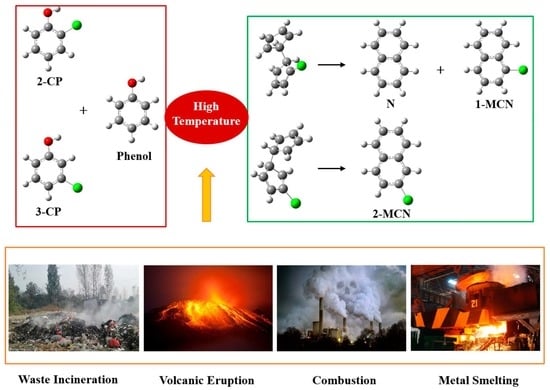
The Homogeneous Gas-Phase Formation Mechanism of PCNs from Cross-Condensation of Phenoxy Radical with 2-CPR and 3-CPR: A Theoretical Mechanistic and Kinetic Study
Abstract
:1. Introduction
2. Results
2.1. Formation of PhR, 2-CPR, and 3-CPR from Phenol, 2-CTP, and 3-CP Molecules
2.2. Formation of Chloro-Bicyclopentadienyl from Cross-Condensation of PhR with 2-CPR/3-CPR
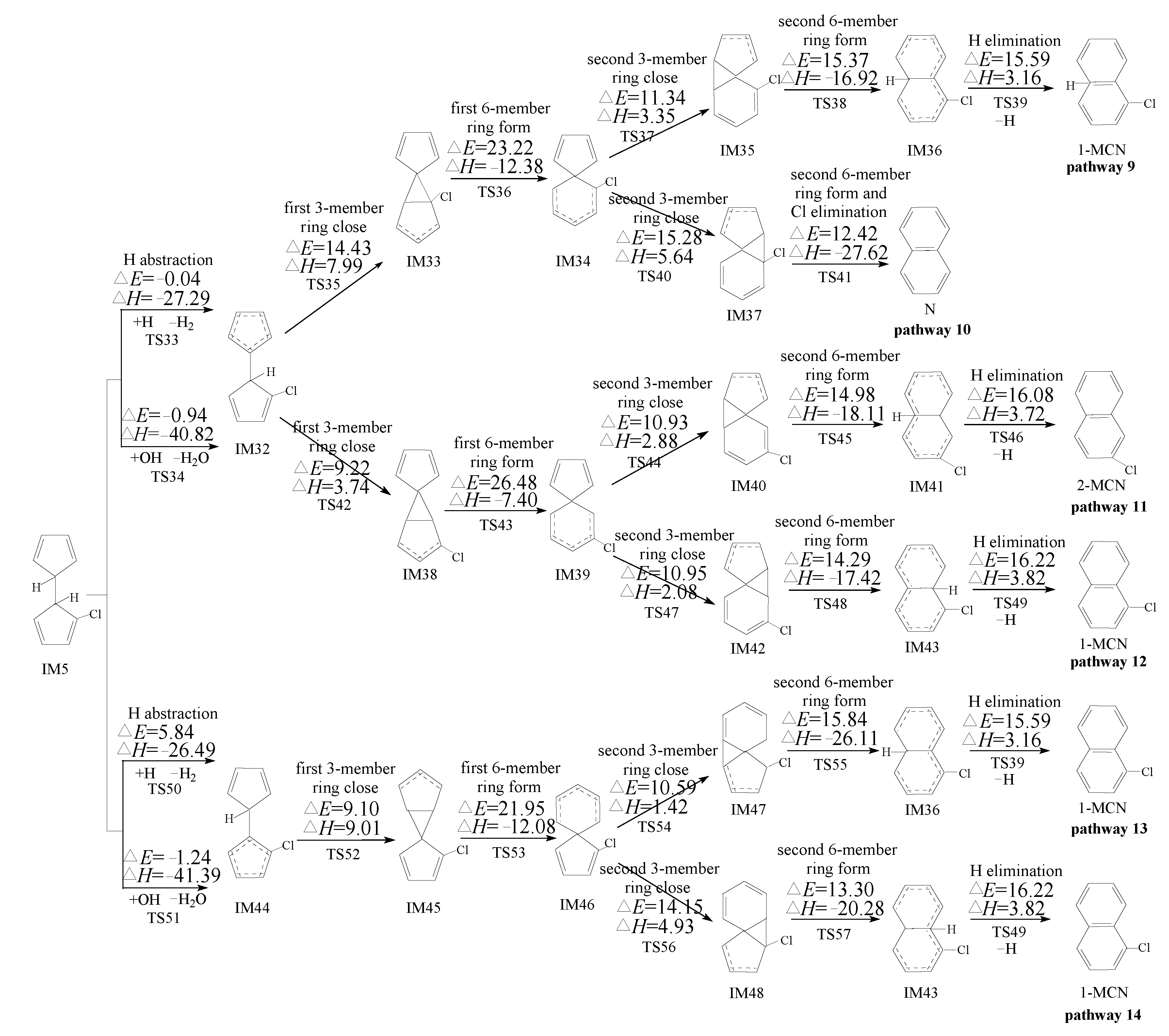
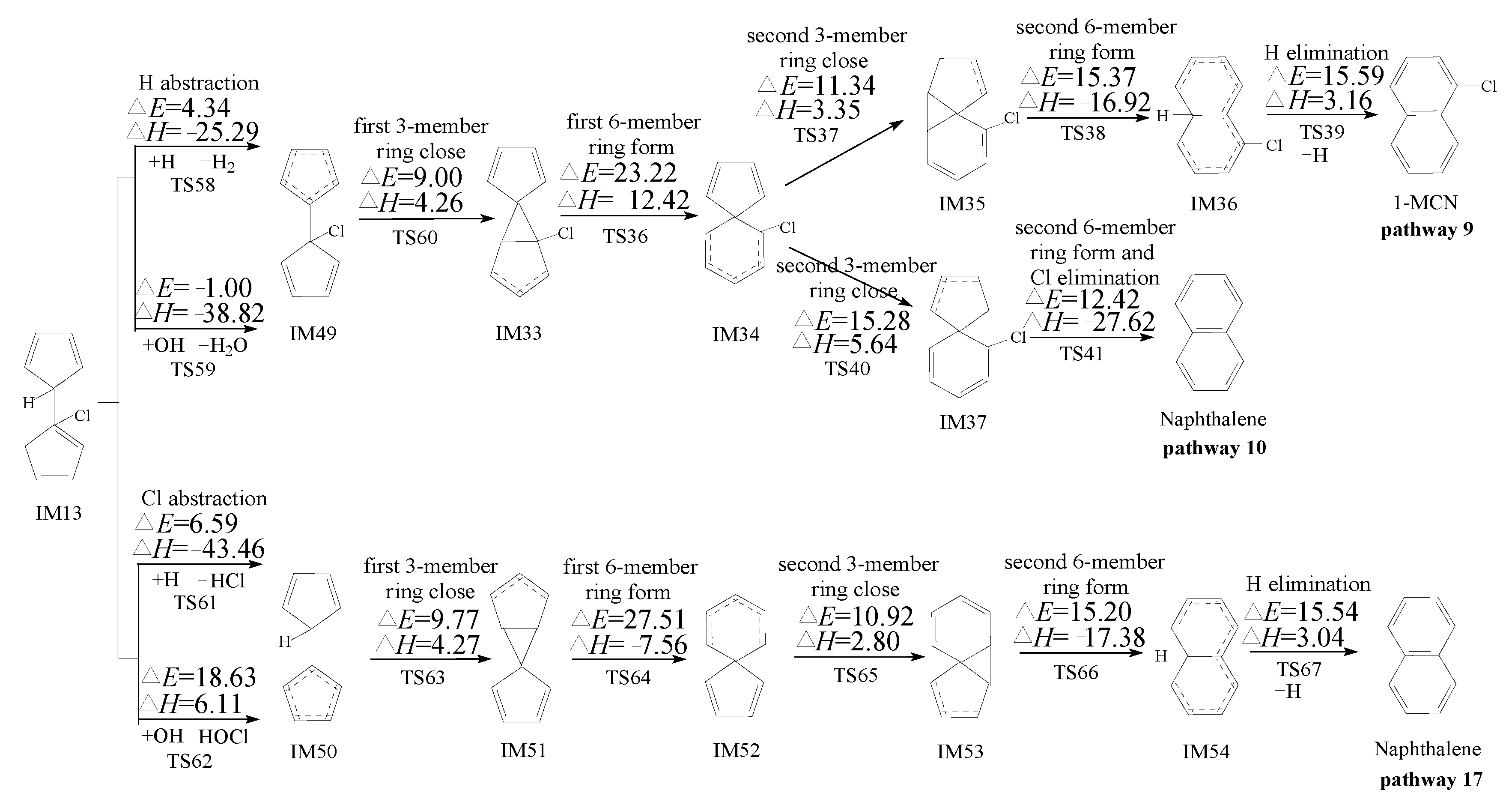
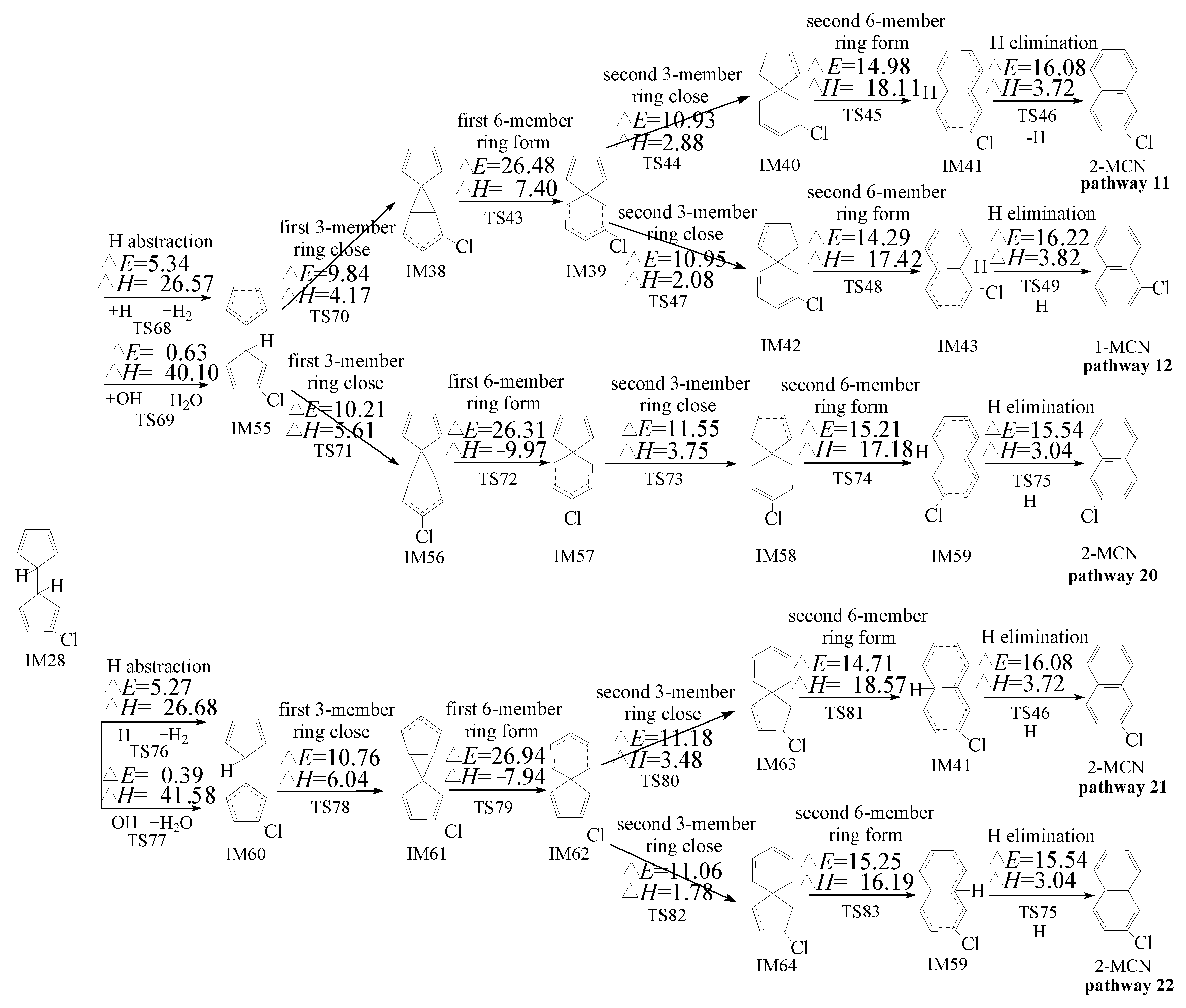
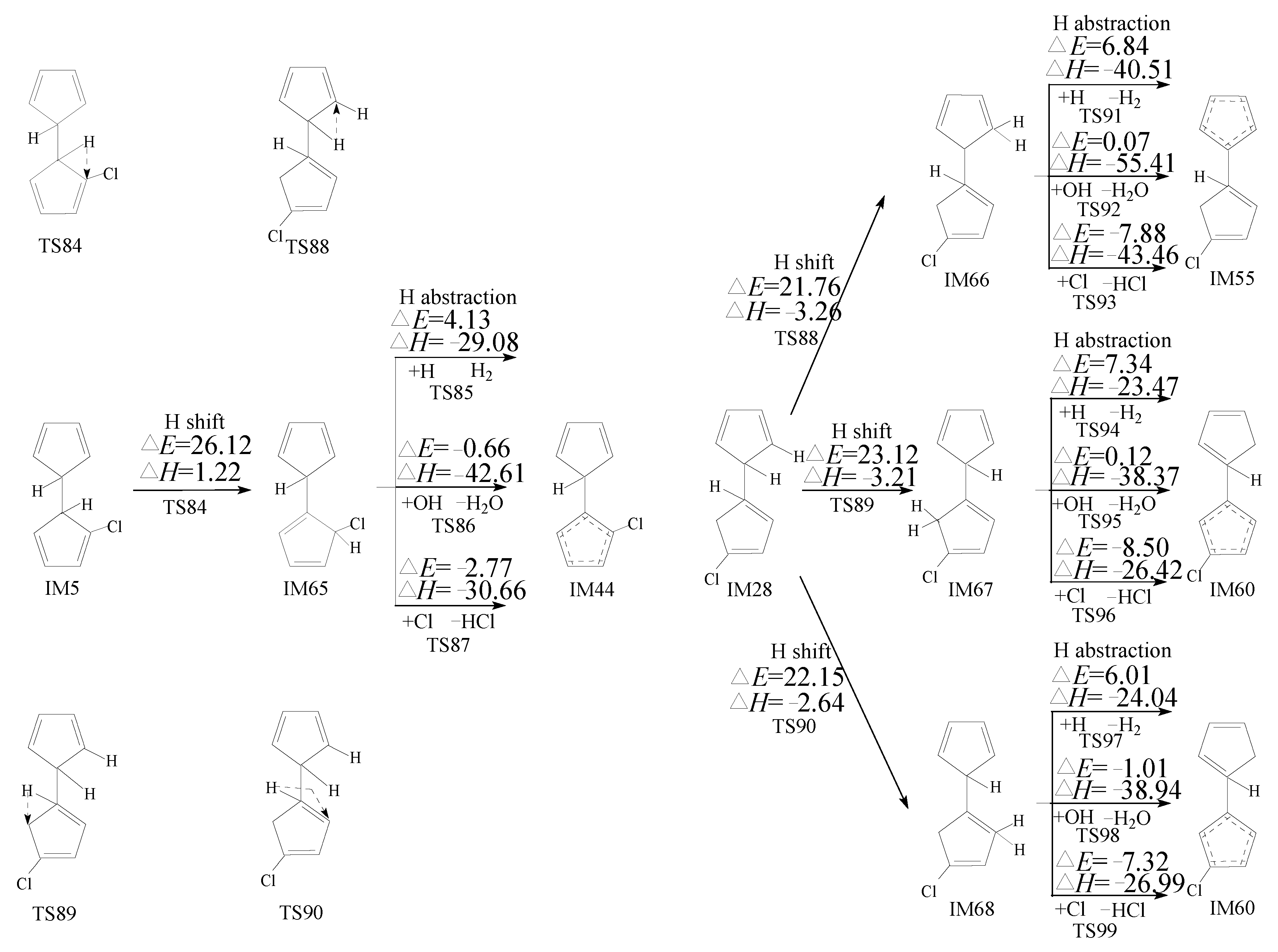
2.3. Formation of PCNs from Pursuant Reactions of Chloro-Bicyclopentadienyls
2.3.1. Formation of PCNs from Pursuant Reactions of Chloro-Bicyclopentadienyls by Direct H/Cl Abstraction Routes
