The Relationship and Expression of miR-451a, miR-25-3p and PTEN in Early Peritoneal Endometriotic Lesions and Their Modulation In Vitro
Abstract
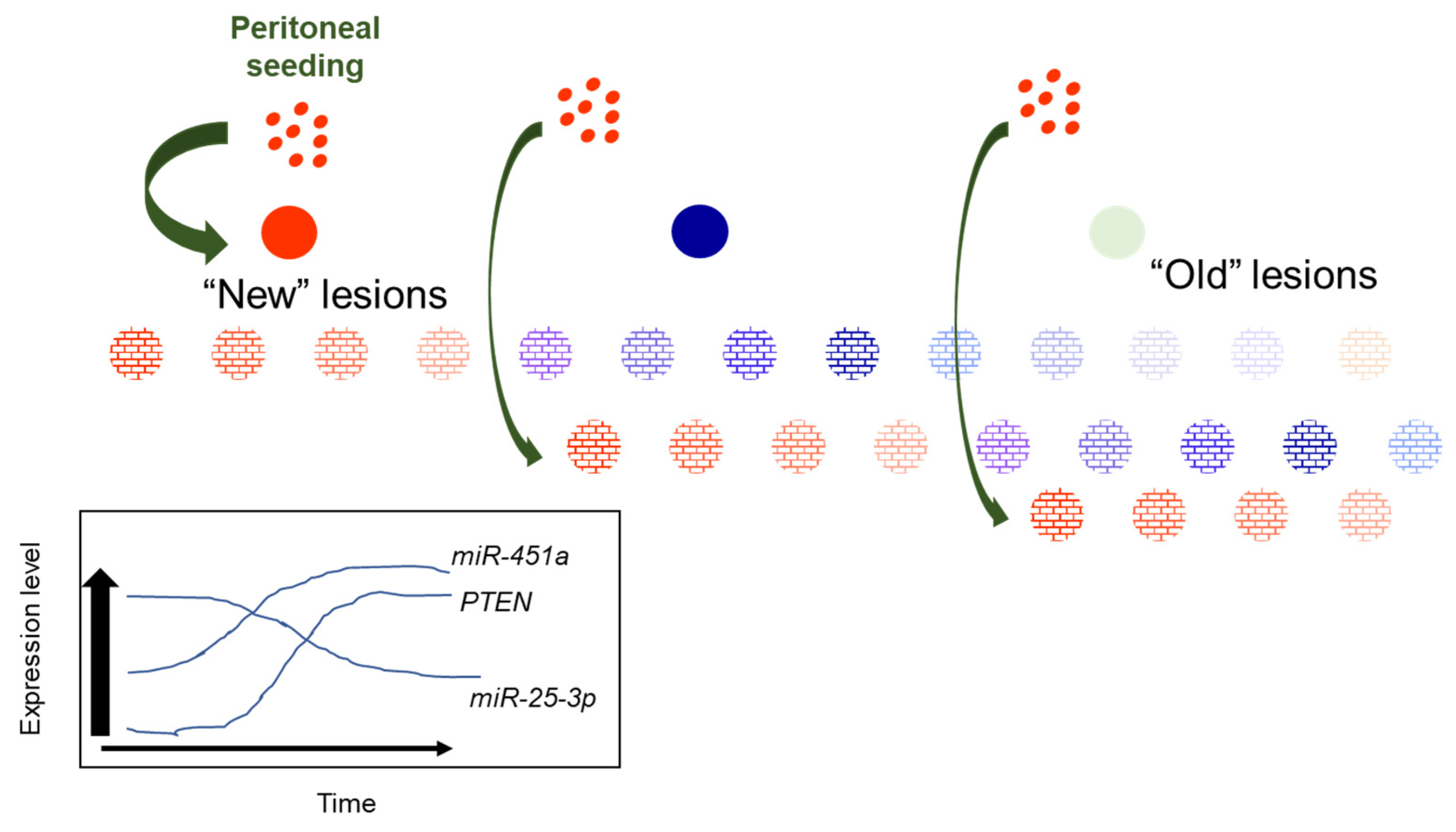
:1. Introduction
2. Results
2.1. Endometriotic Lesion Expression of PTEN Transcript
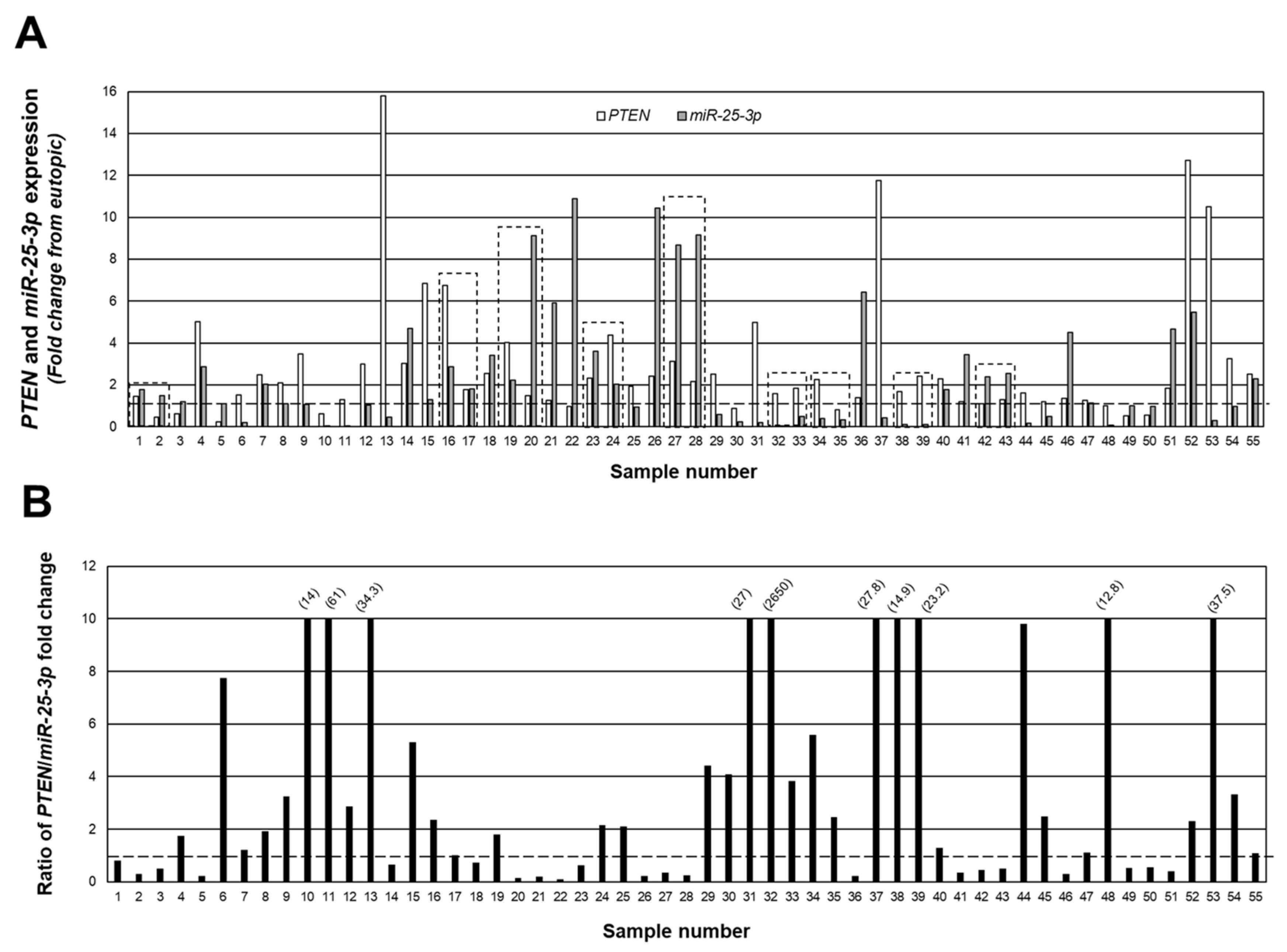
2.2. Endometriotic Lesion Expression of miR-25-3p and Relationship with PTEN
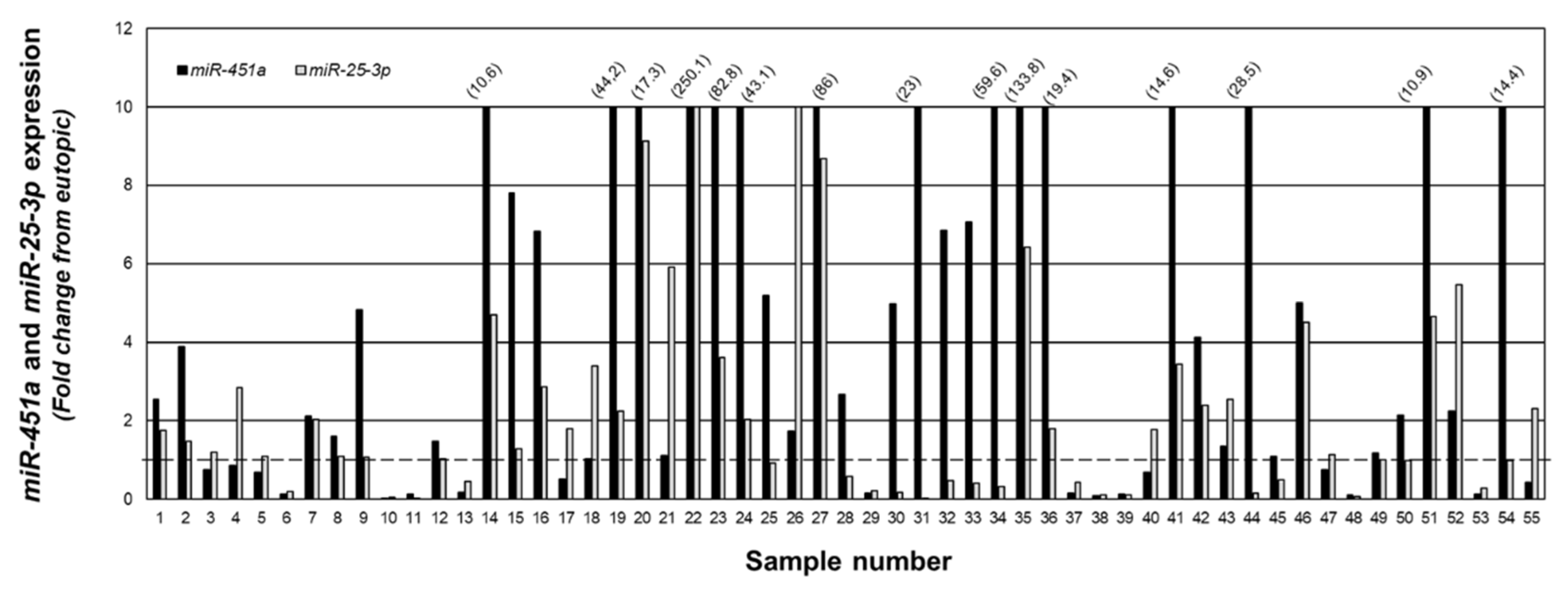
2.3. Relationship between miR-451a and miR-25-3p In Vivo
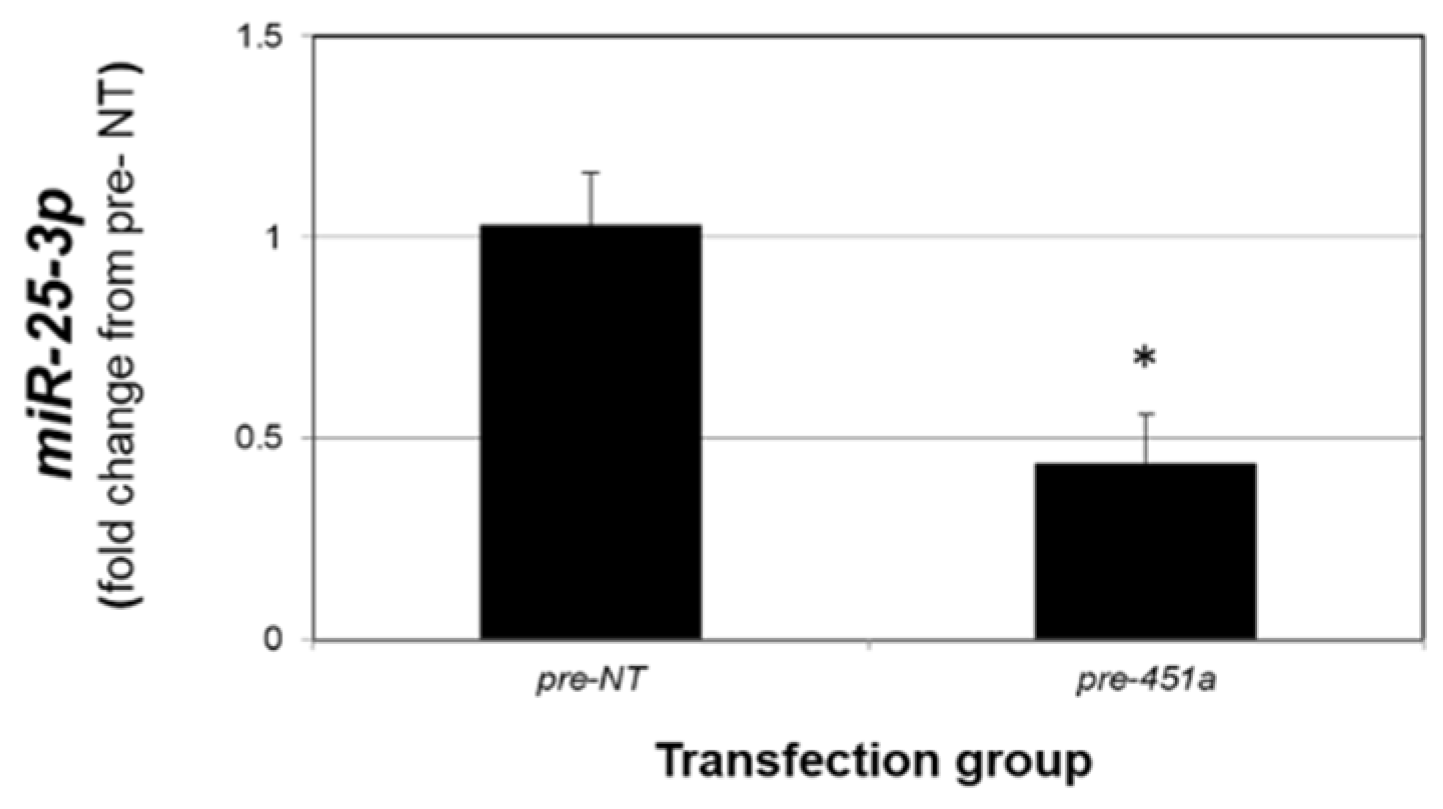
2.4. miR-451a Regulatoin of miR-25-3p In Vitro
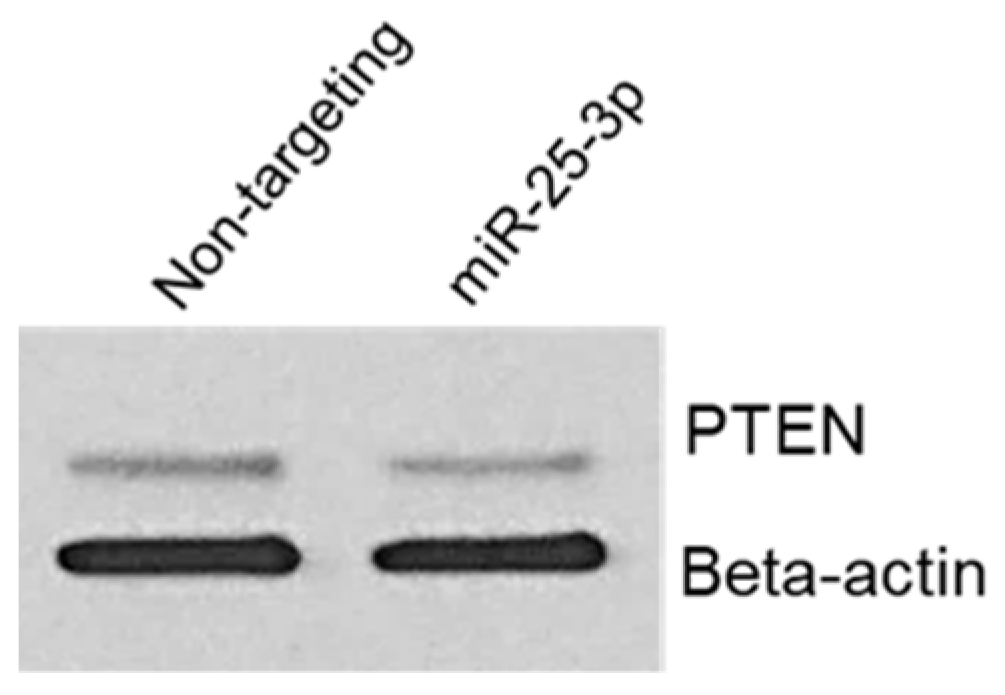
2.5. miR-25-3p Down-Regulates PTEN Protein In Vitro
3. Discussion
4. Materials and Methods
4.1. Human Subjects and Tissue Acquisition
4.2. mRNA Isolation and qRT-PCR
4.3. Cell Culture of Endometriotic Epithelial 12Z Cells and Transfection
4.4. Western Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Sampson, J.A. The development of the implantation theory for the origin of peritoneal endometriosis. Am. J. Obstet. Gynecol. 1940, 40, 549–557. [Google Scholar] [CrossRef]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984, 64, 151–154. [Google Scholar] [PubMed]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, classification, pathogenesis, treatment and genetics (review of the literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Bjorkman, S.; Taylor, H.S. MicroRNAs in endometriosis: Biological function and emerging biomarker candidates. Biol. Reprod. 2019, 100, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Panir, K.; Schjenken, J.E.; Robertson, S.A.; Hull, M.L. Non-coding RNAs in endometriosis: A narrative review. Hum. Reprod. Update 2018, 24, 497–515. [Google Scholar] [CrossRef] [Green Version]
