Beneficial Effects of Linseed Supplementation on Gut Mucosa-Associated Microbiota in a Physically Active Mouse Model of Crohn’s Disease
Abstract
1. Introduction
2. Results
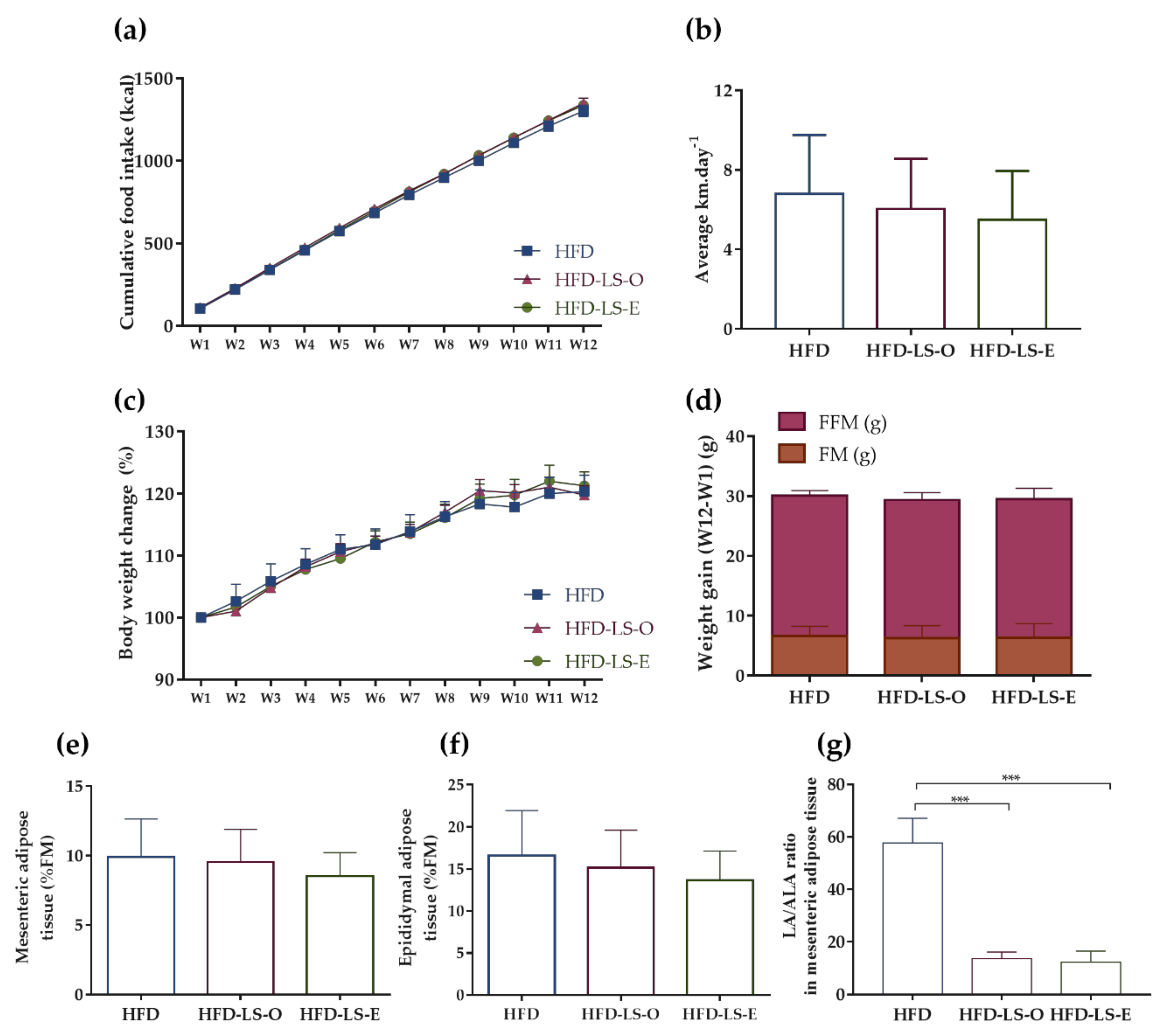
2.1. Body Composition in an Active Mouse Model That Mimics CD Susceptibility
2.2. AIEC-Induced Inflammation
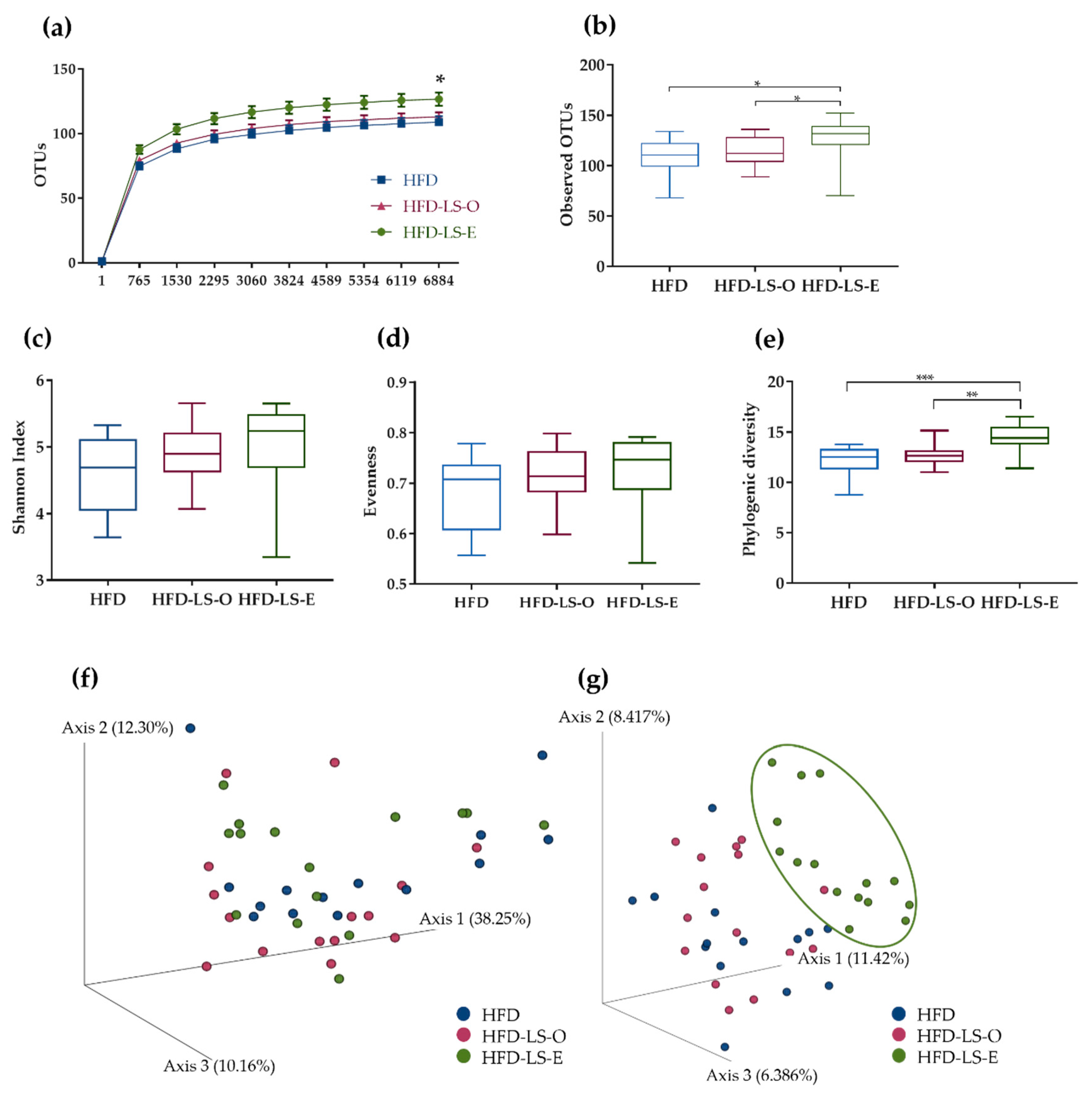
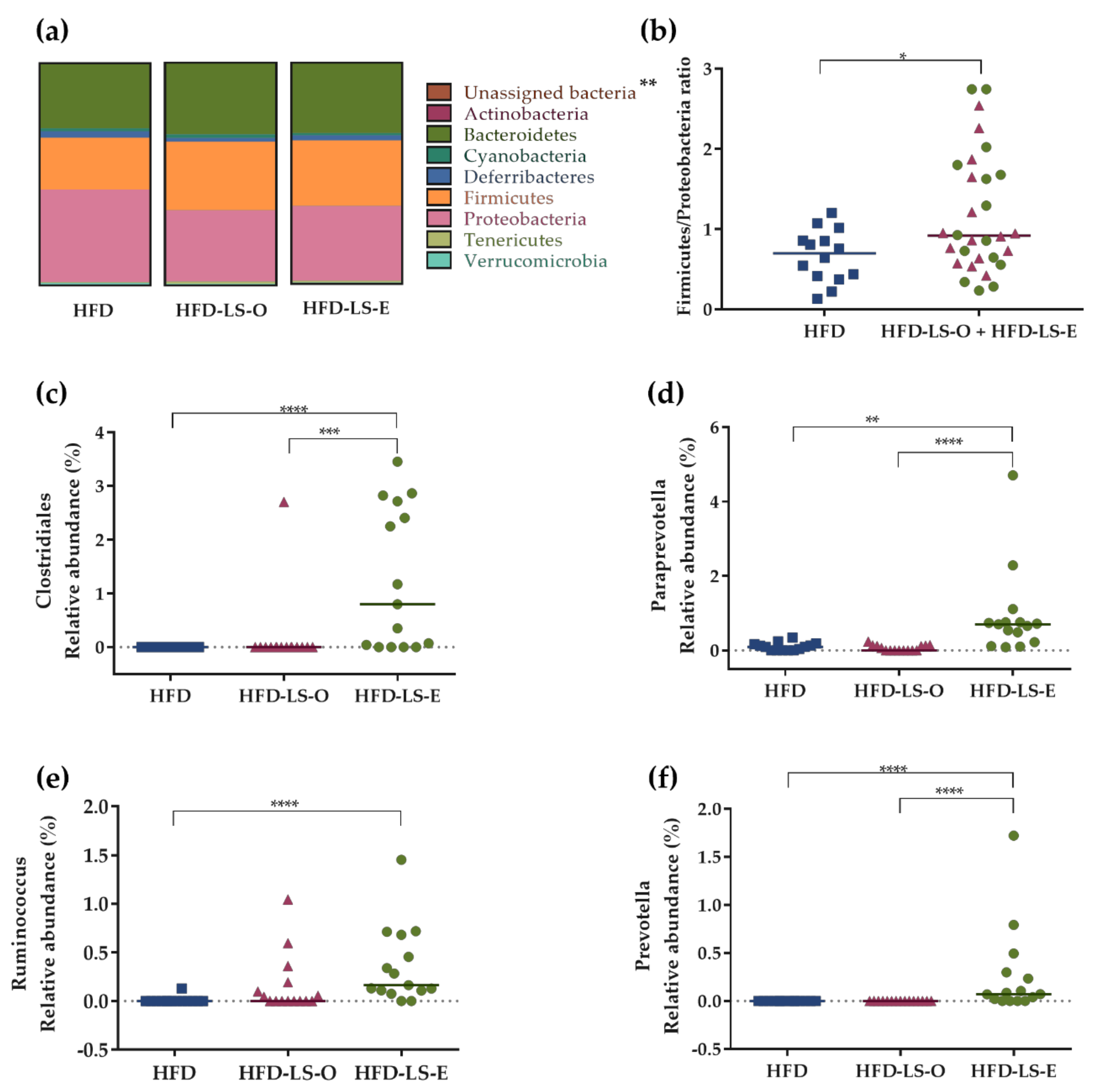
2.3. Intestinal Mucosa-Associated Microbiota Diversity and Composition
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Spontaneous Physical Activity
4.3. Diets
4.4. Study Design
4.5. Weight and Body Composition
4.6. Polyunsaturated Fatty Acids in Mesenteric Adipose Tissue
4.7. Inflammatory Markers
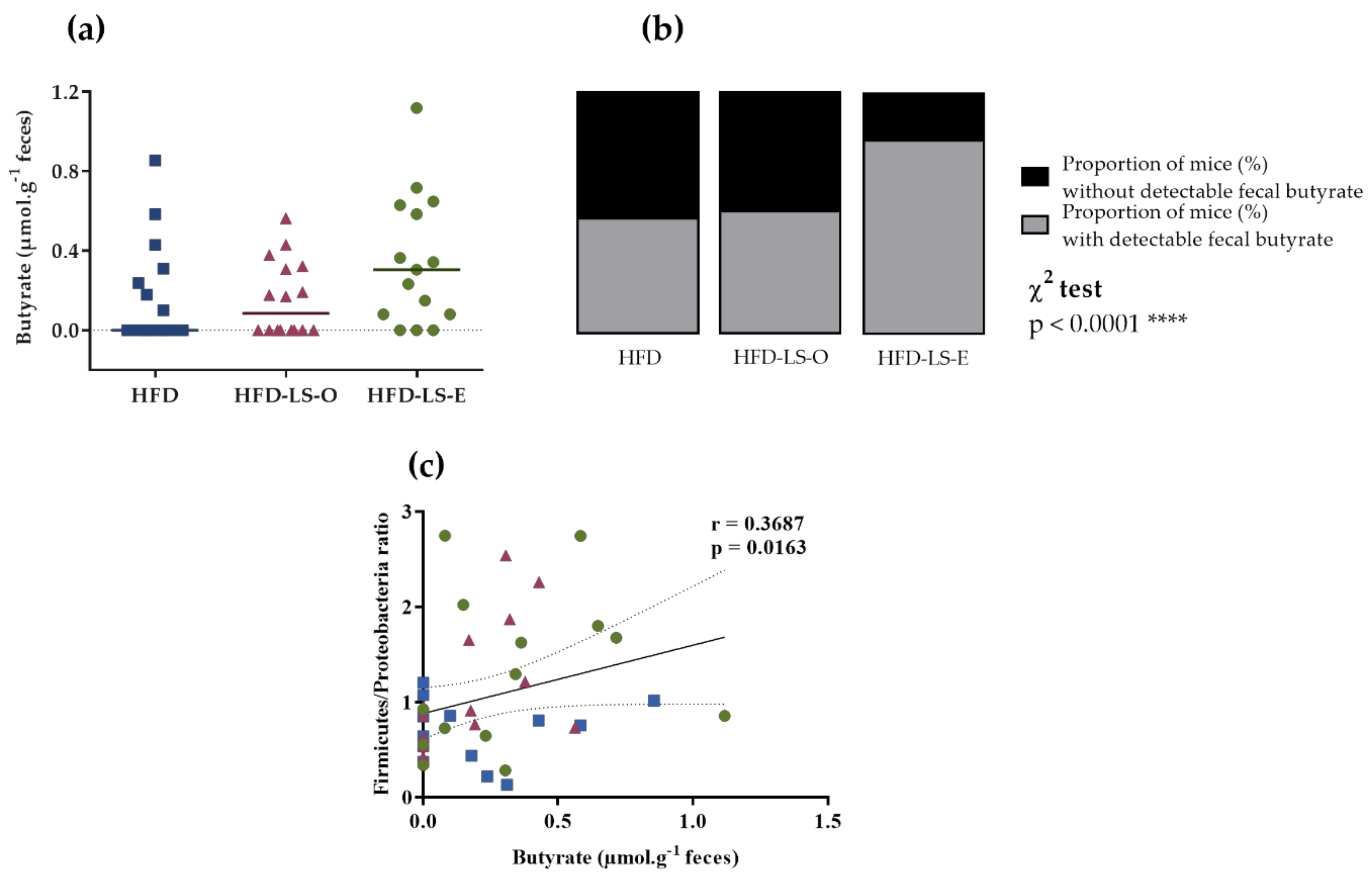
4.8. Fecal Short-Chain Fatty Acids
4.9. Intestinal Permeability: FITC-Dextran
4.10. Microbiota Composition Analyses
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Inflammatory Bowel Disease: Clinical Aspects and Established and Evolving Therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Baumgart, M.; Dogan, B.; Rishniw, M.; Weitzman, G.; Bosworth, B.; Yantiss, R.; Orsi, R.H.; Wiedmann, M.; McDonough, P.; Kim, S.G.; et al. Culture Independent Analysis of Ileal Mucosa Reveals a Selective Increase in Invasive Escherichia Coli of Novel Phylogeny Relative to Depletion of Clostridiales in Crohn’s Disease Involving the Ileum. ISME J. 2007, 1, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced Diversity of Faecal Microbiota in Crohn’s Disease Revealed by a Metagenomic Approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Seksik, P.; Rigottier-Gois, L.; Gramet, G.; Sutren, M.; Pochart, P.; Marteau, P.; Jian, R.; Doré, J. Alterations of the Dominant Faecal Bacterial Groups in Patients with Crohn’s Disease of the Colon. Gut 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low Counts of Faecalibacterium prausnitzii in Colitis Microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Swidsinski, A.; Weber, J.; Loening-Baucke, V.; Hale, L.P.; Lochs, H. Spatial Organization and Composition of the Mucosal Flora in Patients with Inflammatory Bowel Disease. J. Clin. Microbiol. 2005, 43, 3380–3389. [Google Scholar] [CrossRef]
- Darfeuille-Michaud, A.; Neut, C.; Barnich, N.; Lederman, E.; Di Martino, P.; Desreumaux, P.; Gambiez, L.; Joly, B.; Cortot, A.; Colombel, J.F. Presence of Adherent Escherichia Coli Strains in Ileal Mucosa of Patients with Crohn’s Disease. Gastroenterology 1998, 115, 1405–1413. [Google Scholar] [CrossRef]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.-L.; Barnich, N.; Bringer, M.-A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.-F. High Prevalence of Adherent-Invasive Escherichia Coli Associated with Ileal Mucosa in Crohn’s Disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Barnich, N.; Sivignon, A.; Darcha, C.; Chan, C.H.F.; Stanners, C.P.; Darfeuille-Michaud, A. Crohn’s Disease Adherent-Invasive Escherichia Coli Colonize and Induce Strong Gut Inflammation in Transgenic Mice Expressing Human CEACAM. J. Exp. Med. 2009, 206, 2179–2189. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Carvalho, F.A.; Ley, R.E.; Gewirtz, A.T. AIEC Pathobiont Instigates Chronic Colitis in Susceptible Hosts by Altering Microbiota Composition. Gut 2014, 63, 1069–1080. [Google Scholar] [CrossRef]
- Schmitz, J.M.; Tonkonogy, S.L.; Dogan, B.; Leblond, A.; Whitehead, K.J.; Kim, S.C.; Simpson, K.W.; Sartor, R.B. Murine Adherent and Invasive E. Coli Induces Chronic Inflammation and Immune Responses in the Small and Large Intestines of Monoassociated IL-10-/- Mice Independent of Long Polar Fimbriae Adhesin A. Inflamm. Bowel Dis. 2019, 25, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Barnich, N.; Carvalho, F.A.; Glasser, A.-L.; Darcha, C.; Jantscheff, P.; Allez, M.; Peeters, H.; Bommelaer, G.; Desreumaux, P.; Colombel, J.-F.; et al. CEACAM6 Acts as a Receptor for Adherent-Invasive E. Coli, Supporting Ileal Mucosa Colonization in Crohn Disease. J. Clin. Investig. 2007, 117, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Chapman-Kiddell, C.A.; Davies, P.S.W.; Gillen, L.; Radford-Smith, G.L. Role of Diet in the Development of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2010, 16, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western Diet Induces