2.3.2. Formation of PCNs from Pursuant Reactions of Chloro-Bicyclopentadienyls by First H Shift and Then Abstraction Routes
2.4. Rate Constant Calculations
3. Discussion
3.1. Formation of PhR, 2-CPR, and 3-CPR from Phenol, 2-CTP, and 3-CP Molecules
3.2. Formation of Chloro-Bicyclopentadienyl from Cross-Condensation of PhR with 2-CPR/3-CPR
3.3. Formation of PCNs from Pursuant Reactions of Chloro-Bicyclopentadienyls
3.3.1. Formation of PCNs from Pursuant Reactions of Chloro-Bicyclopentadienyls by Direct H/Cl Abstraction Routes
3.3.2. Formation of PCNs from Pursuant Reactions of Chloro-Bicyclopentadienyls by First H Shift and Then Abstraction Routes
3.3.3. Comparing the Cross-Condensation of PhR with 2-CPR/3-CPR and Self-Condensation of 2-CPRs/3-CPRs
3.4. Rate Constant Calculations
4. Materials and Methods
4.1. Density Functional Theory
4.2. Kinetic Calculation
5. Conclusions
- (1)
- The formation of PCNs from the cross-condensation of PhR with 2-CPR/3-CPR contains two processes: the formation of chloro-dihydrofulvlene from the cross-condensation of PhR with 2-CPR/3-CPR, and PCN formation from subsequent reactions of chloro-bicyclopentadienyl. Pathways terminated with Cl elimination (pathways for the naphthalene formation) are preferred over those terminated with H elimination (pathways for the MCN formation). The “direct abstraction” mechanism is energetically favored over the “first H shift, then abstraction” mechanisms.
