Analgesic Effect of Tranilast in an Animal Model of Neuropathic Pain and Its Role in the Regulation of Tetrahydrobiopterin Synthesis
Abstract
:1. Introduction
2. Results
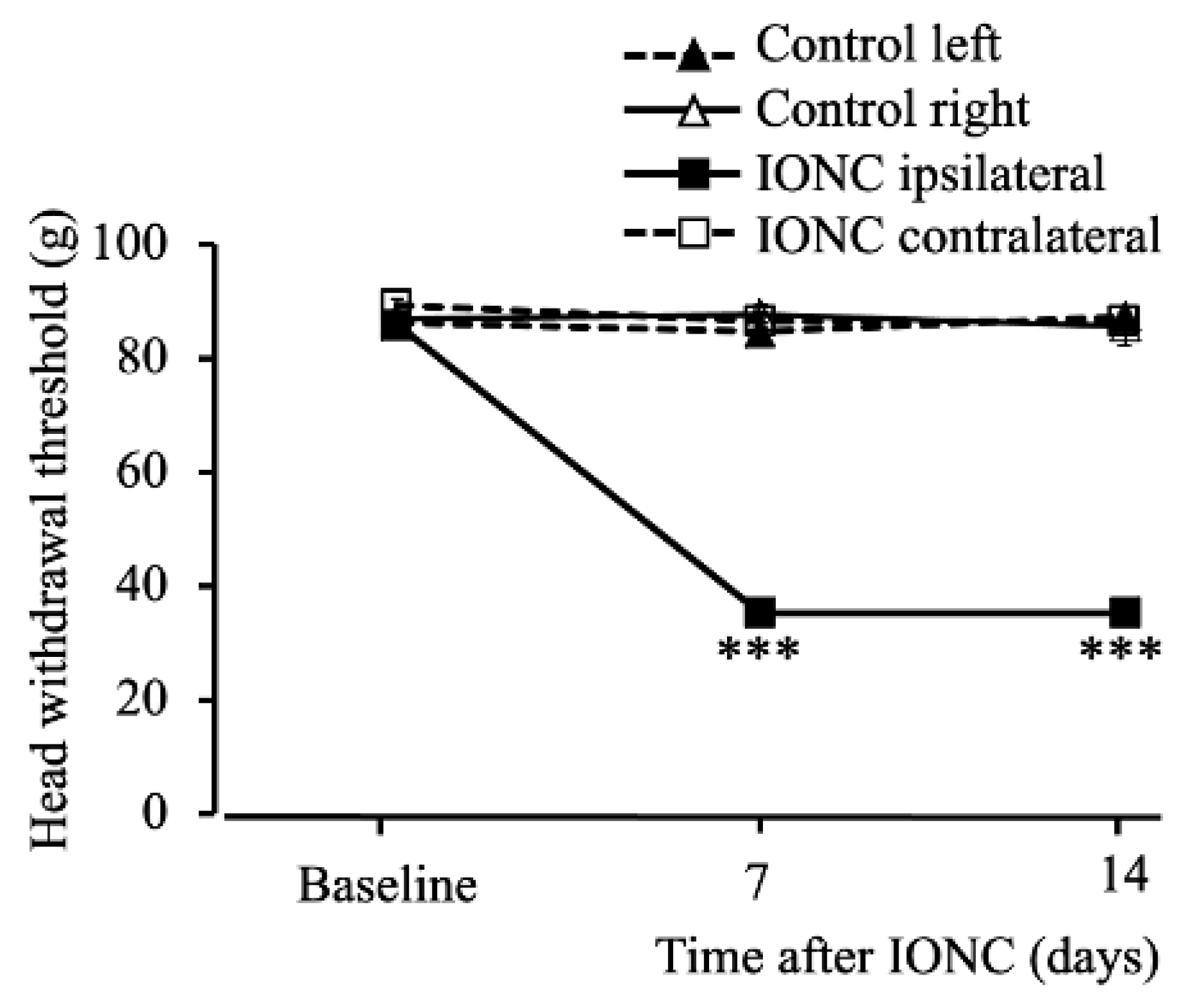
2.1. Infraorbital Nerve-Constriction-Induced Pain Behavior
2.2. Dose Dependent Effect of Tranilast to Mechanical Stimuli Post IONC
2.3. The Effects of Tranilast in Regard to Mechanical Stimuli Post IONC Lasted up to 24 h in Comparison to Carbamazepine
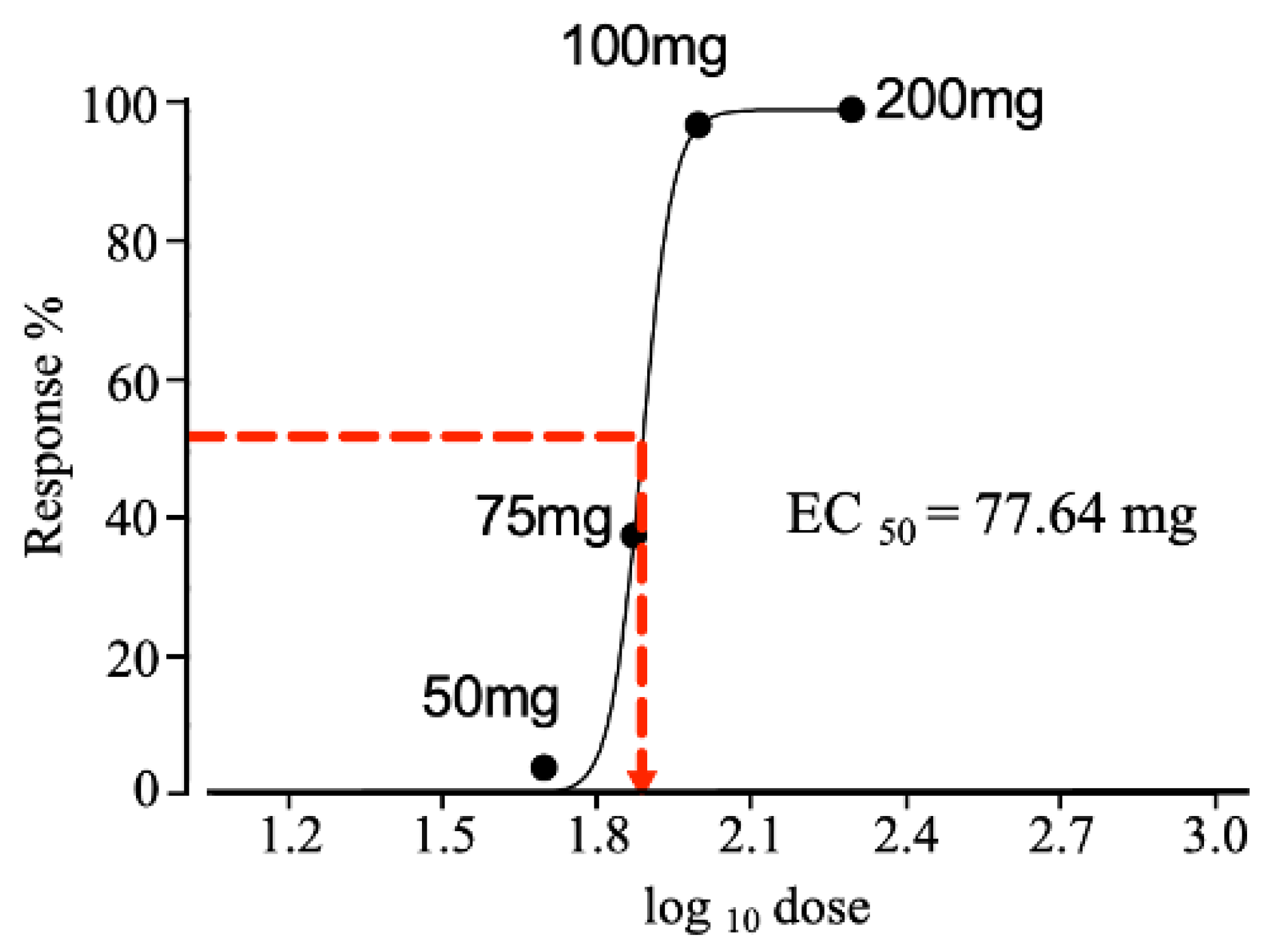
2.4. Effective Dose Required to Produce a 50% Response
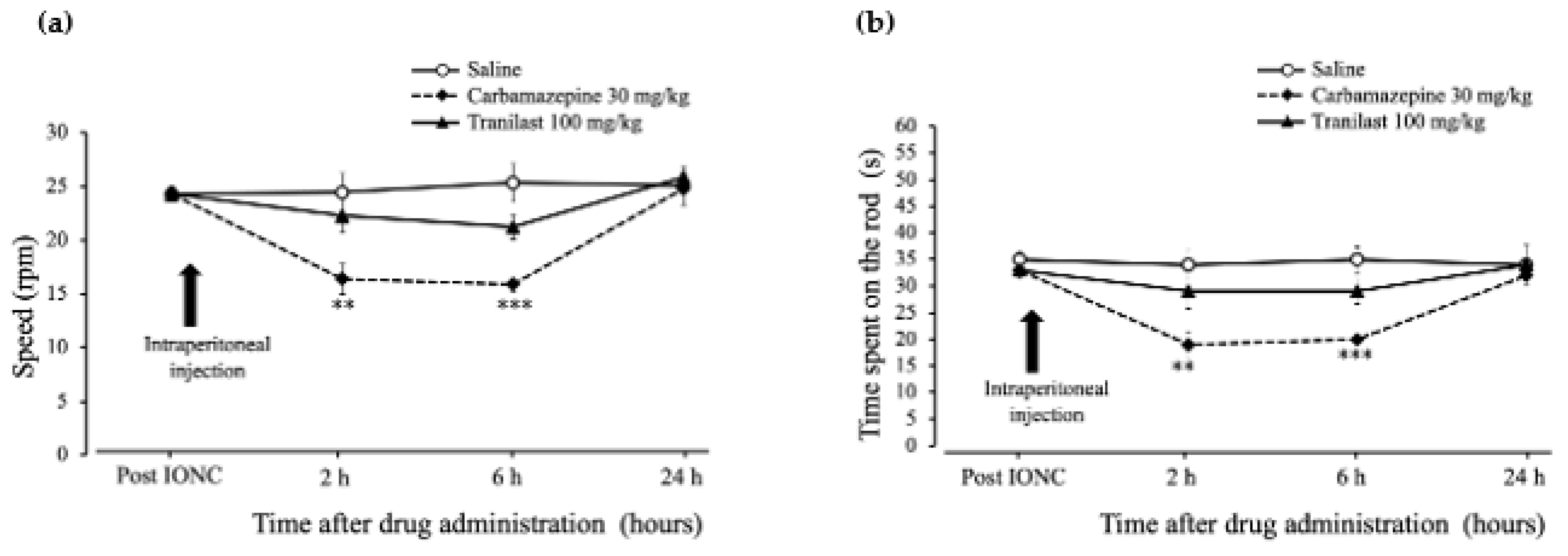
2.5. Rotarod Performance Was Unaffected in the Tranilast Group and Motor Coordination Deficits Were Confirmed in the Carbamazepine Group in IONC Rats
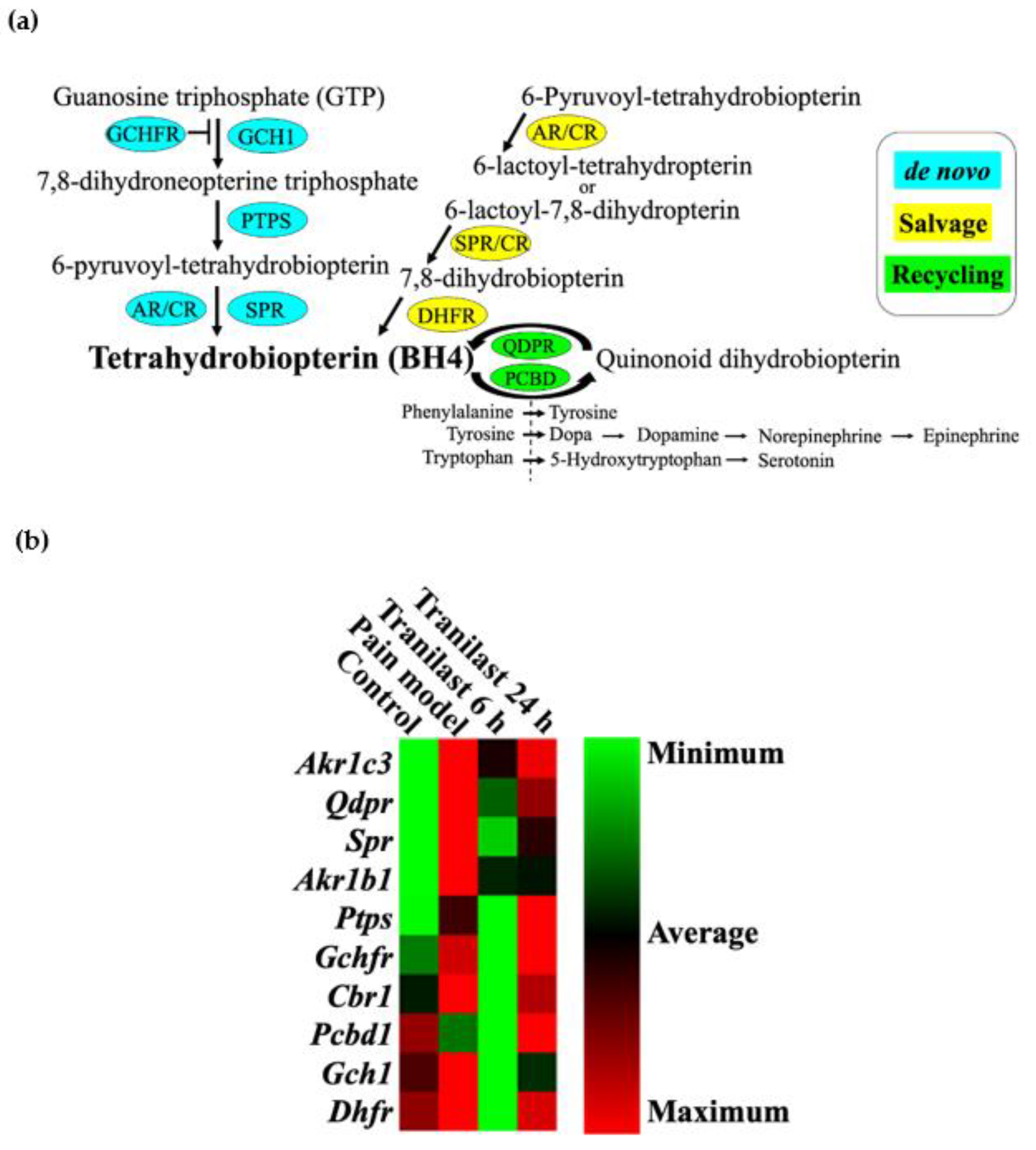
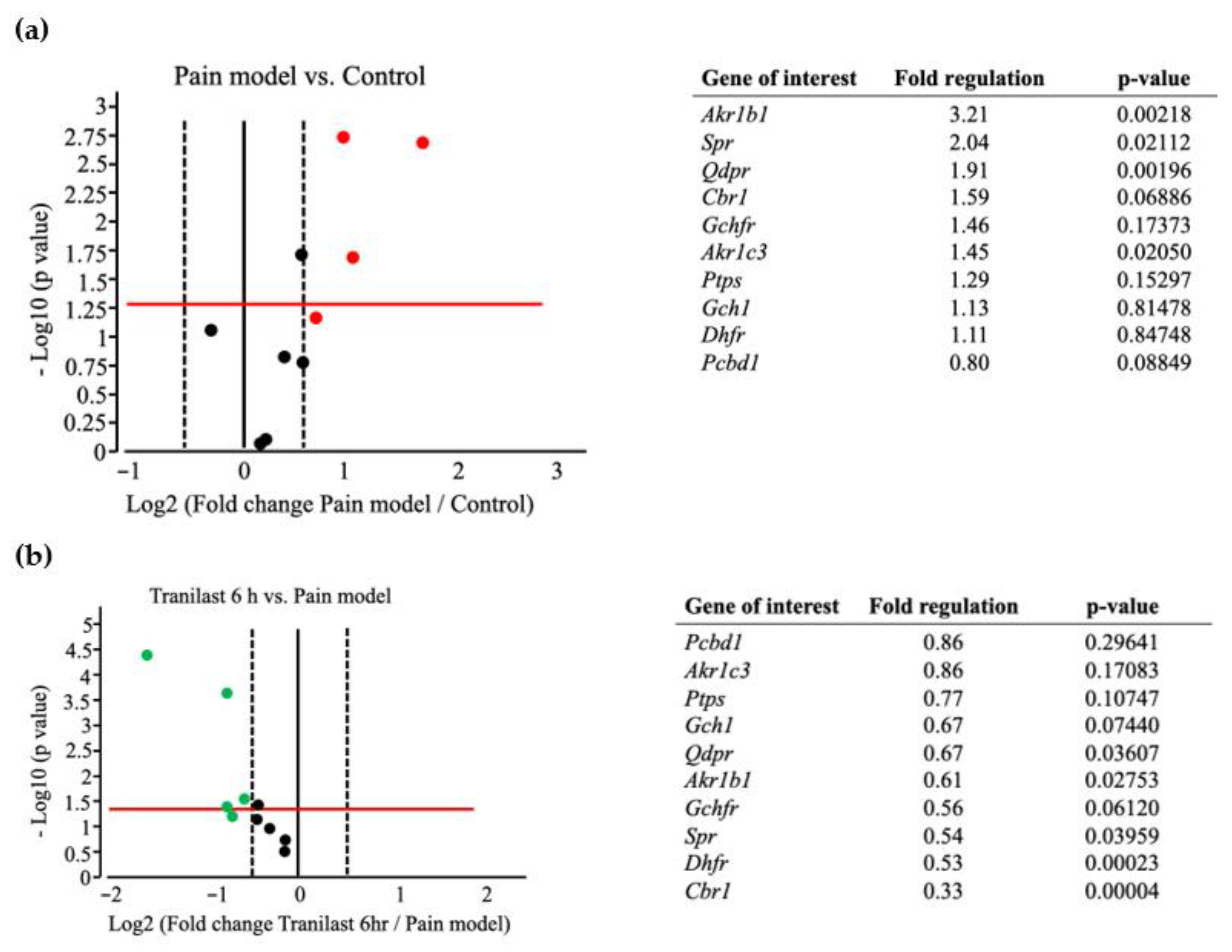
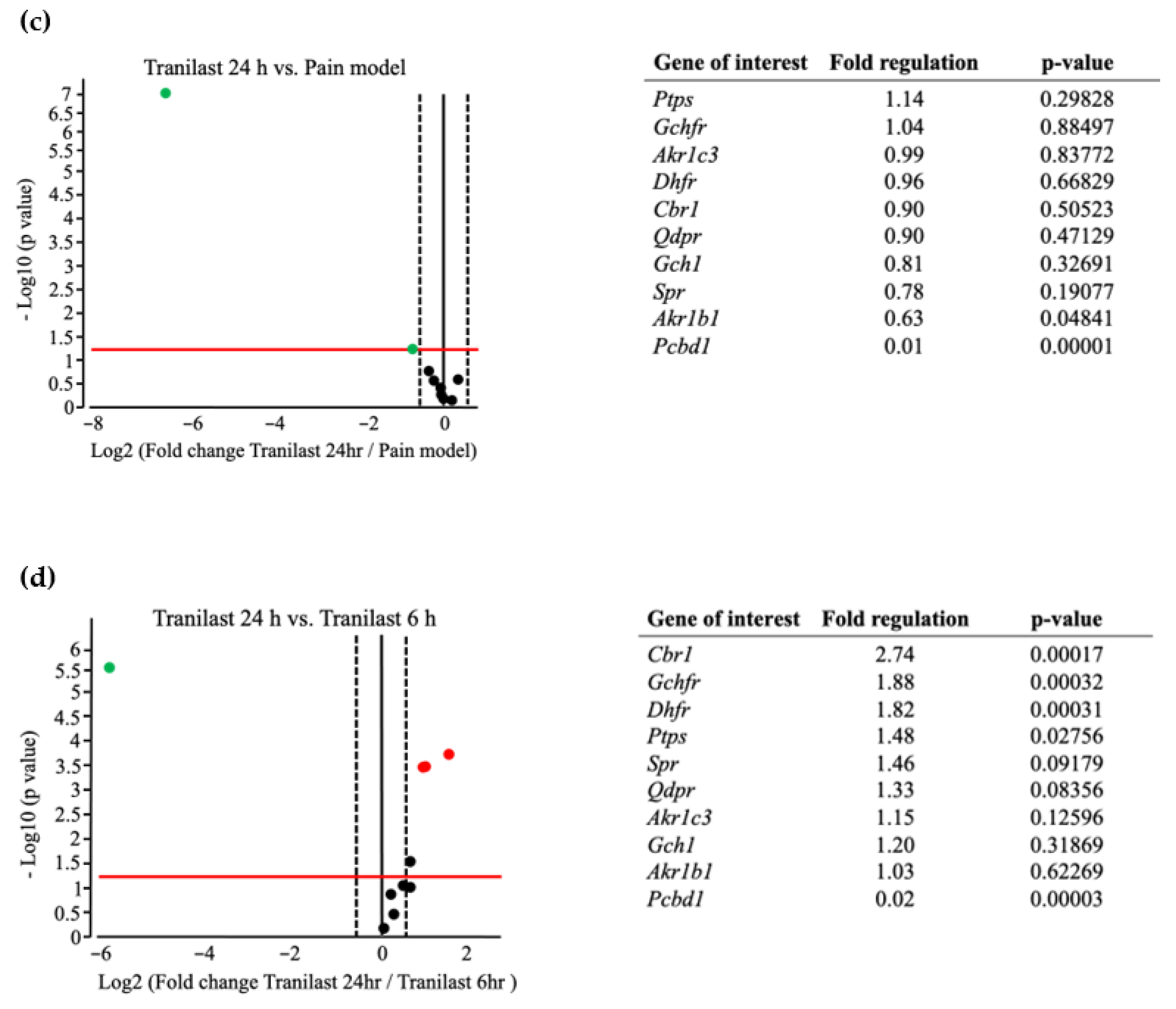
2.6. BH4-Related Gene Expression Is Markedly Upregulated in Trigeminal Ganglions after IONC
2.7. The Expression of Spr and Akr Increases in Trigeminal Ganglions of Nerve Injury Rat Models and Decreases after Tranilast Treatment
3. Discussion
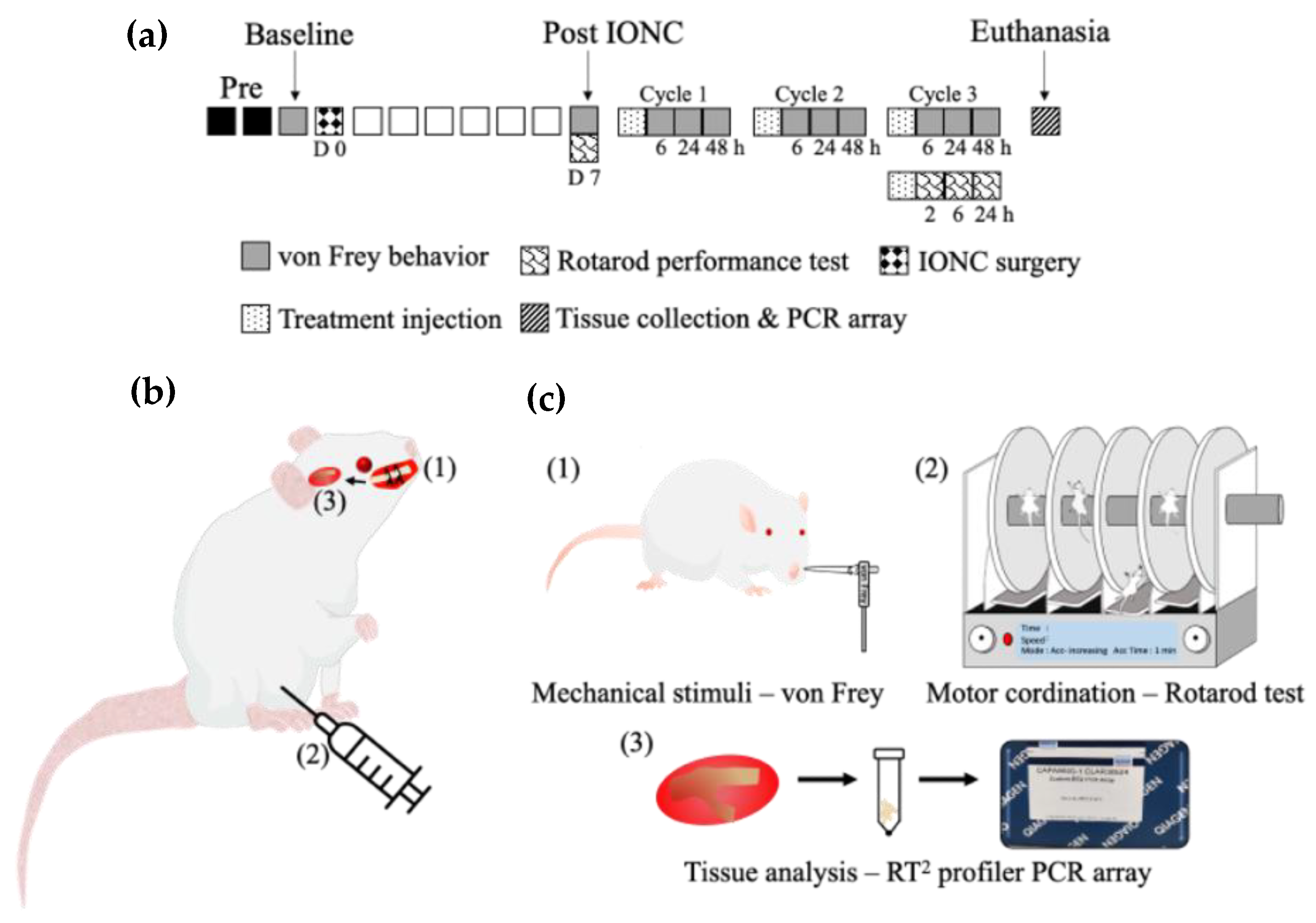
4. Materials and Methods
4.1. Animals
4.2. IONC
4.3. Intraperitoneal Injection of Drugs to the IONC Model
4.4. Behavior Test to Assess Mechanical Sensitivity
4.5. Rotarod Performance Test to Assess Motor Coordination
4.6. BH4 Pathway RT2 Profiler PCR Array
