In Vitro Tumor Cell-Binding Assay to Select High-Binding Antibody and Predict Therapy Response for Personalized 64Cu-Intraperitoneal Radioimmunotherapy against Peritoneal Dissemination of Pancreatic Cancer: A Feasibility Study
Abstract
:1. Introduction
2. Results
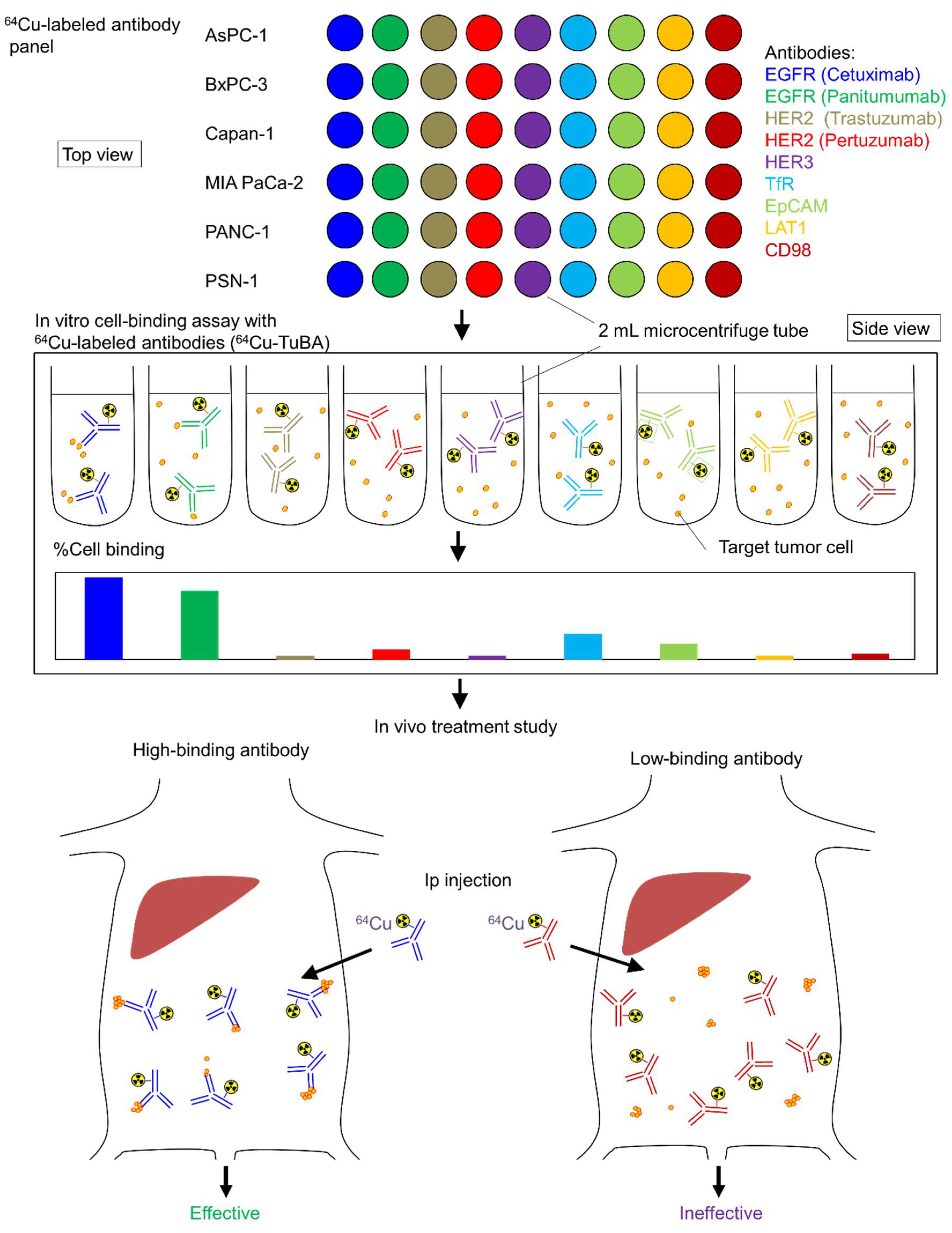
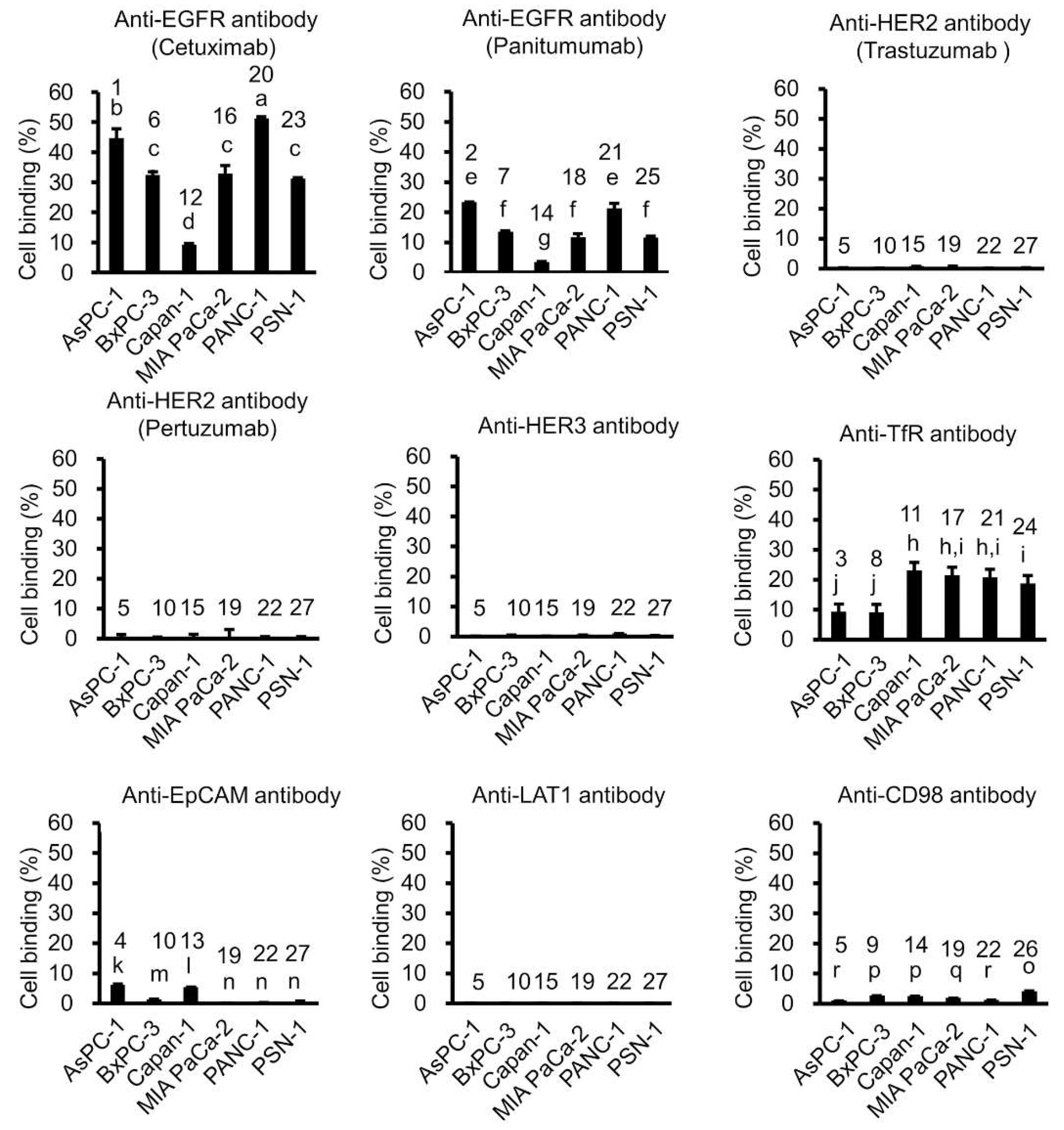
2.1. In Vitro Tumor Cell-Binding Assay with 64Cu-Labeled Antibodies
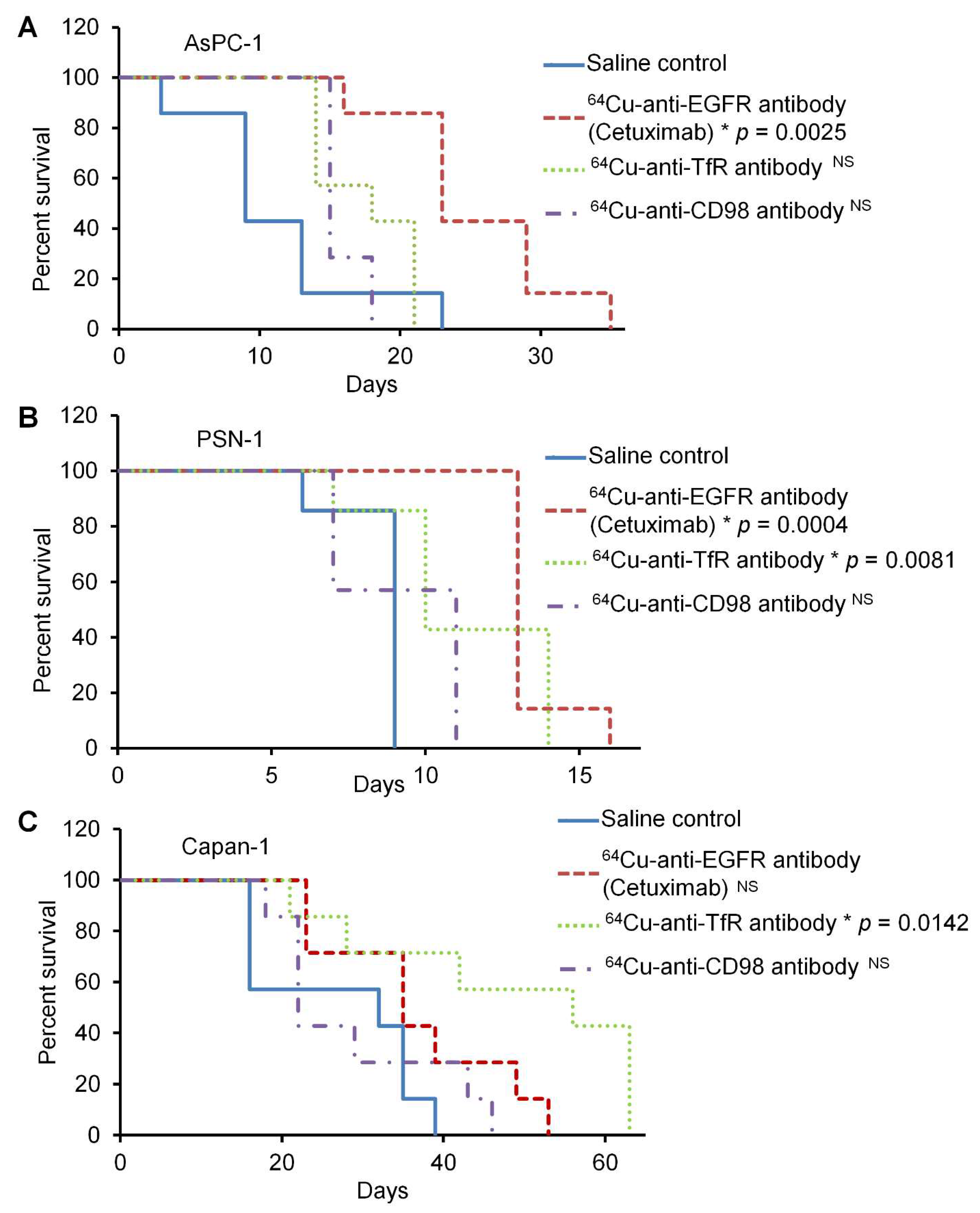
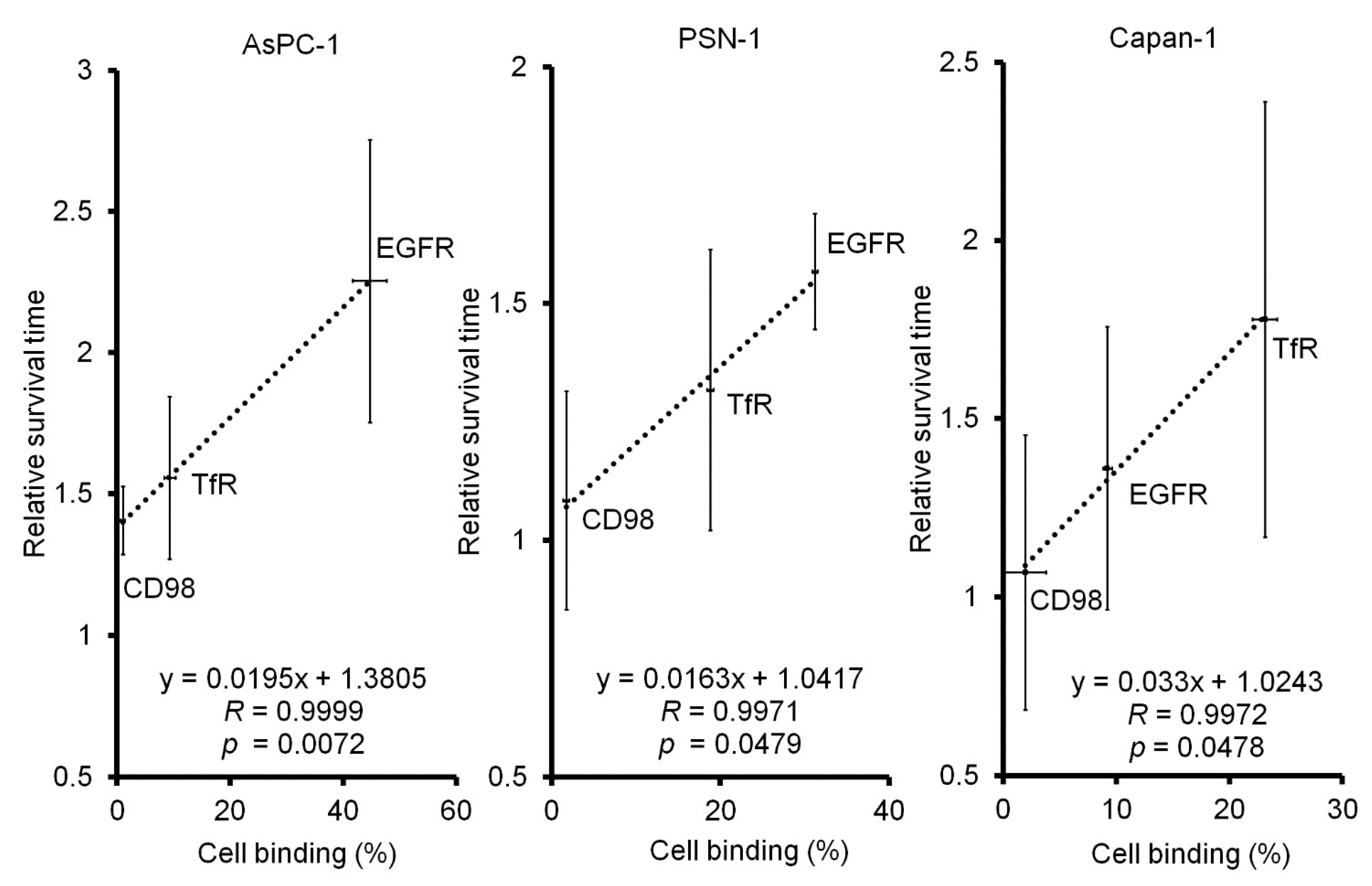
2.2. In Vivo 64Cu-ipRIT Study Using Peritoneal Dissemination Models
3. Discussion
4. Materials and Methods
4.1. Preparation of 64Cu-Labeled Antibody and 64Cu-Labeled Antibody Panel
4.2. Cell Culture
4.3. In Vitro Tumor Cell-Binding Assay with 64Cu-Labeled Antibodies
4.4. Western Blot Analysis
4.5. Animal Experiments
4.6. In Vivo Treatment Study of 64Cu-ipRIT Using the Peritoneal Dissemination Models
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; O’Reilly, E.M. Novel Therapeutics for Pancreatic Adenocarcinoma. Hematol Oncol. Clin. N. Am. 2015, 29, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, W.; Werner, J.; Jager, D.; Debus, J.; Buchler, M.W. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013, 14, e476–e485. [Google Scholar] [CrossRef]
- Nakao, A.; Fujii, T.; Sugimoto, H.; Kanazumi, N.; Nomoto, S.; Kodera, Y.; Inoue, S.; Takeda, S. Oncological problems in pancreatic cancer surgery. World J. Gastroentero 2006, 12, 4466–4472. [Google Scholar] [CrossRef]
- DeNardo, G.L. Concepts in radioimmunotherapy and immunotherapy: Radioimmunotherapy from a Lym-1 perspective. Semin. Oncol. 2005, 32 (Suppl 1.), S27–S35. [Google Scholar] [CrossRef]