- (2)
- (3)
- The theoretical calculation of the PCN formation mechanism from the cross-condensation of PhR with 2-CPR/3-CPR, as well as that from the self-condensation using 2-CPRs/3-CPRs [31], can provide a reasonable explanation of the experimental observations [28,29] that the formation potential of naphthalene is larger than that of 1-MCN from 2-CP as a precursor and almost equal to 1-MCN, and 2-MCN can be produced from 3-CP as a precursor.
- (4)
- PCN formation from the self-condensation of 2-CPRs is more energetically favorable than that from the cross-condensation of PhR with 2-CPR, whereas PCN formation from the cross-condensation of PhR with 3-CPR is favored over that from the self-condensation of 3-CPRs. PCN formation from the cross-condensation of PhR with 3-CPR can occur much easier than that from the cross-condensation of PhR with 2-CPR.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidleman, T.F.; Helm, P.A.; Braune, B.M.; Gabrielsen, G.W. Polychlorinated naphthalenes in polar environments—A review. Sci. Total Environ. 2010, 408, 2919–2935. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, A.L.; Kannan, K.; Villalobos, S.A.; Villeneuve, D.L.; Falandysz, J.; Imagawa, T.; Jakobsson, E.; Giesy, J.P. Relative potencies of individual polychlorinated naphthalenes and halowax mixtures to induce Ah receptor-mediated responses. Environ. Sci. Technol. 2000, 34, 3153–3158. [Google Scholar] [CrossRef]
- Puzyn, T.; Falandysz, J.; Jones, P.D.; Giesy, J.P. Quantitative structure−activity relationships for the prediction of relative in vitro potencies (REPs) for chloronaphthalenes. J. Environ. Sci. Health A 2007, 42, 573–590. [Google Scholar] [CrossRef] [PubMed]
- Harner, T.; Kylin, H.; Bidleman, T.F.; Halsall, C.; Strachan, W.M.J.; Barrie, L.A.; Fellin, P. Polychlorinated naphthalenes and coplanar polychlorinated biphenyls in Arctic air. Environ. Sci. Technol. 1998, 32, 3257–3265. [Google Scholar] [CrossRef]
- Falandysz, J.; Strandberg, B.; Strandberg, L.; Bergqvist, P.A.; Rappe, C. Concentrations and biomagnification of polychlorinated naphthalenes in black cormorants Phalacrocorax carbo sinensis from the Gulf of Gdańsk, Baltic Sea. Sci. Total Environ. 1997, 204, 97–106. [Google Scholar] [CrossRef]
- Villeneuve, D.L.; Kannan, K.; Khim, J.S.; Falandysz, J.; Nikiforov, V.A.; Blankenship, A.L.; Giesy, J.P. Relative potencies of individual polychlorinated naphthalenes to induce dioxin-like responses in fish and mammalian in vitro bioassays. Arch. Environ. Contam. Toxicol. 2000, 39, 273–281. [Google Scholar]
- Falandysz, J. Polychlorinated naphthalenes: An environmental update. Environ. Pollut. 1998, 101, 77–90. [Google Scholar] [CrossRef]
- Yamashita, N.; Taniyasu, S.; Hanari, N.; Horii, Y.; Falandysz, J. Polychlorinated naphthalene contamination of some recently manufactured industrial products and commercial goods in Japan. J. Environ. Sci. Health A 2003, 38, 1745–1759. [Google Scholar] [CrossRef]
- Helm, P.A.; Bidleman, T.F. Current combustion-related sources contribute to polychlorinated naphthalene and dioxin-like polychlorinated biphenyl levels and profiles in air in Toronto, Canada. Environ. Sci. Technol. 2003, 37, 1075–1082. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, M.; Liu, W.; Li, C.; Nie, Z.; Liu, G.; Zhang, B.; Xiao, K.; Gao, L. Characterization of polychlorinated naphthalenes in stack gas emissions from waste incinerators. Environ. Sci. Pollut. Res. 2013, 20, 2905–2911. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, G.; Liu, W.; Zhang, B.; Zheng, M. Characterization and quantification of unintentional POP emissions from primary and secondary copper metallurgical processes in China. Atmos. Environ. 2012, 57, 109–115. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, M.; Lv, P.; Liu, W.; Wang, C.; Zhang, B.; Xiao, K. Estimation and characterization of polychlorinated naphthalene emission from coking industries. Environ. Sci. Technol. 2010, 44, 8156–8161. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zheng, M.; Du, B.; Nie, Z.; Zhang, B.; Liu, W.; Li, C.; Hu, J. Atmospheric emission of polychlorinated naphthalenes from iron ore sintering processes. Chemosphere 2012, 89, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lv, P.; Jiang, X.; Nie, Z.; Zheng, M. Identifying iron foundries as a new source of unintentional polychlorinated naphthalenes and characterizing their emission profiles. Environ. Sci. Technol. 2014, 48, 13165–13172. [Google Scholar] [CrossRef] [PubMed]
- Järnberg, U.; Asplund, L.; De Wit, C.; Egebäck, A.L.; Wideqvist, U.; Jakobsson, E. Distribution of polychlorinated naphthalene congeners in environmental and source-related samples. Arch. Environ. Contam. Toxicol. 1997, 32, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, M.; Bayram, A.; Elbir, T.; Seyfioglu, R.; Dumanoglu, Y.; Ornektekin, S. Investigation of soil concentrations of persistent organic pollutants, trace elements, and anions due to iron–steel plant emissions in an industrial region in Turkey. Water Air Soil Pollut. 2010, 213, 375–388. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, G.; Wang, M.; Liu, W.; Tang, C.; Li, L.; Zheng, M. Case study of polychlorinated naphthalene emissions and factors influencing emission variations in secondary aluminum production. J. Hazard. Mater. 2015, 286, 545–552. [Google Scholar] [CrossRef]
