Bone Marrow MSC Secretome Increases Equine Articular Chondrocyte Collagen Accumulation and Their Migratory Capacities
Abstract
:1. Introduction
2. Results
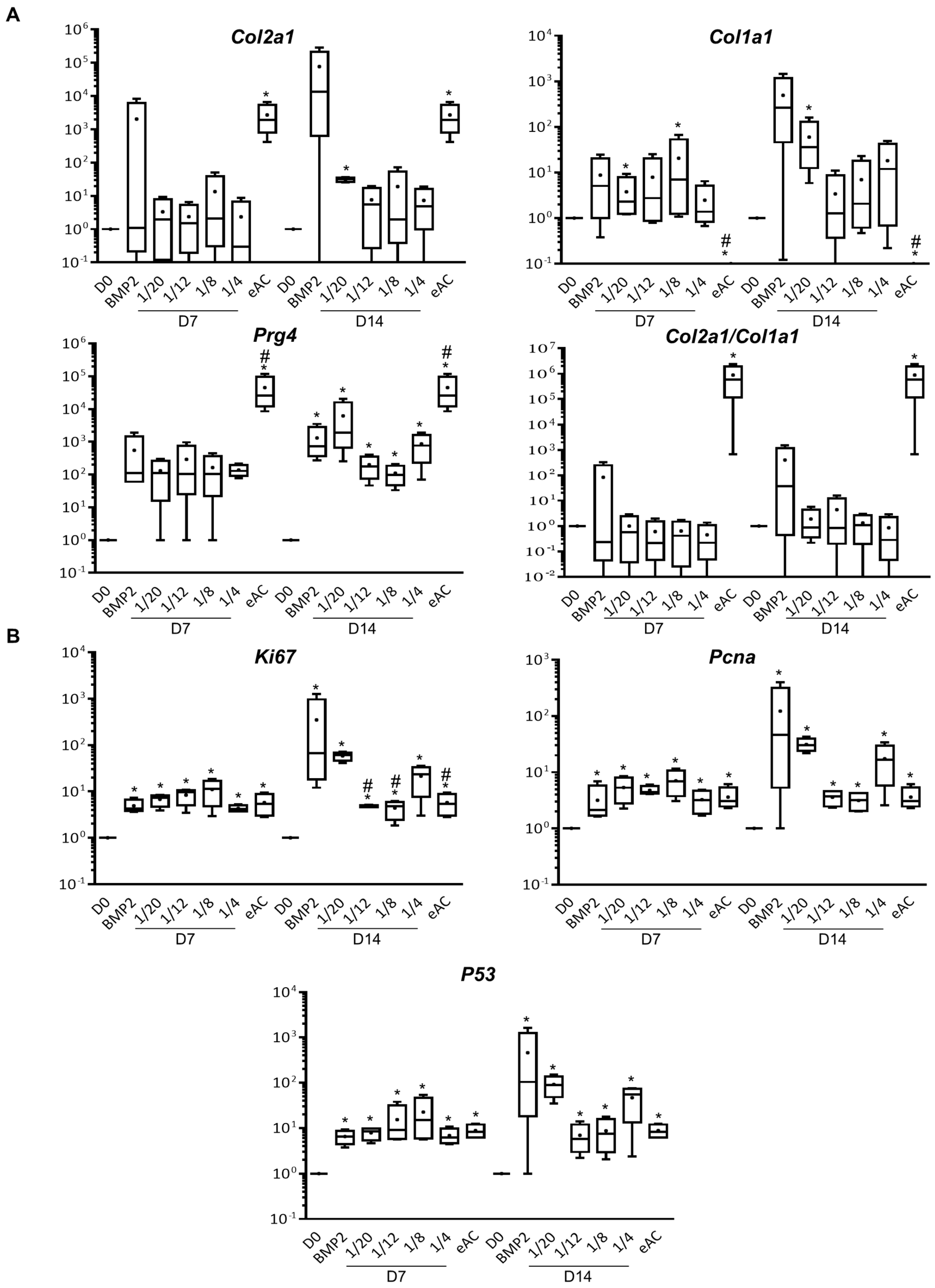
2.1. MSCs Increase eAC mRNA Levels of Hyaline Cartilage and Fibrocartilage Markers Collagens, Prg4 and Proliferation Associated Molecules
2.2. MSC-CM Are Not Cytotoxic and do Not Affect eAC Proliferation
2.3. MSC-CM Favor Collagen Synthesis
2.4. eACs Cultured in the Presence of MSC-CM have Increased Migratory Capacities
2.5. MSC-CM Contain Small Particles
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Co-Culture Experiments
4.3. Preparation of Conditioned Media
4.4. XTT Assay
4.5. Cytotoxicity Evaluation
4.6. RNA Isolation and RT-qPCR
4.7. Protein Extraction and Western Blots
4.8. Scratch Wound Healing Assay
4.9. Nanoparticle Tracking Analysis (NTA)
4.10. Transmission Electron Microscopy (TEM)
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosinska, M.K.; Ludwig, T.E.; Liebisch, G.; Zhang, R.; Siebert, H.C.; Wilhelm, J.; Kaesser, U.; Dettmeyer, R.B.; Klein, H.; Ishaque, B.; et al. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS ONE 2015, 5, e0125192. [Google Scholar]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 14, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Diehl, P.; Kolombe, T.; Fay, J.; Siebold, R.; Fickert, S. Clinical outcome and success rates of ACI for cartilage defects of the patella: A subgroup analysis from a controlled randomized clinical phase II trial (CODIS study). Arch. Orthop. Trauma Surg. 2020, 6, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Yamato, M.; Mitani, G.; Takagaki, T.; Hamahashi, K.; Nakamura, Y.; Ishihara, M.; Matoba, R.; Kobayashi, H.; Okano, T.; et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen. Med. 2019, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef]
- Velot, É.; Madry, H.; Venkatesan, J.K.; Bianchi, A.; Cucchiarini, M. Is extracellular vesicle-based therapy the next answer for cartilage regeneration? Front. Bioeng. Biotechnol. 2021, 9, 645039. [Google Scholar] [CrossRef]
- Lee, K.B.L.; Hui, J.H.P.; Song, I.C.; Ardany, L.; Lee, E.H. Injectable mesenchymal stem cell therapy for large cartilage defects-a porcine model. Stem Cells 2007, 2511, 2964–2971. [Google Scholar] [CrossRef] [Green Version]
- Ortved, K.F.; Nixon, A.J. Cell-based cartilage repair strategies in the horse. Vet. J. 2015, 208, 1–12. [Google Scholar] [CrossRef]
- Kong, L.; Zheng, L.Z.; Qin, L.; Ho, K.K.W. Role of mesenchymal stem cells in osteoarthritis treatment. J. Orthop. Trans. 2017, 9, 89–103. [Google Scholar] [CrossRef]
- Bertoni, L.; Branly, T.; Jacquet, S.; Desancé, M.; Desquilbet, L.; Rivory, P.; Hartmann, D.J.; Denoix, J.M.; Audigié, F.; Galéra, P.; et al. Intra-articular injection of 2 different dosages of autologous and allogeneic bone marrow- and umbilical cord-derived mesenchymal stem cells triggers a variable inflammatory response of the fetlock joint on 12 sound experimental horses. Stem Cells Int. 2019, 2019, 9431894. [Google Scholar] [CrossRef]