- Nothnick, W.B. MicroRNAs and Endometriosis: Distinguishing drivers from passengers in disease pathogenesis. Semin. Reprod. Med. 2017, 35, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Graham, A.; Falcone, T.; Nothnick, W.B. The expression of microRNA-451 in human endometriotic lesions is inversely related to that of macrophage migration inhibitory factor (MIF) and regulates MIF expression and modulation of epithelial cell survival. Hum. Reprod. 2015, 30, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Nothnick, W.B.; Swan, K.; Flyckt, R.; Falcone, T.; Graham, A. Human endometriotic lesion expression of the miR-144-3p/miR-451a cluster, its correlation with markers of cell survival and origin of lesion content. Sci. Rep. 2019, 9, 8823. [Google Scholar] [CrossRef]
- Nothnick, W.B.; Falcone, T.; Joshi NFazleabas, A.T.; Graham, A. Serum miR-451a levels are significantly elevated in women with endometriosis and recapitulated in, baboons (Papio anubis) with experimentally-induced disease. Reprod. Sci. 2017, 24, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B.; Graham, A.; Holbert, J.; Weiss, M.J. miR-451 deficiency is associated with altered endometrial fibrinogen alpha chain expression and reduced endometriotic implant establishment in an experimental mouse model. PLoS ONE 2014, 9, e100336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Steck, P.A.; Pershouse, M.A.; Jasser, S.A.; Yung, W.K.; Lin, H.; Ligon, A.H.; Langford, L.A.; Baumgard, M.L.; Hattier, T.; Davis, T.; et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997, 15, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Q.; Teng, H.; Xu, X.H.; Liu, S.Y.; Wang, Y.H.; Guo, F.J.; Lin, X.J. Microarray analysis of microRNA deregulation and angiogenesis-related proteins in endometriosis. Genet. Mol. Res. 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Peng, J.; Shi, Y.; Sun, P. miR-92a promotes progesterone resistance in endometriosis through PTEN/AKT pathway. Life Sci. 2020, 242, 117190. [Google Scholar] [CrossRef]
- Madanes, D.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I.; Ricci, A.G. PI3K/AKT pathway is altered in the endometriosis patient’s endometrium and presents differences according to severity stage. Gynecol. Endocrinol. 2020, 36, 436–440. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- Tokar, T.; Pastrello, C.; Rossos, A.E.M.; Abovsky, M.; Hauschild, A.-C.; Tsay, M.; Li, R.; Jurisica, L. mirDIP4.1–integrative database of human microRNA target predictions. Nucleic Acids Res. 2018, 46, D360–D370. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X. Prediction of functional miRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Wan, W.; Wan, W.; Long, Y.; Li, Q.; Jin, X.; Wan, G.; Zhang, F.; Lv, Y.; Zheng, G.; Li, Z.; et al. MiR-25-3p promotes malignant phenotypes of retinoblastoma by regulating PTEN/Akt pathway. Biomed. Pharmacother. 