- Oliveira-Cunha, M.; Newman, W.G.; Siriwardena, A.K. Epidermal growth factor receptor in pancreatic cancer. Cancers 2011, 3, 1513–1526. [Google Scholar] [CrossRef]
- Lewis, J.; Laforest, R.; Buettner, T.; Song, S.; Fujibayashi, Y.; Connett, J.; Welch, M. Copper-64-diacetyl-bis(N4-methylthiosemicarbazone): An agent for radiotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 1206–1211. [Google Scholar] [CrossRef] [Green Version]
- McMillan, D.D.; Maeda, J.; Bell, J.J.; Genet, M.D.; Phoonswadi, G.; Mann, K.A.; Kraft, S.L.; Kitamura, H.; Fujimori, A.; Yoshii, Y.; et al. Validation of 64Cu-ATSM damaging DNA via high-LET Auger electron emission. J. Radiat. Res. 2015, 56, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, Y.; Matsumoto, H.; Yoshimoto, M.; Oe, Y.; Zhang, M.R.; Nagatsu, K.; Sugyo, A.; Tsuji, A.B.; Higashi, T. 64Cu-Intraperitoneal Radioimmunotherapy: A Novel Approach for Adjuvant Treatment in a Clinically Relevant Preclinical Model of Pancreatic Cancer. J. Nucl. Med. 2019, 60, 1437–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshii, Y.; Yoshimoto, M.; Matsumoto, H.; Tashima, H.; Iwao, Y.; Takuwa, H.; Yoshida, E.; Wakizaka, H.; Yamaya, T.; Zhang, M.R.; et al. Integrated treatment using intraperitoneal radioimmunotherapy and positron emission tomography-guided surgery with 64Cu-labeled cetuximab to treat early- and late-phase peritoneal dissemination in human gastrointestinal cancer xenografts. Oncotarget 2018, 9, 28935–28950. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Wang, X.G.; Jiang, Z.; Goldberg, G.L.; Casadevall, A.; Dadachova, E. Radioimmunotherapy of experimental head and neck squamous cell carcinoma (HNSCC) with E6-specific antibody using a novel HPV-16 positive HNSCC cell line. Head Neck Oncol. 