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Board Review
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakrzewska, J.M.; Hamlyn, P.J. Facial pain. In Epidemiology of Pain Seattle; IASP Press: Washington, DC, USA, 1999; pp. 171–202. [Google Scholar]
- Dubner, R.; Ren, K. Brainstem mechanisms of persistent pain following injury. J. Orofac. Pain 2004, 18, 299–305. [Google Scholar] [PubMed]
- Shinoda, M.; Imamura, Y.; Hayashi, Y.; Noma, N.; Okada-Ogawa, A.; Hitomi, S.; Iwata, K. Orofacial neuropathic pain-basic research and their clinical relevancies. Front. Mol. Neurosci. 2021, 14, 691396. [Google Scholar] [CrossRef] [PubMed]
- Obermann, M. Treatment options in trigeminal neuralgia. Ther. Adv. Neurol. Disord. 2010, 3, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.A.; Chi, C.C.; Wiffen, P.J.; Derry, S.; Rice, A.S. Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Database Syst. Rev. 2015, 10, CD010902. [Google Scholar] [CrossRef]
- Sidebottom, A.; Maxwell, S. The medical and surgical management of trigeminal neuralgia. J. Clin. Pharm. Ther. 1995, 20, 31–35. [Google Scholar] [CrossRef]
- Sessle, B.J. Chronic orofacial pain: Models, mechanisms, and genetic and related environmental influences. Int. J. Mol. Sci. 2021, 22, 7112. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.J.; Islam, B.; Ward, S.; Jackson, O.; Armitage, R.; Blackburn, J.; McHugh, P.C. Repurposing of tranilast for potential neuropathic pain treatment by inhibition of sepiapterin reductase in the BH4 pathway. ACS Omega 2019, 4, 11960–11972. [Google Scholar] [CrossRef] [Green Version]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef]
