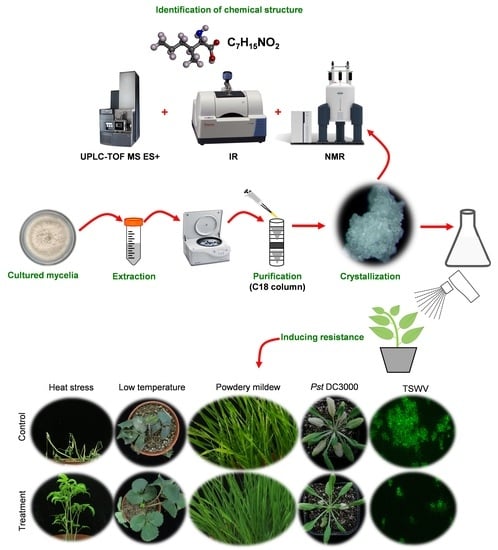
Natural 2-Amino-3-Methylhexanoic Acid as Plant Elicitor Inducing Resistance against Temperature Stress and Pathogen Attack
Abstract
:1. Introduction
2. Results and Discussion
2.1. AMHA Is a Naturally Occurring α-Amino Acid
2.2. Free AMHA Is Produced by Different Fungal Species
2.3. AMHA Induces Plant Resistance to Temperature Stress
2.4. AMHA Induces Plant Resistance to Pathogen Infection
3. Materials and Methods
3.1. Fungus Strains and Chemicals
3.2. Cultivation of Fungi
3.3. Extraction, Purification and Structure Analysis
3.4. The Physical and Spectroscopic Data of AMHA
3.5. Conformational Analysis, Geometrical Optimization and ECD Calculation of (2S, 3S)-AMHA
3.6. Test of AMHA in Different Fungus Strains and Plants
3.7. High/Low-Temperature Resistance Assays
3.8. Wheat Resistance to Fungus Powdery Mildew
3.9. Arabidopsis Resistance to Bacterial Infection
3.10. Tobacco Resistance to Virus Infection
3.11. Statistical Analysis
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Hura, T. Wheat and barley: Acclimatization to abiotic and biotic stress. Int. J. Mol. Sci. 2020, 21, 7423–7428. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Ji, Y.; Shi, Y.; Zhao, Z.; Zhu, W.; Xu, Y.; Li, B.; Qian, X. Floro-pyrazolo[3,4-d]pyrimidine derivative as a novel plant activator induces two-pathway immune system. Phytochemistry 2021, 184, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Marolleau, B.; Gaucher, M.; Heintz, C.; Degrave, A.; Warneys, R.; Orain, G.; Lemarquand, A.; Brisset, M.-N. When a plant resistance inducer leaves the lab for the field: Integrating ASM into routine apple protection practices. Front. Plant Sci. 2017, 8, 1938. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.; Dong, Y.; Zhang, Y.; Li, S.; Shi, F. Plant immunity inducer development and application. Mol. Plant-Microbe Interact. 2017, 30, 356–360. [Google Scholar]
- Conrath, U. Molecular aspects of defence priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- De Kesel, J.; Conrath, U.; Flors, V.; Luna, E.; Mageroy, M.; Mauch-Mani, B.; Kyndt, T. The induced resistance lexicon: Do’s and don’ts. Trends Plant Sci. 2021, 26, 685–691. [Google Scholar] [CrossRef]
- Apone, F.; Tito, A.; Carola, A.; Arciello, S.; Tortora, A.; Filippini, L.; Monoli, I.; Cucchiara, M.; Gibertoni, S.; Chrispeels, M.; et al. A mixture of peptides and sugars derived from plant cell walls increases plant defense responses to stress and attenuates ageing-associated molecular changes in cultured skin cells. J. Biotechnol. 2010, 145, 367–376. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Verkleij, F. Seaweed extracts in agriculture and horticulture: A review. Biol. Agric. Hortic. 1992, 8, 309–324. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 30, 39–48. [Google Scholar] [CrossRef]
- Santosh, K.B.; Prianka, H.; Jia, X.; Wang, W.; Yin, H. Alginate oligosaccharide postharvest treatment preserve fruit quality and increase storage life via abscisic acid signaling in strawberry. Food Chem. 2019, 283, 665–674. [Google Scholar]
- Zhang, C.; Howlader, P.; Liu, T.; Sun, X.; Jia, X.; Zhao, X.; Shen, P.; Qin, Y.; Wang, W.; Yin, H. Alginate oligosaccharide (AOS) induced resistance to Pst DC3000 via salicylic acid-mediated signaling pathway in Arabidopsis thaliana. Carbohydr. Polym. 2019, 225, 115–221. [Google Scholar] [CrossRef]
- Raho, N.; Ramirez, L.; Lanteri, M.; Gonorazky, G.; Lamattina, L.; ten Have, A.; Laxalt, A. Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. J. Plant Physiol. 2011, 168, 534–539. [Google Scholar] [CrossRef]
- Gagnon, P.; Boivin, J. Synthesis of amino acid from substituted cyanoacetic esters. Can. J. Res. 1948, 26, 503–510. [Google Scholar] [CrossRef]
- Kisumi, M.; Komatsubara, S.; Chibata, I. Multivalent repression and genetic derepression of isoleucine-valine biosynthetic enzymes in Serratia marcescens. J. Bacteriol. 1971, 107, 824–827. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, M.; Kisumi, M.; Chibata, I. β-methylnorleucine, an antimetabolite produced by Serratia marcescens. J. Antibiot. 1981, 34, 1278–1282. [Google Scholar] [CrossRef]
- Sugiura, M.; Kisumi, M.; Chibata, I. Biosynthetic pathway of β-methylnorleucine, an antimetabolite produced by Serratia marcescens. J. Antibiot. 1981, 34, 1283–1289. [Google Scholar] [CrossRef]