2011, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Song, I.H.; Noh, Y.; Kwon, J.; Jung, J.H.; Lee, B.C.; Kim, K.I.; Lee, Y.J.; Kang, J.H.; Rhee, C.S.; Lee, C.H.; et al. Immuno-PET imaging based radioimmunotherapy in head and neck squamous cell carcinoma model. Oncotarget 2017, 8, 92090–92105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [PubMed]
- Ly, L.D.; Ly, D.D.; Nguyen, N.T.; Kim, J.H.; Yoo, H.; Chung, J.; Lee, M.S.; Cha, S.K.; Park, K.S. Mitochondrial Ca(2+) Uptake Relieves Palmitate-Induced Cytosolic Ca(2+) Overload in MIN6 Cells. Mol. Cells 2020, 43, 66–75. [Google Scholar] [PubMed]
- Sakahara, H.; Endo, K.; Koizumi, M.; Nakashima, T.; Kunimatsu, M.; Watanabe, Y.; Kawamura, Y.; Nakamura, T.; Tanaka, H.; Kotoura, Y.; et al. Relationship between in vitro binding activity and in vivo tumor accumulation of radiolabeled monoclonal antibodies. J. Nucl. Med. 1988, 29, 235–240. [Google Scholar] [PubMed]
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017, 7, 462–477. [Google Scholar] [CrossRef] [Green Version]
- Chou, A.; Waddell, N.; Cowley, M.J.; Gill, A.J.; Chang, D.K.; Patch, A.M.; Nones, K.; Wu, J.; Pinese, M.; Johns, A.L.; et al. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhang, L.; Li, X.; Yan, H.; Yang, L.; Li, Y.; Li, T.; Wang, J.; Cao, B. The prognostic significance of human epidermal growth factor receptor family protein expression in operable pancreatic cancer: HER1-4 protein expression and prognosis in pancreatic cancer. BMC Cancer 2016, 16, 910. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.M.; Hwang, S.; Seong, R.H. Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Biochem. Biophys. Res. Commun. 