2019, 118, 109111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tong, Z.; Sun, Z.; Zhu, G.; Shen, E.; Huang, Y. MiR-25-3p targets PTEN to regulate the migration, invasion, and apoptosis of esophageal cancer cells via the PI3K/AKT pathway. Biosci. Rep. 2020, 40, BSR20201901. [Google Scholar] [CrossRef] [PubMed]
- Zi, Y.; Zhang, Y.; Wu, Y.; Zhang, L.; Yang, R.; Huang, Y. Downregulation of microRNA-25-3p inhibits the proliferation and promotes the apoptosis of multiple myeloma cells via targeting the PTEN/PI3K/AKT signaling pathway. Int. J. Mol. Med. 2021, 47, 8. [Google Scholar] [CrossRef] [PubMed]
- Filigheddu, N.; Gregnanin, I.; Porporato, P.E.; Surico, D.; Perego, B.; Galli, L.; Patrignani, C.; Graziani, A.; Surico, N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J. Biomed. Biotechnol. 2010, 2010, 369549. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Gu, L.; Chen, J.; Guo, X.R.; Shi, Y.L. Downregulation of miR-183 inhibits apoptosis and enhances the invasive potential of endometrial stromal cells in endometriosis. Int. J. Mol. Med. 2014, 33, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Hong, X.; Liu, Y.; Zhou, W.; Zhang, Y. The miR-25-3p/Sp1 pathway is dysregulated in ovarian endometriosis. J. Int. Med. Res. 2020, 48, 300060520918437. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson Teague, E.M.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. MicroRNAs regulating microRNAs in cancer. Trends Cancer 2018, 4, 465–468. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- Forrest, A.R.; Kanamori-Katayama, M.; Tomaru, Y.; Lassmann, T.; Ninomiya, N.; Takahashi, Y.; de Hoon, M.J.L.; Kubosaki, A.; Kaiho, A.; Suzuki, M.; et al. Induction of microRNAs, mir-155, mir-222, mir-424 and mir-503, promotes monocytic differentiation through combinatorial regulation. Leukemia 2010, 24, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Li, L.; Zhu, D.; Hou, D.; Cao, T.; Gu, H.; Zhang, J.; Chen, J.; Zhang, C.-Y.; Zen, K. Mouse miRNA-709 directly regulates miRNA-15a/16-1 biogenesis at the posttranscriptional level in the nucleus: Evidence for a microRNA hierarchy system. Cell Res. 2012, 22, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-S.; Su, J.-L.; Cha, S.-T.; Tarn, W.-Y.; Wang, M.-Y.; Hsu, H.-C.; Lin, M.-T.; Chu, C.-Y.; Hua, K.-T.; Chen, T.-N.; et al. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J. Clin. Investig. 2011, 121, 3442–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosar, E.; Mamillapalli, R.; Ersoy, G.S.; Cho, S.; Seifer, B.; Taylor, H.S. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil. Steril. 2016, 106, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obstet. Gynecol. 2020, 223, 557.e1–557.e11. [Google Scholar] [CrossRef]
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Alali, Z.; Graham, A.; Swan, K.; Flyckt, R.; Falcone, T.; Cui, W.; Yang, X.; Christianson, J.; Nothnick, W.B. 60S acidic ribosomal protein P1 (RPLP1) is elevated in human endometriotic tissue and in a murine model of endometriosis and is essential for endometriotic epithelial cell survival in vitro. Mol. Hum. Reprod. 