- Bertoni, L.; Jacquet-Guibon, S.; Branly, T.; Desancé, M.; Legendre, F.; Melin, M.; Rivory, P.; Hartmann, D.J.; Schmutz, A.; Denoix, J.M.; et al. Evaluation of allogeneic bone-marrow-derived and umbilical cord blood-derived mesenchymal stem cells to prevent the development of osteoarthritis in an equine model. Int. J. Mol. Sci. 2021, 22, 2499. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.; Al Jumah, M.; Pace, R.A.; Kalionis, B. The immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. Rep. 2012, 8, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lai, R.C.; Po Hui, J.H.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2016, 67, 56–64. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214–16226. [Google Scholar] [CrossRef]
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Mauck, R.L.; Burdick, J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng. Part A 2011, 17, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Duan, L.; Liang, Y.; Zhu, W.; Xiong, J.; Wang, D. Human umbilical cord blood-derived mesenchymal stem cells contribute to chondrogenesis in coculture with chondrocytes. BioMed Res. Int. 2016, 2016, 3827057. [Google Scholar] [CrossRef]
- Levorson, E.J.; Santoro, M.; Kasper, F.K.; Mikos, A.G. Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs. Acta Biomater. 2014, 10, 1824–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oke, S.L.; McIlwrailth, C.W. Review of the economic impact of osteoarthritis and oral joint-health supplements in horses. Joints 2010, 56, 12–16. [Google Scholar]
- Galera, P.; Ollitrault, D.; Legendre, F.; Demoor, M.; Mallein-Gerin, F.; Bonmediene, K.; Herbage, B.; Duterque-Coquillaud, M.; Damour, O. Method for obtaining differentiated articular chondrocytes in vitro or ex vivo and use of same. National Patent WO 2012/038668-22/09/2011, 29 March 2012. [Google Scholar]
- Legendre, F.; Ollitrault, D.; Hervieu, M.; Baugé, C.; Maneix, L.; Goux, D.; Chajra, H.; Mallein-Gerin, F.; Boumediene, K.; Galera, P.; et al. Enhanced hyaline cartilage matrix synthesis in collagen sponge scaffolds by using siRNA to stabilize chondrocytes phenotype cultured with bone morphogenetic protein-2 under hypoxia. Tissue Eng. Part C Methods 2013, 19, 550–567. [Google Scholar] [CrossRef]
- Ollitrault, D.; Legendre, F.; Drougard, C.; Briand, M.; Benateau, H.; Goux, D.; Chajra, H.; Poulain, L.; Hartmann, D.; Vivien, D.; et al. BMP-2, hypoxia, and COL1A1/HTRA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes. Tissue Eng. Part C Methods 2015, 21, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Rakic, R.; Bourdon, B.; Hervieu, M.; Branly, T.; Legendre, F.; Saulnier, N.; Audigié, F.; Maddens, S.; Demoor, M.; Galera, P. RNA interference and BMP-2 stimulation allows equine chondrocytes redifferentiation in 3D-hypoxia cell culture model: Application for matrix-induced autologous chondrocyte implantation. Int. J. Mol. Sci. 2017, 18, 1842. [Google Scholar] [CrossRef]