2016, 471, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, F.; Struck, L.; Tachezy, M.; Vashist, Y.; Wicklein, D.; Schumacher, U.; Izbicki, J.R.; Bockhorn, M. Serum EpCAM expression in pancreatic cancer. Anticancer Res. 2014, 34, 4741–4746. [Google Scholar] [PubMed]
- Yanagisawa, N.; Ichinoe, M.; Mikami, T.; Nakada, N.; Hana, K.; Koizumi, W.; Endou, H.; Okayasu, I. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J. Clin. Pathol. 2012, 65, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Sunose, Y.; Arakawa, K.; Ogawa, T.; Sunaga, N.; Shimizu, K.; Tominaga, H.; Oriuchi, N.; Itoh, H.; Nagamori, S.; et al. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br. J. Cancer 2012, 107, 632–638. [Google Scholar] [CrossRef]
- Okita, K.; Imai, K.; Kato, K.; Sugiura, R.; Endo, Y.; Masuko, K.; Tomioka, Y.; Masuko, T. Altered binding avidities and improved growth inhibitory effects of novel anti-HER3 mAb against human cancers in the presence of HER1-or HER2-targeted drugs. Biochem. Biophys. Res. Commun. 2021, 576, 59–65. [Google Scholar] [CrossRef]
- Okita, K.; Okazaki, S.; Uejima, S.; Yamada, E.; Kaminaka, H.; Kondo, M.; Ueda, S.; Tokiwa, R.; Iwata, N.; Yamasaki, A.; et al. Novel functional anti-HER3 monoclonal antibodies with potent anti-cancer effects on various human epithelial cancers. Oncotarget 2020, 11, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Furukawa, T.; Tashiro, T.; Okita, K.; Jin, Z.H.; Aung, W.; Sugyo, A.; Nagatsu, K.; Endo, H.; Tsuji, A.B.; et al. Immuno-PET Imaging of HER3 in a Model in which HER3 Signaling Plays a Critical Role. PLoS ONE 2015, 10, e0143076. [Google Scholar] [CrossRef] [Green Version]