2020, 26, 53–64. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nothnick, W.B.; Peterson, R.; Minchella, P.; Falcone, T.; Graham, A.; Findley, A. The Relationship and Expression of miR-451a, miR-25-3p and PTEN in Early Peritoneal Endometriotic Lesions and Their Modulation In Vitro. Int. J. Mol. Sci. 2022, 23, 5862. https://doi.org/10.3390/ijms23115862
Nothnick WB, Peterson R, Minchella P, Falcone T, Graham A, Findley A. The Relationship and Expression of miR-451a, miR-25-3p and PTEN in Early Peritoneal Endometriotic Lesions and Their Modulation In Vitro. International Journal of Molecular Sciences. 2022; 23(11):5862. https://doi.org/10.3390/ijms23115862
Chicago/Turabian StyleNothnick, Warren B., Riley Peterson, Paige Minchella, Tommaso Falcone, Amanda Graham, and Austin Findley. 2022. "The Relationship and Expression of miR-451a, miR-25-3p and PTEN in Early Peritoneal Endometriotic Lesions and Their Modulation In Vitro" International Journal of Molecular Sciences 23, no. 11: 5862. https://doi.org/10.3390/ijms23115862
APA StyleNothnick, W. B., Peterson, R., Minchella, P., Falcone, T., Graham, A., & Findley, A. (2022). The Relationship and Expression of miR-451a, miR-25-3p and PTEN in Early Peritoneal Endometriotic Lesions and Their Modulation In Vitro. International Journal of Molecular Sciences, 23(11), 5862. https://doi.org/10.3390/ijms23115862