- Muramatsu, R.; Negishi, T.; Mimoto, T.; Miura, A.; Misawa, S. Existence of beta-methylnorleucine in recombinant hirudin produced by Escherichia coli. J. Biotechnol. 2002, 93, 131–142. [Google Scholar] [CrossRef]
- Reitz, C.; Fan, Q.; Neubauer, P. Synthesis of non-canonical branched-chain amino acids in Escherichia coli and approaches to avoid their incorporation into recombinant proteins. Curr. Opin. Biotechnol. 2018, 53, 248–253. [Google Scholar] [CrossRef]
- Sugiura, M.; Kisumi, M.; Chibata, I. β-Methylnorleucine, a novel antagonist of isoleucine. Agric. Biol. Chem. 1985, 49, 1889–1890. [Google Scholar] [CrossRef]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Gilliham, M.; Tyerman, S.D. Linking metabolism to membrane signaling: The GABA-malate connection. Trends Plant Sci. 2016, 21, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not just a metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef]
- Cohen, Y.; Vaknin, M.; Mauch-Mani, B. BABA-induced resistance: Milestones along a 55-year journey. Phytoparasitica 2016, 44, 513–538. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. γ-Aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell. Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.-M.; Lin, H.-X.; Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotech. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, M.; Gao, L.; Yang, Q.; Kalaji, H.M.; Qiang, S.; Strasser, R.J.; Chen, S. Tenuazonic acid-triggered cell death is the essential prerequisite for Alternaria alternata (Fr.) Keissler to infect successfully host Ageratina adenophora. Cells 2021, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, X.-F.; Kvitko, B.; He, S. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Ishiga, Y.; Ishiga, T.; Uppalapati, S.R.; Mysore, K.S. Arabidopsis seedling flood-inoculation technique: A rapid and reliable assay for studying plant-bacterial interactions. Plant Methods 2011, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Feng, M.; Cheng, R.; Guo, M.; Li, L.; Feng, Z.; Wu, J.; Xie, L.; Zhang, Z.; Kormelinkd, R.; Tao, X. Rescue of tomato spotted wilt virus entirely from complementary DNA clones. Proc. Natl. Acad. Sci. USA 2020, 117, 1181–1190. [Google Scholar] [CrossRef]
- Kang, Y.; Feng, H.; Zhang, J.; Chen, S.; Valverde, B.E.; Qiang, S. TeA is a key virulence factor for Alternaria alternata (Fr.) Keissler infection of its host. Plant. Physiol. Biochem. 2017, 115, 73–82. [Google Scholar] [CrossRef]
- Gatenbeck, S.; Sierankiewicz, J. Microbial production of tenuazonic acid analogues. Antimicrob. Agents Chemother. 1973, 3, 308–309. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, A.; Arabi, M.; Ghaedi, M.; Ostovan, A.; Chen, L. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 2018, 195, 390–400. [Google Scholar] [CrossRef]
- Xia, W.; Rui, W.; Zhao, W.; Sheng, S.; Lei, L.; Feng, Y.; Zhao, S. Stable isotope labeling and 2,3,5,4’-tetrahydroxystilbene-2-O-β-d-glucopyranoside biosynthetic pathway characterization in Fallopia multiflora. Planta 2018, 247, 613–623. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C. 01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, J.; Yang, Q.; Wang, L.; Wang, J.; Zhang, Y.; Guo, Y.; Li, R.; Zhang, R.; Tao, X.; et al. Natural 2-Amino-3-Methylhexanoic Acid as Plant Elicitor Inducing Resistance against Temperature Stress and Pathogen Attack. Int. J. Mol. Sci. 2022, 23, 5715. https://doi.org/10.3390/ijms23105715
Wang H, Li J, Yang Q, Wang L, Wang J, Zhang Y, Guo Y, Li R, Zhang R, Tao X, et al. Natural 2-Amino-3-Methylhexanoic Acid as Plant Elicitor Inducing Resistance against Temperature Stress and Pathogen Attack. International Journal of Molecular Sciences. 2022; 23(10):5715. https://doi.org/10.3390/ijms23105715
Chicago/Turabian StyleWang, He, Jingjing Li, Qian Yang, Lan Wang, Jing Wang, Yaxin Zhang, Yanjing Guo, Rui Li, Ruiqi Zhang, Xiaorong Tao, and et al. 2022. "Natural 2-Amino-3-Methylhexanoic Acid as Plant Elicitor Inducing Resistance against Temperature Stress and Pathogen Attack" International Journal of Molecular Sciences 23, no. 10: 5715. https://doi.org/10.3390/ijms23105715
APA StyleWang, H., Li, J., Yang, Q., Wang, L., Wang, J., Zhang, Y., Guo, Y., Li, R., Zhang, R., Tao, X., E. Valverde, B., Qiang, S., Kalaji, H. M., & Chen, S. (2022). Natural 2-Amino-3-Methylhexanoic Acid as Plant Elicitor Inducing Resistance against Temperature Stress and Pathogen Attack. International Journal of Molecular Sciences, 23(10), 5715. https://doi.org/10.3390/ijms23105715