- Kurosawa, G.; Akahori, Y.; Morita, M.; Sumitomo, M.; Sato, N.; Muramatsu, C.; Eguchi, K.; Matsuda, K.; Takasaki, A.; Tanaka, M.; et al. Comprehensive screening for antigens overexpressed on carcinomas via isolation of human mAbs that may be therapeutic. Proc. Natl. Acad. Sci. USA 2008, 105, 7287–7292. [Google Scholar] [CrossRef] [Green Version]
- Kurosawa, G.; Sumitomo, M.; Akahori, Y.; Matsuda, K.; Muramatsu, C.; Takasaki, A.; Iba, Y.; Eguchi, K.; Tanaka, M.; Suzuki, K.; et al. Methods for comprehensive identification of membrane proteins recognized by a large number of monoclonal antibodies. J. Immunol. Methods 2009, 351, 1–12. [Google Scholar] [CrossRef]
- Kurosawa, G.; Sumitomo, M.; Ukai, Y.; Subere, J.; Muramatsu, C.; Eguchi, K.; Tanaka-Hashiba, M.; Sugiura, M.; Ando, M.; Sato, N.; et al. Selection and analysis of anti-cancer antibodies for cancer therapy obtained from antibody phage library. Cancer Sci. 2011, 102, 175–181. [Google Scholar] [CrossRef]
- Masuko, T. Analysis of Target Molecules towards Anti-cancer Therapeutic Antibodies. Yakugaku Zasshi 2021, 141, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Yamasaki, A.; Ueda, S.; Okazaki, S.; Ohno, Y.; Tanaka, T.; Endo, Y.; Tomioka, Y.; Masuko, K.; Masuko, T.; et al. Oncogenic transformation of NIH/3T3 cells by the overexpression of L-type amino acid transporter 1, a promising anti-cancer target. Oncotarget 2021, 12, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Ikotun, O.F.; Marquez, B.V.; Huang, C.; Masuko, K.; Daiji, M.; Masuko, T.; McConathy, J.; Lapi, S.E. Imaging the L-type amino acid transporter-1 (LAT1) with Zr-89 immunoPET. PLoS ONE 2013, 8, e77476. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Hayashi, H.; Miyamoto, T.; Abe, S.; Hirai, K.; Matsukura, K.; Yagi, H.; Hara, Y.; Yoshida, K.; Okazaki, S.; et al. Anti-tumor effects of mAb against L-type amino acid transporter 1 (LAT1) bound to human and monkey LAT1 with dual avidity modes. Cancer Sci. 2019, 110, 674–685. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Inoue, K.; Hayashi, H.; Suzuki, T.; Masuko, T. Identification of cell proliferation-associated epitope on CD98 oncoprotein using phage display random peptide library. Cancer Sci. 2007, 98, 1696–1700. [Google Scholar] [CrossRef]
- Mirus, J.E.; Zhang, Y.; Li, C.I.; Lokshin, A.E.; Prentice, R.L.; Hingorani, S.R.; Lampe, P.D. Cross-species antibody microarray interrogation identifies a 3-protein panel of plasma biomarkers for early diagnosis of pancreas cancer. Clin. Cancer Res. 2015, 21, 1764–1771. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, P.; Cao, B. Development and optimization of an antibody array method for potential cancer biomarker detection. J. Biomed. Res. 2011, 25, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Arias-Pinilla, G.A.; Modjtahedi, H. Therapeutic Application of Monoclonal Antibodies in Pancreatic Cancer: Advances, Challenges and Future Opportunities. Cancers 2021, 13, 1781. [Google Scholar] [CrossRef]
- Tomoko Tachibana, Y.Y.; Matsumoto, H.; Zhang, M.-R.; Nagatsu, K.; Hihara, F.; Igarashi, C.; Sugyo, A.; Tsuji, A.; Higashi, T. Efficacy of vorinostat-sensitized intraperitoneal radioimmunotherapy with 64Cu-labeled cetuximab against peritoneal dissemination of gastric cancer in a mouse model. J. Cancer Res. Ther. 2020. [Google Scholar] [CrossRef]
- Ohya, T.; Nagatsu, K.; Suzuki, H.; Fukada, M.; Minegishi, K.; Hanyu, M.; Fukumura, T.; Zhang, M.R. Efficient preparation of high-quality 64Cu for routine use. Nucl. Med. Biol. 2016, 43, 685–691. [Google Scholar] [CrossRef]