Dysbiosis with Increased E Coli in CEABAC10 Mice, Alters Host Barrier Function Favouring AIEC Colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western Diet Induces a Shift in Microbiota Composition Enhancing Susceptibility to Adherent-Invasive E. Coli Infection and Intestinal Inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.-Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Human Requirement for N-3 Polyunsaturated Fatty Acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 Fatty Acids in Obesity and Metabolic Syndrome: A Mechanistic Update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 Polyunsaturated Fatty Acids and Inflammatory Processes: Nutrition or Pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased Gut Permeability and Microbiota Change Associate with Mesenteric Fat Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish Oil Attenuates Omega-6 Polyunsaturated Fatty Acid-Induced Dysbiosis and Infectious Colitis but Impairs LPS Dephosphorylation Activity Causing Sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef] [PubMed]
- Pulkrabek, M.; Rhee, Y.; Gibbs, P.; Hall, C. Flaxseed- and Buckwheat-Supplemented Diets Altered Enterobacteriaceae Diversity and Prevalence in the Cecum and Feces of Obese Mice. J. Diet. Suppl. 2017, 14, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Deficiency of Essential Dietary N-3 PUFA Disrupts the Caecal Microbiome and Metabolome in Mice. Br. J. Nutr. 2017, 118, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Mourot, J.; Tonnac, A. de The Bleu Blanc Cœur Path: Impacts on Animal Products and Human Health. OCL 2015, 22, D610. [Google Scholar] [CrossRef][Green Version]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- Shafie, S.R.; Wanyonyi, S.; Panchal, S.K.; Brown, L. Linseed Components Are More Effective Than Whole Linseed in Reversing Diet-Induced Metabolic Syndrome in Rats. Nutrients 2019, 11, 1677. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Maillard, F.; Pereira, B.; Boisseau, N. Effect of High-Intensity Interval Training on Total, Abdominal and Visceral Fat Mass: A Meta-Analysis. Sports Med. 2018, 48, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Dupuit, M.; Maillard, F.; Pereira, B.; Marcelo, L.M.; Antonio, H.L.; Boisseau, N. Effect of High Intensity Interval Training on Body Composition in Women before and after Menopause: A Meta-Analysis. Exp. Physiol. 2020, 105, 1470–1490. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.P.; Madhusudhan, K.S.; Kedia, S.; Sharma, R.; Pratap Mouli, V.; Bopanna, S.; Dhingra, R.; Pradhan, R.; Goyal, S.; Sreenivas, V.; et al. Development and Validation of Visceral Fat Quantification as a Surrogate Marker for Differentiation of Crohn’s Disease and Intestinal Tuberculosis. J. Gastroenterol. Hepatol. 2017, 32, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Crohn, B.B.; Ginzburg, L.; Oppenheimer, G.D. Regional Ileitis: A Pathologic and Clinical Entity. 1932. Mt. Sinai J. Med. 2000, 67, 263–268. [Google Scholar]