- Perkins, N.R.; Reid, S.W.; Morris, R.S. Profiling the New Zealand Thoroughbred racing industry. 2. Conditions interfering with training and racing. N. Z. Vet. J. 2005, 53, 69–76. [Google Scholar] [CrossRef]
- Preston, S.A.; Trumble, T.N.; Zimmel, D.N.; Chmielewski, T.L.; Brown, M.P.; Hernandez, J.A. Lameness, athletic performance, and financial returns in yearling Thoroughbreds bought for the purpose of resale for profit. J. Am. Vet. Med. Assoc. 2008, 232, 85–90. [Google Scholar] [CrossRef]
- Malda, J.; Benders, K.E.; Klein, T.J.; de Grauw, J.C.; Kik, M.J.; Hutmacher, D.W.; Saris, D.B.; van Weeren, P.R.; Dhert, W.J. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr. Cartil. 2012, 20, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 2015, 142, 817–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, S.M.; Leonard, C.; Regmi, S.C.; De Rantere, D.; Tailor, P.; Ren, G.; Ishida, H.; Hsu, C.; Abubacker, S.; Pang, D.S.; et al. Lubricin/proteoglycan 4 binds to and regulates the activity of toll-like receptors in vitro. Sci. Rep. 2016, 6, 18910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reesink, H.L.; Watts, A.E.; Mohammed, H.O.; Jay, G.D.; Nixon, A.J. Lubricin/proteoglycan 4 increases in both experimental and naturally occurring equine osteoarthritis. Osteoarthr. Cartil. 2017, 25, 128–137. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.L.A.; Costa-Pinto, A.R.; Martins, A.; Correlo, V.M.; Sol, P.; Bhattacharya, M.; Faria, S.; Reis, R.L.; Neves, N.M. Conditioned medium as a strategy for human stem cells chondrogenic differentiation. J. Tissue Eng. Regen. Med. 2015, 9, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Bay-Jensen, A.C.; Pap, T.; Dvir-Ginzberg, M.; Quasnichka, H.; Barrett-Jolley, R.; Mobasheri, A.; Henrotin, Y. Chondrocyte secretome: A source of novel insights and exploratory biomarkers of osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1199–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, A.P.; Bernardi, A.; Frozza, R.L.; Grudzinski, P.B.; Hoppe, J.B.; de Souza, L.F.; Chagastelles, P.; de Souza Wyse, A.T.; Bernard, E.A.; Battastini, A.M.; et al. Mesenchymal stem cell-conditioned medium triggers neuroinflammation and reactive species generation in organotypic cultures of rat hippocampus. Stem Cells Dev. 2011, 20, 1171–1181. [Google Scholar] [CrossRef]
- Man, G.S.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41. [Google Scholar]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and strategies for improving the regenerative effects of mesenchymal stromal cell-based therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef] [Green Version]
