, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.H.; Sun, D.; Nevo, E. Domestication evolution, genetics and genomics in wheat. Mol. Breed. 2011, 28, 281–301. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Gietz, D.; Jean, A.S.; Woods, R.A.; Schiestl, R.H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992, 20, 1425. [Google Scholar] [CrossRef]
- Batoko, H.; Zheng, H.Q.; Hawes, C.; Moore, I. A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 2000, 12, 2201–2217. [Google Scholar] [CrossRef] [Green Version]
- Brandizzi, F.; Snapp, E.; Roberts, A.G.; Lippincott-Schwartz, J.; Hawes, C. Membrane Protein Transport between the Endoplasmic Reticulum and the Golgi in Tobacco Leaves Is Energy Dependent but Cytoskeleton Independent: Evidence from Selective Photobleaching. Plant Cell 2002, 14, 1293–1309. [Google Scholar] [CrossRef] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, S.; Giehl, R.F.; Meier, B.; Takahashi, M.; Terada, Y.; Ignatyev, K.; Andresen, E.; Küpper, H.; Peiter, E.; Von Wirén, N. Metal Tolerance Protein 8 Mediates Manganese Homeostasis and Iron Reallocation during Seed Development and Germination. Plant Physiol. 2017, 174, 1633–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migocka, M.; Papierniak, A.; Maciaszczyk-Dziubińska, E.; Poździk, P.; Posyniak, E.; Garbiec, A.; Filleur, S. Cucumber metal transport protein MTP8 confers increased tolerance to manganese when expressed in yeast and Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 5367–5384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delhaize, E.; Kataoka, T.; Hebb, D.M.; White, R.; Ryan, P.R. Genes Encoding Proteins of the Cation Diffusion Facilitator Family That Confer Manganese Tolerance. Plant Cell 2003, 15, 1131–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Li, Y.; Wu, X.; Zhou, L.; Zhu, X.; Fang, W. Metal transport protein 8 in Camellia sinensis confers superior manganese tolerance when expressed in yeast and Arabidopsis thaliana. Sci. Rep. 2017, 7, 39915. [Google Scholar] [CrossRef]
- Pedas, P.; Stokholm, M.S.; Hegelund, J.N.; Ladegård, A.H.; Schjoerring, J.K.; Husted, S. Golgi Localized Barley MTP8 Proteins Facilitate Mn Transport. PLoS ONE 2014, 9, e113759. [Google Scholar] [CrossRef]
- Tsunemitsu, Y.; Genga, M.; Okada, T.; Yamaji, N.; Ma, J.F.; Miyazaki, A.; Kato, S.-I.; Iwasaki, K.; Ueno, D. A member of cation diffusion facilitator family, MTP11, is required for manganese tolerance and high fertility in rice. Planta 2018, 248, 231–241. [Google Scholar] [CrossRef]
- Wang, D.; Yu, K.; Jin, D.; Sun, L.; Chu, J.; Wu, W.; Xin, P.; Gregová, E.; Li, X.; Sun, J.; et al. Natural variations in the promoter of Awn Length Inhibitor 1 (ALI-1) are associated with awn elongation and grain length in common wheat. Plant J. 2019, 101, 1075–1090. [Google Scholar] [CrossRef]
- Pearson, J.N.; Rengel, Z.; Jenner, C.F.; Graham, R.D. Manipulation of xylem transport affects Zn and Mn transport into developing wheat grains of cultured ears. Physiol. Plant. 1996, 98, 229–234. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Xia, J.; Ma, J.F. OsYSL6 Is Involved in the Detoxification of Excess Manganese in Rice. Plant Physiol. 2011, 157, 1832–1840. [Google Scholar] [CrossRef] [Green Version]
- Yamaji, N.; Sasaki, A.; Xia, J.X.; Yokosho, K.; Ma, J.F. A node-based switch for preferential distribution of manganese in rice. Nat. Commun. 2013, 4, 2442. [Google Scholar] [CrossRef]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of Iron in Arabidopsis Seed Requires the Vacuolar Membrane Transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, O.S.; Ward, D.M.; Kaplan, J. CCC1 Is a Transporter That Mediates Vacuolar Iron Storage in Yeast. J. Biol. Chem. 2001, 276, 29515–29519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Kraemer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Qiao, K.; Wang, H.; Wang, H.; Chai, T. MTP8 from Triticum urartu Is Primarily Responsible for Manganese Tolerance. Int. J. Mol. Sci. 2022, 23, 5683. https://doi.org/10.3390/ijms23105683
Wang F, Qiao K, Wang H, Wang H, Chai T. MTP8 from Triticum urartu Is Primarily Responsible for Manganese Tolerance. International Journal of Molecular Sciences. 2022; 23(10):5683. https://doi.org/10.3390/ijms23105683
Chicago/Turabian StyleWang, Fanhong, Kun Qiao, Huanhuan Wang, Hong Wang, and Tuanyao Chai. 2022. "MTP8 from Triticum urartu Is Primarily Responsible for Manganese Tolerance" International Journal of Molecular Sciences 23, no. 10: 5683. https://doi.org/10.3390/ijms23105683
APA StyleWang, F., Qiao, K., Wang, H., Wang, H., & Chai, T. (2022). MTP8 from Triticum urartu Is Primarily Responsible for Manganese Tolerance. International Journal of Molecular Sciences, 23(10), 5683. https://doi.org/10.3390/ijms23105683