- Komatsu, H.; Kojima, M.; Tsutsumi, N.; Hamano, S.; Kusama, H.; Ujiie, A.; Ikeda, S.; Nakazawa, M. Study of the Mechanism of Inhibitory Action of Tranilast on Chemical Mediator Release. Jpn. J. Pharmacol. 1988, 46, 43–51. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Danks, R.; Kardash, E. Therapeutic potential of tranilast, an anti-allergy drug, in proliferative disorders. Anticancer Res. 2012, 32, 2471–2478. [Google Scholar] [PubMed]
- Honda, R.; Honda, T.; Tashiro, H.; Saya, H.; Yoshimura, Y.; Katabuchi, H. Evaluating the effect of tranilast for pelvic pain caused by endometriosis. Fertil. Steril. 2013, 100, 372–373. [Google Scholar] [CrossRef]
- Inglis, J.J.; Criado, G.; Andrews, M.; Feldmann, M.; Williams, R.O.; Selley, M.L. The anti-allergic drug, N-(3′,4′-dimethoxycinna-monyl) anthranilic acid, exhibits potent anti-inflammatory and analgesic properties in arthritis. Rheumatology 2007, 46, 1428–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, E.R.; Blau, N.; Thöny, B. Tetrahydrobiopterin: Biochemistry and pathophysiology. Biochem. J. 2011, 438, 397–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [Green Version]
- Tegeder, I.; Costigan, M.; Griffin, R.S.; Abele, A.; Belfer, I.; Schmidt, H.; Woolf, C.J. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat. Med. 2006, 12, 1269–1277. [Google Scholar] [CrossRef]