- Noronha, N.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res.Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Caffi, V.; Espinosa, G.; Gajardo, G.; Morales, N.; Durán, M.C.; Uberti, B.; Morán, G.; Plaza, A.; Henríquez, C. Pre-conditioning of equine bone marrow-derived mesenchymal stromal cells increases their immunomodulatory capacity. Front. Vet. Sci. 2020, 7, 318. [Google Scholar] [CrossRef]
- Pourgholaminejad, A.; Aghdami, N.; Baharvand, H.; Moazzeni, S.M. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine 2016, 85, 51–60. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.; Laurencin, C.T. Emergence of the stem cell secretome in regenerative engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef]
- Chang, C.P.; Chio, C.C.; Cheong, C.U.; Chao, C.M.; Cheng, B.C.; Lin, M.T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. 2013, 124, 165–176. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef]
- Bourdon, B.; Contentin, R.; Cassé, F.; Maspimby, C.; Oddoux, S.; Noël, A.; Legendre, F.; Gruchy, N.; Galéra, P. Marine collagen hydrolysates downregulate the synthesis of pro-catabolic and pro-inflammatory markers of osteoarthritis and favor collagen production and metabolic activity in equine articular chondrocyte organoids. Int. J. Mol. Sci. 2021, 22, 580. [Google Scholar] [CrossRef]
- Pigott, J.H.; Ishihara, A.; Wellman, M.L.; Russell, D.S.; Bertone, A.L. Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet. Comp. Orthopaed. 2013, 26, 453–460. [Google Scholar]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2012, 32, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Branly, T.; Bertoni, L.; Contentin, R.; Rakic, R.; Gomez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Jacquet, S.; Audigié, F.; et al. Characterization and use of equine bone marrow mesenchymal stem cells in equine cartilage engineering. Study of their hyaline cartilage forming potential when cultured under hypoxia within a biomaterial in the presence of BMP-2 and TGF-ß1. Stem Cell Rev. Rep. 2017, 5, 611–630. [Google Scholar] [CrossRef]
- Branly, T.; Contentin, R.; Desancé, M.; Jacquel, T.; Bertoni, L.; Jacquet, S.; Mallein-Gerin, F.; Denoix, J.M.; Audigié, F.; Demoor, M.; et al. Improvement of the chondrocyte-specific phenotype upon equine bone marrow mesenchymal stem cell differentiation: Influence of culture time, transforming growth factors and type i collagen siRNAs on the differentiation index. Int. J. Mol. Sci. 2018, 2, 435. [Google Scholar] [CrossRef] [Green Version]