| Antigens | Abbreviations | Antibodies | Source |
|---|---|---|---|
| Epidermal growth factor receptor | EGFR | cetuximab | Merck Serono |
| panitumumab | Takeda | ||
| Human epidermal growth factor receptor 2 | HER2 | trastuzumab | Chugai Pharmaceutical |
| pertuzumab | Chugai Pharmaceutical | ||
| Human epidermal growth factor receptor 3 | HER3 | Ab3-1 | [25,26,27] |
| Transferrin receptor | TfR | 066-188 | [28,29,30] |
| Epithelial cell adhesion molecule | EpCAM | 1D12 | [31] |
| L-type amino acid transporter 1 | LAT1 | Ab1 | [32,33,34] |
| 4F2 heavy chain | CD98 | HBJ127 | [35] |
| Groups | AsPC-1 | |||
|---|---|---|---|---|
| Mean Survival Time | %MST | |||
| Saline control | 11 | ± | 6 | 100 |
| 64Cu-cetuximab | 25 | ± | 6 | 225 |
| 64Cu-anti-TfR antibody | 18 | ± | 3 | 156 |
| 64Cu-anti-CD98 antibody | 16 | ± | 1 | 141 |
| Groups | PSN-1 | |||
| Mean survival time | %MST | |||
| Saline control | 9 | ± | 1 | 100 |
| 64Cu-cetuximab | 13 | ± | 1 | 157 |
| 64Cu-anti-TfR antibody | 11 | ± | 3 | 132 |
| 64Cu-anti-CD98 antibody | 9 | ± | 2 | 108 |
| Groups | CAPAN-1 | |||
| Mean survival time | %MST | |||
| Saline control | 27 | ± | 10 | 100 |
| 64Cu-cetuximab | 37 | ± | 11 | 136 |
| 64Cu-anti-TfR antibody | 48 | ± | 16 | 178 |
| 64Cu-anti-CD98 antibody | 29 | ± | 10 | 107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hihara, F.; Matsumoto, H.; Yoshimoto, M.; Masuko, T.; Endo, Y.; Igarashi, C.; Tachibana, T.; Shinada, M.; Zhang, M.-R.; Kurosawa, G.; et al. In Vitro Tumor Cell-Binding Assay to Select High-Binding Antibody and Predict Therapy Response for Personalized 64Cu-Intraperitoneal Radioimmunotherapy against Peritoneal Dissemination of Pancreatic Cancer: A Feasibility Study. Int. J. Mol. Sci. 2022, 23, 5807. https://doi.org/10.3390/ijms23105807