- Vos, B.P.; Strassman, A.M.; Maciewicz, R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J. Neurosci. 1994, 14, 2708–2723. [Google Scholar] [CrossRef] [Green Version]
- Imamura, Y.; Kawamoto, H.; Nakanishi, O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp. Brain Res. 1997, 116, 97–103. [Google Scholar] [CrossRef]
- Iwasa, T.; Afroz, S.; Inoue, M.; Arakaki, R.; Oshima, M.; Raju, R.; Waskitho, A.; Inoue, M.; Baba, O.; Matsuka, Y. IL-10 and CXCL2 in trigeminal ganglia in neuropathic pain. Neurosci. Lett. 2019, 703, 132–138. [Google Scholar] [CrossRef]
- Waskitho, A.; Yamamoto, Y.; Raman, S.; Kano, F.; Yan, H.; Raju, R.; Matsuka, Y. Peripherally administered botulinum toxin type A localizes bilaterally in trigeminal ganglia of animal model. Toxins 2021, 13, 704. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Isaji, M.; Miyata, H.; Ajisawa, Y. Tranilast: A new application in the cardiovascular field as an antiproliferative drug. Cardiovasc. Drug Rev. 1998, 16, 288–299. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, S.; Shinoda, M.; Hayashi, Y.; Saito, H.; Asano, S.; Kubo, A.; Iwata, K. Increase in IGF-1 expression in the injured infraorbital nerve and possible implications for orofacial neuropathic pain. Int. J. Mol. Sci. 2019, 20, 6360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; Mc Quay, H.J. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst. Rev. 2011, 1, CD005451. [Google Scholar]