- Liu, G.; Zhan, J.; Zhao, Y.; Li, L.; Jiang, X.; Fu, J.; Li, C.; Zheng, M. Distributions, profiles and formation mechanisms of polychlorinated naphthalenes in cement kilns co-processing municipal waste incinerator fly ash. Chemosphere 2016, 155, 348–357. [Google Scholar] [CrossRef]
- Wang, Y.J.; Ho, Y.S.; Jeng, J.H.; Su, H.J.; Lee, C.C. Different cell death mechanisms and gene expression in human cells induced by pentachlorophenol and its major metabolite, tetrachlorohydroquinone. Chem. Biol. Interact. 2000, 128, 173–188. [Google Scholar] [CrossRef]
- Kovács, Á.; Kende, A.; Mörtl, M.; Volk, G.; Rikker, T.; Torkos, K. Determination of phenols and chlorophenols as trimethylsilyl derivatives using gas chromatography—Mass spectrometry. J. Chromatogr. A 2008, 1194, 139–142. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols—Sources and Toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Xu, X.; Zou, H.; Zhang, J. Formation of strong mutagen [3-chloro-4-(dichloromethyl)-5-hydroxy-2 (5H)-furanone] MX by chlorination of fractions of lake water. Water Res. 1997, 31, 1021–1026. [Google Scholar] [CrossRef]
- Subhan, M.A.; Saha, P.C.; Rahman, M.M.; Ahmed, J.; Asiri, A.M.; Al-Mamun, M. Fabrication of a 2,4-dinitrophenol sensor based on Fe3O4@Ag@Ni nanomaterials and studies on their antibacterial properties. New J. Chem. 2018, 42, 872–881. [Google Scholar] [CrossRef]
- Ahmed, J.; Rahman, M.M.; Siddiquey, I.A.; Asiri, A.M.; Hasnat, M.A. Efficient Bisphenol—A detection based on the ternary metal oxide (TMO) composite by electrochemical approaches. Electrochim. Acta 2017, 246, 597–605. [Google Scholar] [CrossRef]
- Liu, H.; Lin, L.; Zou, S.; Yang, L.; Zhang, Y.; Luan, T. Determination of chlorophenols and their degradation metabolites in environmental samples by spme and onfibre silylation with GC-MS. LC GC Eur. 2010, 23, 560–570. [Google Scholar]
- Wang, T.; Chen, T.; Lin, X.; Zhan, M.; Li, X. Emission and distribution of PCDD/Fs, chlorobenzenes, chlorophenols, and PAHs from stack gas of a fluidized bed and a stoker waste incinerator in China. Environ. Sci. Pollut. Res. 2017, 24, 5607–5618. [Google Scholar] [CrossRef]
- Yang, Y.; Mulholland, J.A.; Akki, U. Formation of furans by gas-phase reactions of chlorophenols. Symp. (Int.) Combust. 1998, 27, 1761–1768. [Google Scholar] [CrossRef]
- Kim, D.H.; Mulholland, J.A.; Ryu, J.Y. Formation of polychlorinated naphthalenes from chlorophenols. Proc. Combust. Inst. 2005, 30, 1245–1253. [Google Scholar] [CrossRef]
- Kim, D.H.; Mulholland, J.A. Temperature-dependent formation of polychlorinated naphthalenes and dibenzofurans from chlorophenols. Environ. Sci. Technol. 2005, 39, 5831–5836. [Google Scholar] [CrossRef]
- Kim, D.H.; Mulholland, J.A.; Ryu, J.Y. Chlorinated naphthalene formation from the oxidation of dichlorophenols. Chemosphere 2007, 67, S135–S143. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, R.M.; Li, Y.; Zhang, Q.Z.; Wang, W.X. Theoretical mechanistic and kinetic studies on homogeneous gas-phase formation of polychlorinated naphthalene from 2-chlorophenol as forerunner. Int. J. Mol. Sci. 2015, 16, 25641–25656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Shi, X.L.; Zhang, Q.Z. Quantum chemical and kinetic study on polychlorinated naphthalene formation from 3-chlorophenol precursor. Int. J. Mol. Sci. 2015, 16, 20620–20640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, R.; Blumenstock, M.; Heger, H.J.; Schramm, K.W.; Kettrup, A. Emission of nonchlorinated and chlorinated aromatics in the flue gas of incineration plants during and after transient disturbances of combustion conditions: Delayed emission effects. Environ. Sci. Technol. 2001, 35, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, H.; Zhang, Q.Z.; Qu, X.H.; Wang, W.X. Kinetic properties for the complete series reactions of chlorophenols with OH radicals relevance for dioxin formation. Environ. Sci. Technol. 2010, 44, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, K.K.; Gordon, M.S.; Steckler, R.; Truhlar, D.G. Ab initio reaction paths and direct dynamics calculations. J. Phys. Chem. 1989, 93, 5107–5119. [Google Scholar] [CrossRef]
- Gonzalez-Lafont, A.; Truong, T.N.; Truhlar, D.G. Interpolated variational transition-state theory: Practical methods for estimating variational transition-state properties and tunneling contributions to chemical reaction rates from electronic structure calculations. J. Phys. Chem. 1991, 95, 8875–8894. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Generalized transition state theory. Classical mechanical theory and applications to collinear reactions of hydrogen molecules. J. Phys. Chem. 1979, 83, 1052–1079. [Google Scholar] [CrossRef]
- Fernandez-Ramos, A.; Ellingson, B.A.; Garrett, B.C.; Truhlar, D.G. Variational transition state theory with multidimensional tunneling. Rev. Comput. Chem. 2007, 23, 125–232. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: The MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J. Phys. Chem. A 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision A.1. 2009. Available online: https://www.researchgate.net/publication/292069487_Gaussian_09_Revision_A1_Gaussian_Inc (accessed on 3 April 2022).