Hihara F, Matsumoto H, Yoshimoto M, Masuko T, Endo Y, Igarashi C, Tachibana T, Shinada M, Zhang M-R, Kurosawa G, et al. In Vitro Tumor Cell-Binding Assay to Select High-Binding Antibody and Predict Therapy Response for Personalized 64Cu-Intraperitoneal Radioimmunotherapy against Peritoneal Dissemination of Pancreatic Cancer: A Feasibility Study. International Journal of Molecular Sciences. 2022; 23(10):5807. https://doi.org/10.3390/ijms23105807
Chicago/Turabian StyleHihara, Fukiko, Hiroki Matsumoto, Mitsuyoshi Yoshimoto, Takashi Masuko, Yuichi Endo, Chika Igarashi, Tomoko Tachibana, Mitsuhiro Shinada, Ming-Rong Zhang, Gene Kurosawa, and et al. 2022. "In Vitro Tumor Cell-Binding Assay to Select High-Binding Antibody and Predict Therapy Response for Personalized 64Cu-Intraperitoneal Radioimmunotherapy against Peritoneal Dissemination of Pancreatic Cancer: A Feasibility Study" International Journal of Molecular Sciences 23, no. 10: 5807. https://doi.org/10.3390/ijms23105807
APA StyleHihara, F., Matsumoto, H., Yoshimoto, M., Masuko, T., Endo, Y., Igarashi, C., Tachibana, T., Shinada, M., Zhang, M.-R., Kurosawa, G., Sugyo, A., Tsuji, A. B., Higashi, T., Kurihara, H., Ueno, M., & Yoshii, Y. (2022). In Vitro Tumor Cell-Binding Assay to Select High-Binding Antibody and Predict Therapy Response for Personalized 64Cu-Intraperitoneal Radioimmunotherapy against Peritoneal Dissemination of Pancreatic Cancer: A Feasibility Study. International Journal of Molecular Sciences, 23(10), 5807. https://doi.org/10.3390/ijms23105807