- Hahm, T.S.; Ahn, H.J.; Ryu, S.; Gwak, M.S.; Choi, S.J.; Kim, J.K.; Yu, J.M. Combined carbamazepine and pregabalin therapy in a rat model of neuropathic pain. Br. J. Anaesth. 2012, 109, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, K.; Kawasaki, A.; Mizushima, Y.; Yano, S. Effect of antiallergic agents and bronchial hypersensitivity in short-term bronchial asthma. Chest 1991, 100, 57–62. [Google Scholar] [CrossRef]
- Koda, A.; Nagai, H.; Watanabe, S.; Yanagihara, Y.; Sakamoto, K. Inhibition of hypersensitivity reactions by a new drug, N (3′, 4′-dimethoxycinnamoyl) anthranilic acid (N-5′). J. Allergy Clin. Immunol. 1976, 57, 396–407. [Google Scholar] [CrossRef]
- Araya, E.I.; Claudino, R.F.; Piovesan, E.J.; Chichorro, J.G. Trigeminal neuralgia: Basic and clinical aspects. Curr. Neuropharmacol. 2020, 18, 109–119. [Google Scholar] [CrossRef]
- Pritchett, K.; Mulder, G.B. The rotarod. J. Am. Assoc. Lab. Anim. Sci. 2003, 42, 49. [Google Scholar]
- Sheahan, T.D.; Siuda, E.R.; Bruchas, M.R.; Shepherd, A.J.; Mohapatra, D.P.; Gereau, R.W.; Golden, J.P. Inflammation and nerve injury minimally affect mouse voluntary behaviors proposed as indicators of pain. Neurobiol. Pain 2017, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic-Petrovic, R.M.; Tomic, M.A.; Vuckovic, S.M.; Paranos, S.; Ugrešic, N.D.; Prostran, M.