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Corchado, J.C.; Chuang, Y.Y.; Fast, P.L.; Hu, W.P.; Liu, Y.P.; Lynch, G.C.; Nguyen, K.A.; Jackels, C.F.; Fernandez-Ramos, A.; Ellingson, B.A. POLYRATE, Version 9.7. University of Minnesota, Minneapolis, MN, USA. 2007. Available online: https://www.researchgate.net/publication/260052498_POLYRATE_version_97 (accessed on 3 April 2022).
- Xu, F.; Zhang, R.M.; Li, Y.F.; Zhang, Q.Z. Homogeneous gas-phase formation of polychlorinated naphthalene from dimerization of 4−chlorophenoxy radicals and cross-condensation of phenoxy radical with 4-chlorophenoxy radical: Mechanism and kinetics study. Chem. Phys. Lett. 2015, 638, 153–160. [Google Scholar] [CrossRef]


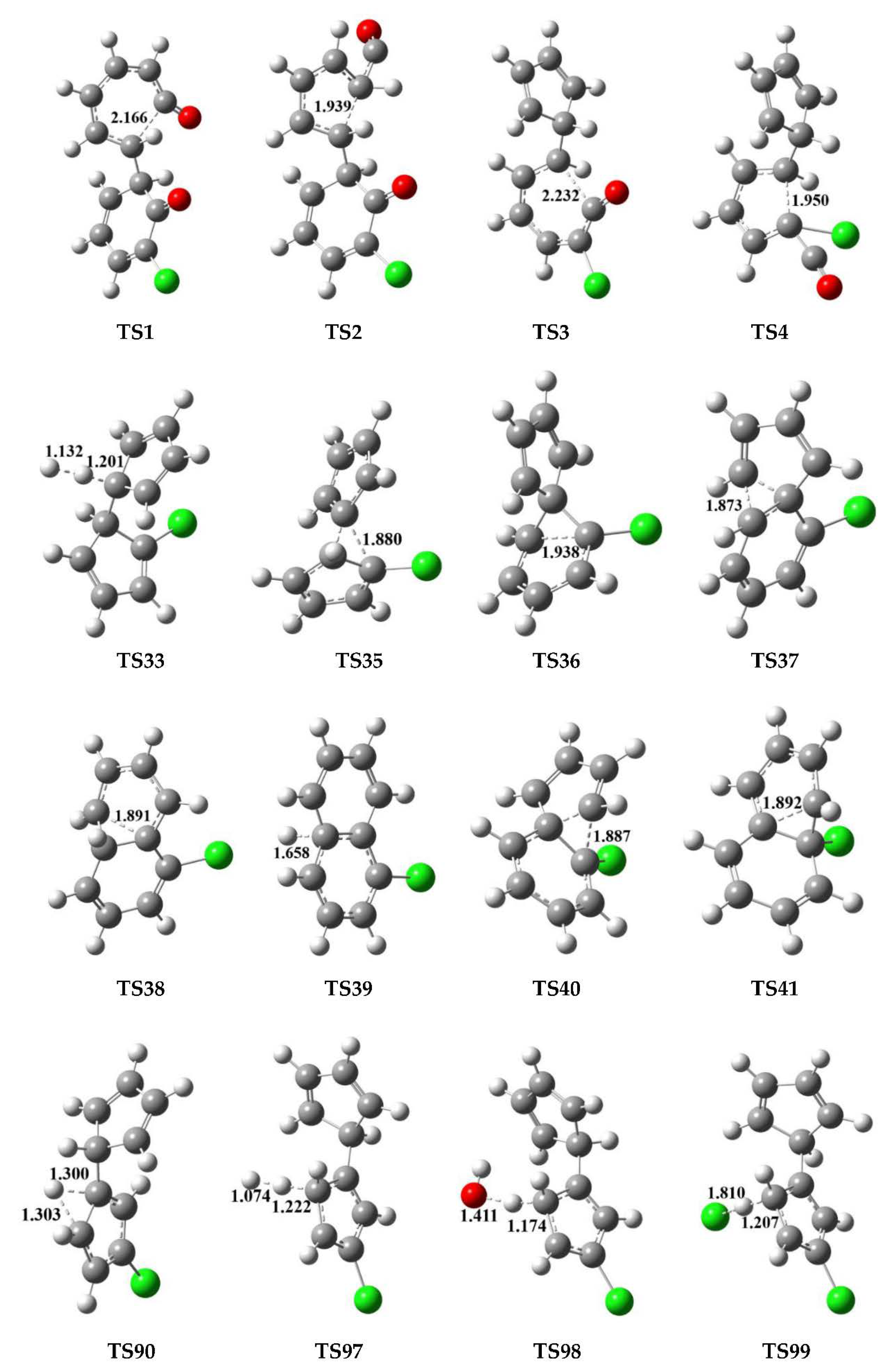
| Reactions Arrhenius Formulas | Arrhenius Formulas |
|---|---|
| IM1→IM2 via TS1 | k(T) = (2.54 × 1013) exp(−19,455.46/T) |
| IM2→IM3 + CO via TS2 | k(T) = (8.29 × 1010) exp(−22,071.16/T) |
| IM3→IM4 via TS3 | k(T) = (3.05 × 1013) exp(−21,165.47/T) |
| IM4→IM5 + CO via TS4 | k(T) = (6.50 × 1011) exp(−20,913.80/T) |
| IM1→IM6 via TS5 | k(T) = (2.72 × 1011) exp(−27,739.54/T) |
| IM6→IM7 + CO via TS6 | k(T) = (1.59 × 1012) exp(−20,905.12/T) |
| IM7→IM8 via TS7 | k(T) = (2.08 × 1013) exp(−19,698.59/T) |
| IM8→IM5 + CO via TS8 | k(T) = (2.20 × 1011) exp(−24,734.37/T) |
| IM9→IM10 via TS9 | k(T) = (1.78 × 1013) exp(−19,076.64/T) |
| IM10→IM11 + CO via TS10 | k(T) = (3.82 × 1011) exp(−24,130.47/T) |
| IM11→IM12 via TS11 | k(T) = (2.06 × 1013) exp(−21,131.16/T) |
| IM12→IM13 + CO via TS12 | k(T) = (3.29 × 1011) exp(−28,711.62/T) |
| IM9→IM14 via TS13 | k(T) = (2.34 × 1013) exp(−21,481.07/T) |
| IM14→IM15 + CO via TS14 | k(T) = (2.14 × 1011) exp(−28,645.35/T) |
| IM15→IM16 via TS15 | k(T) = (1.54 × 1013) exp(−18,260.72/T) |
| IM16→IM13 + CO via TS16 | k(T) = (4.97 × 1011) exp(−24,263.04/T) |
| IM17→IM18 via TS17 | k(T) = (2.40 × 1013) exp(−19,312.41/T) |
| IM18→IM19 + CO via TS18 | k(T) = (4.42 × 1011) exp(−24,236.82/T) |
| IM19→IM20 via TS19 | k(T) = (2.59 × 1013) exp(−18,506.41/T) |
| IM20→IM5 + CO via TS20 | k(T) = (6.45 × 1011) exp(−25,174.58/T) |
| IM17→IM21 via TS21 | k(T) = (4.05 × 1013) exp(−23,466.51/T) |
| IM21→IM22 + CO via TS22 | k(T) = (4.22 × 1011) exp(−25,584.87/T) |
| IM22→IM23 via TS23 | k(T) = (1.00 × 1011) exp(−4309.34/T) |
| IM23→IM5 + CO via TS24 | k(T) = (2.23 × 1011) exp(−24,680.07/T) |
| IM24→IM25 via TS25 | k(T) = (1.52 × 1012) exp(−19,589.40/T) |
| IM25→IM26 + CO via TS26 | k(T) = (2.15 × 1011) exp(−23,682.22/T) |