- Desancé, M.; Contentin, R.; Bertoni, L.; Gomez-Leduc, T.; Branly, T.; Jacquet, S.; Betsch, J.M.; Batho, A.; Legendre, F.; Audigié, F.; et al. Chondrogenic differentiation of defined equine mesenchymal stem cells derived from umbilical cord blood for use in cartilage repair therapy. Int. J. Mol. Sci. 2018, 19, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contentin, R.; Demoor, M.; Concari, M.; Desancé, M.; Audigié, F.; Branly, T.; Galéra, P. Comparison of the chondrogenic potential of mesenchymal stem cells derived from bone marrow and umbilical cord blood intended for cartilage tissue engineering. Stem Cell Rev. Rep. 2020, 16, 126–143. [Google Scholar] [CrossRef] [PubMed]





| ß-Actin. | Forward | GATGATGATATCGCCGCGCTC |
| Reverse | TGCCCCACGTATGAGTCCTT | |
| Col2a1 | Forward | GGCAATAGCAGGTTCACGTACA |
| Reverse | CGATAACAGTCTTGCCCCACTT | |
| Prg4 | Forward | CTACCACCCAACGCAACAAA |
| Reverse | ACTGTTGTCTCCTTATTGGGTGT | |
| Col1a1 | Forward | TGCCGTGACCTCAAGATGTG |
| Reverse | CGTCTCCATGTTGCAGAAGA | |
| Ki67 | Forward | AAGCTGCACGTTCATGGAGA |
| Reverse | ACCCACAGTTCTTCCTCCGA | |
| Pcna | Forward | GCGTGAACCTCACCAGTATGT |
| Reverse | GCAAATTGCCCAGAAGGCAT | |
| P53 | Forward | CACCTGAGGTTGGCTCTGAC |
| Reverse | GCACAAACACGCACCTCAAA |
| Antibody-Dilution | Supplier |
|---|---|
| Rabbit anti-bovine type I collagen—1/3000 | Novotec; Bron, France |
| Rabbit anti-human type II collagen—1/1500 | |
| Rabbit anti-human GAPDH—1/5000 | Santa Cruz Biotechnology; Dallas, TX, USA |
| Rabbit anti-human type IIB collagen—1/1500 | Covalab; Villeurbanne, France |
| HRP-conjugated goat anti-rabbit antibody— 1/5000 | Jackson Immunoresearch; West Grove, PA, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contentin, R.; Jammes, M.; Bourdon, B.; Cassé, F.; Bianchi, A.; Audigié, F.; Branly, T.; Velot, É.; Galéra, P. Bone Marrow MSC Secretome Increases Equine Articular Chondrocyte Collagen Accumulation and Their Migratory Capacities. Int. J. Mol. Sci. 2022, 23, 5795. https://doi.org/10.3390/ijms23105795
Contentin R, Jammes M, Bourdon B, Cassé F, Bianchi A, Audigié F, Branly T, Velot É, Galéra P. Bone Marrow MSC Secretome Increases Equine Articular Chondrocyte Collagen Accumulation and Their Migratory Capacities. International Journal of Molecular Sciences. 2022; 23(10):5795. https://doi.org/10.3390/ijms23105795
Chicago/Turabian StyleContentin, Romain, Manon Jammes, Bastien Bourdon, Frédéric Cassé, Arnaud Bianchi, Fabrice Audigié, Thomas Branly, Émilie Velot, and Philippe Galéra. 2022. "Bone Marrow MSC Secretome Increases Equine Articular Chondrocyte Collagen Accumulation and Their Migratory Capacities" International Journal of Molecular Sciences 23, no. 10: 5795. https://doi.org/10.3390/ijms23105795
APA StyleContentin, R., Jammes, M., Bourdon, B., Cassé, F., Bianchi, A., Audigié, F., Branly, T., Velot, É., & Galéra, P. (2022). Bone Marrow MSC Secretome Increases Equine Articular Chondrocyte Collagen Accumulation and Their Migratory Capacities. International Journal of Molecular Sciences, 23(10), 5795. https://doi.org/10.3390/ijms23105795