Š.; Boškovic, B. The antinociceptive effects of anticonvulsants in a mouse visceral pain model. Anesth. Analg. 2008, 106, 1897–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afroz, S.; Arakaki, R.; Iwasa, T.; Oshima, M.; Hosoki, M.; Inoue, M.; Matsuka, Y. CGRP induces differential regulation of cytokines from satellite glial cells in trigeminal ganglia and orofacial nociception. Int. J. Mol. Sci. 2019, 20, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latremoliere, A.; Latini, A.; Andrews, N.; Cronin, S.J.; Fujita, M.; Gorska, K.; Hovius, R.; Romero, C.; Chuaiphichai, S.; Painter, M.; et al. Reduction of neuropathic and inflammatory pain through inhibition of the tetrahydrobiopterin pathway. Neuron 2015, 86, 1393–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, K.L.; Nicholl, B.I.; Macfarlane, G.J.; Thomson, W.; Davies, K.A.; McBeth, J. Do genetic predictors of pain sensitivity associate with persistent widespread pain. Mol. Pain 2009, 5, 56. [Google Scholar] [CrossRef]
- Doehring, A.; Freynhagen, R.; Griessinger, N.; Zimmermann, M.; Sittl, R.; Hentig, N.; Geisslinger, G.; Lötsch, J. Cross-sectional assessment of the consequences of a GTP cyclohydrolase 1 haplotype for specialized tertiary outpatient pain care. Clin. J. Pain 2009, 25, 781–785. [Google Scholar] [CrossRef]
- Lötsch, J.; Klepstad, P.; Doehring, A.; Dale, O.A. GTP cyclohydrolase 1 genetic variant delays cancer pain. Pain 2010, 148, 103–106. [Google Scholar] [CrossRef]