| IM26→IM27 via TS27 | k(T) = (1.42 × 1013) exp(−19,927.13/T) |
| IM27→IM28 + CO via TS28 | k(T) = (9.55 × 1011) exp(−23,833.30/T) |
| IM24→IM29 via TS29 | k(T) = (2.69 × 1013) exp(−25,090.63/T) |
| IM29→IM30 + CO via TS30 | k(T) = (1.40 × 1010) exp(−21,048.41/T) |
| IM30→IM31 via TS31 | k(T) = (7.50 × 1012) exp(−19,656.05/T) |
| IM31→IM28 + CO via TS32 | k(T) = (6.05 × 1011) exp(−24,859.70/T) |
| Reactions Arrhenius Formulas | Arrhenius Formulas |
|---|---|
| IM5 + H→IM32 + H2 via TS33 | k(T) = (9.91 × 10−12) exp(−1436.77/T) |
| IM32→IM33 via TS35 | k(T) = (2.30 × 1012) exp(−18,463.38/T) |
| IM33→IM34 via TS36 | k(T) = (5.29× 1012) exp(−9166.55/T) |
| IM34→IM35 via TS37 | k(T) = (5.87× 1012) exp(−6278.72/T) |
| IM35→IM36 via TS38 | k(T) = (4.51 × 1012) exp(−8007.32/T) |
| IM36→1–MCN + H via TS39 | k(T) = (1.80 × 1013) exp(−8511.99/T) |
| IM34→IM37 via TS40 | k(T) = (1.48 × 1012) exp(−8686.54/T) |
| IM37→naphthalene via TS41 | k(T) = (1.96 × 1013) exp(−7007.83/T) |
| IM32→IM38 via TS42 | k(T) = (7.48 × 1011) exp(−7882.77/T) |
| IM38→IM39 via TS43 | k(T) = (1.80 × 1013) exp(−14,352.16/T) |
| IM39→IM40 via TS44 | k(T) = (1.94 × 1012) exp(−5928.21/T) |
| IM40→IM41 via TS45 | k(T) = (5.17 × 1012) exp(−7830.77/T) |
| IM41→2-MCN + H via TS46 | k(T) = (7.07 × 1012) exp(−8307.29/T) |
| IM39→IM42 via TS47 | k(T) = (2.37 × 1012) exp(−5684.42/T) |
| IM42→IM43 via TS48 | k(T) = (7.61 × 1012) exp(−7631.97/T) |
| IM43→1-MCN + H via TS49 | k(T) = (1.72 × 1013) exp(−8895.75/T) |
| IM5 + H→IM44 + H2 via TS50 | k(T) = (6.74 × 10−11) exp(−3328.55/T) |
| IM44→IM45 via TS52 | k(T) = (2.49 × 1012) exp(−5056.83/T) |
| IM45→IM46 via TS53 | k(T) = (9.36 × 1012) exp(−8844.00/T) |
| IM46→IM47 via TS54 | k(T) = (1.74 × 1012) exp(−5737.01/T) |
| IM47→IM36 via TS55 | k(T) = (6.33 × 1012) exp(−5056.83/T) |
| IM46→IM48 via TS56 | k(T) = (9.72 × 1011) exp(−7692.64/T) |
| IM48→IM43 via TS57 | k(T) = (1.12 × 1013) exp(−7110.50/T) |
| IM13 + H→IM49 + H2 via TS58 | k(T) = (1.87 × 10−11) exp(−2331.16/T) |
| IM49→IM33 via TS60 | k(T) = (4.19 × 1011) exp(−5100.98/T) |
| IM13 + H→IM50 + HCl via TS61 | k(T) = (6.66 × 10−11) exp(−4051.86/T) |
| IM13 + OH→IM50 + HOCl via TS62 | k(T) = (2.90 × 10−11) exp(−6850.19/T) |
| IM50→IM51 via TS63 | k(T) = (8.57 × 1011) exp(−5187.97/T) |
| IM51→IM52 via TS64 | k(T) = (1.60 × 1012) exp(−14,041.51/T) |
| IM52→IM53 via TS65 | k(T) = (1.14 × 1012) exp(−6156.97/T) |
| IM53→IM54 via TS66 | k(T) = (4.70 × 1012) exp(−7819.81/T) |