- Kim, D.H.; Dai, F.; Belfer, I.; Banco, R.J.; Martha, J.F.; Tighiouart, H.; Tromanhauser, S.G.; Jenis, L.G.; Hunter, D.J.; Schwartz, C.E. Polymorphic variation of the guanosine triphosphate cyclohydrolase 1 gene predicts outcome in patients undergoing surgical treatment for lumbar degenerative disc disease. Spine 2010, 35, 1909–1914. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Channon, K.M. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 2011, 25, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, M.J.; Tatham, A.L.; Hale, A.B.; Alp, N.J.; Channon, K.M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling. J. Biol. Chem. 2009, 284, 28128–28136. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Jan, Y.H.; Mishin, V.; Richardson, J.R.; Hossain, M.M.; Heindel, N.D.; Heck, D.E.; Laskin, D.L.; Laskin, J.D. Sulfa drugs inhibit sepiapterin reduction and chemical redox cycling by sepiapterin reductase. J. Pharmacol. Exp. Ther. 2015, 352, 529–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, Y.; Matsuka, Y.; Spigelman, I.; Ishihara, Y.; Yamamoto, Y.; Sonoyama, W.; Kuboki, T.; Oguma, K. Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience 2009, 159, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Afroz, S.; Arakaki, R.; Iwasa, T.; Waskitho, A.; Oshima, M.; Matsuka, Y. Role of CGRP in neuroimmune interaction via NF-κB signaling genes in glial cells of trigeminal ganglia. Int. J. Mol. Sci. 2020, 21, 6005. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raman, S.; Waskitho, A.; Raju, R.; Iwasa, T.; Ikutame, D.; Okura, K.; Oshima, M.; Matsuka, Y. Analgesic Effect of Tranilast in an Animal Model of Neuropathic Pain and Its Role in the Regulation of Tetrahydrobiopterin Synthesis. Int. J. Mol. Sci. 2022, 23, 5878. https://doi.org/10.3390/ijms23115878
Raman S, Waskitho A, Raju R, Iwasa T, Ikutame D, Okura K, Oshima M, Matsuka Y. Analgesic Effect of Tranilast in an Animal Model of Neuropathic Pain and Its Role in the Regulation of Tetrahydrobiopterin Synthesis. International Journal of Molecular Sciences. 2022; 23(11):5878. https://doi.org/10.3390/ijms23115878
Chicago/Turabian StyleRaman, Swarnalakshmi, Arief Waskitho, Resmi Raju, Takuma Iwasa, Daisuke Ikutame, Kazuo Okura, Masamitsu Oshima, and Yoshizo Matsuka. 2022. "Analgesic Effect of Tranilast in an Animal Model of Neuropathic Pain and Its Role in the Regulation of Tetrahydrobiopterin Synthesis" International Journal of Molecular Sciences 23, no. 11: 5878. https://doi.org/10.3390/ijms23115878
APA StyleRaman, S., Waskitho, A., Raju, R., Iwasa, T., Ikutame, D., Okura, K., Oshima, M., & Matsuka, Y. (2022). Analgesic Effect of Tranilast in an Animal Model of Neuropathic Pain and Its Role in the Regulation of Tetrahydrobiopterin Synthesis. International Journal of Molecular Sciences, 23(11), 5878. https://doi.org/10.3390/ijms23115878