| IM54→naphthalene + H via TS67 | k(T) = (1.61 × 1013) exp(−8459.84/T) |
| IM28 + H→IM55 + H2 via TS68 | k(T) = (5.16 × 10−11) exp(−3030.02/T) |
| IM28 + OH→IM55 + H2O via TS69 | k(T) = (9.71 × 10−14) exp(−2135.19/T) |
| IM55→IM38 via TS70 | k(T) = (1.36 × 1012) exp(−5053.71/T) |
| IM55→IM56 via TS71 | k(T) = (1.21 × 1012) exp(−5103.87/T) |
| IM56→IM57 via TS72 | k(T) = (1.90 × 1013) exp(−13,757.06/T) |
| IM57→IM58 via TS73 | k(T) = (2.88 × 1012) exp(−8011.41/T) |
| IM58→IM59 via TS74 | k(T) = (1.86 × 1013) exp(−8105.33/T) |
| IM59→2-MCN + H via TS75 | k(T) = (1.13 × 1013) exp(−4381.07/T) |
| IM28 + H→IM60 + H2 via TS76 | k(T) = (2.77 × 10−12) exp(−4600.55/T) |
| IM60→IM61 via TS78 | k(T) = (8.07 × 1011) exp(−5830.65/T) |
| IM61→IM62 via TS79 | k(T) = (1.00 × 1013) exp(−14,059.47/T) |
| IM62→IM63 via TS80 | k(T) = (2.01 × 1012) exp(−6103.57/T) |
| IM63→IM41 via TS81 | k(T) = (7.30 × 1012) exp(−7837.39/T) |
| IM62→IM64 via TS82 | k(T) = (1.99 × 1012) exp(−5912.17/T) |
| IM64→IM59 via TS83 | k(T) = (1.50 × 1013) exp(−8098.93/T) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Z.; Han, Y.; He, S.; Hadizadeh, M.H.; Zhang, Q.; Bai, X.; Wang, X.; Sun, Y.; Xu, F. The Homogeneous Gas-Phase Formation Mechanism of PCNs from Cross-Condensation of Phenoxy Radical with 2-CPR and 3-CPR: A Theoretical Mechanistic and Kinetic Study. Int. J. Mol. Sci. 2022, 23, 5866. https://doi.org/10.3390/ijms23115866
Teng Z, Han Y, He S, Hadizadeh MH, Zhang Q, Bai X, Wang X, Sun Y, Xu F. The Homogeneous Gas-Phase Formation Mechanism of PCNs from Cross-Condensation of Phenoxy Radical with 2-CPR and 3-CPR: A Theoretical Mechanistic and Kinetic Study. International Journal of Molecular Sciences. 2022; 23(11):5866. https://doi.org/10.3390/ijms23115866
Chicago/Turabian StyleTeng, Zhuochao, Yanan Han, Shuming He, Mohammad Hassan Hadizadeh, Qi Zhang, Xurong Bai, Xiaotong Wang, Yanhui Sun, and Fei Xu. 2022. "The Homogeneous Gas-Phase Formation Mechanism of PCNs from Cross-Condensation of Phenoxy Radical with 2-CPR and 3-CPR: A Theoretical Mechanistic and Kinetic Study" International Journal of Molecular Sciences 23, no. 11: 5866. https://doi.org/10.3390/ijms23115866
APA StyleTeng, Z., Han, Y., He, S., Hadizadeh, M. H., Zhang, Q., Bai, X., Wang, X., Sun, Y., & Xu, F. (2022). The Homogeneous Gas-Phase Formation Mechanism of PCNs from Cross-Condensation of Phenoxy Radical with 2-CPR and 3-CPR: A Theoretical Mechanistic and Kinetic Study. International Journal of Molecular Sciences, 23(11), 5866. https://doi.org/10.3390/ijms23115866