- Serena, C.; Keiran, N.; Madeira, A.; Maymó-Masip, E.; Ejarque, M.; Terrón-Puig, M.; Espin, E.; Martí, M.; Borruel, N.; Guarner, F.; et al. Crohn’s Disease Disturbs the Immune Properties of Human Adipose-Derived Stem Cells Related to Inflammasome Activation. Stem Cell Rep. 2017, 9, 1109–1123. [Google Scholar] [CrossRef]
- Desreumaux, P.; Ernst, O.; Geboes, K.; Gambiez, L.; Berrebi, D.; Müller-Alouf, H.; Hafraoui, S.; Emilie, D.; Ectors, N.; Peuchmaur, M.; et al. Inflammatory Alterations in Mesenteric Adipose Tissue in Crohn’s Disease. Gastroenterology 1999, 117, 73–81. [Google Scholar] [CrossRef]
- Drouet, M.; Dubuquoy, L.; Desreumaux, P.; Bertin, B. Visceral Fat and Gut Inflammation. Nutrition 2012, 28, 113–117. [Google Scholar] [CrossRef]
- Allen, N.G.; Higham, S.M.; Mendham, A.E.; Kastelein, T.E.; Larsen, P.S.; Duffield, R. The Effect of High-Intensity Aerobic Interval Training on Markers of Systemic Inflammation in Sedentary Populations. Eur. J. Appl. Physiol. 2017, 117, 1249–1256. [Google Scholar] [CrossRef]
- Maillard, F.; Vazeille, E.; Sauvanet, P.; Sirvent, P.; Bonnet, R.; Combaret, L.; Chausse, P.; Chevarin, C.; Otero, Y.F.; Delcros, G.; et al. Preventive Effect of Spontaneous Physical Activity on the Gut-Adipose Tissue in a Mouse Model That Mimics Crohn’s Disease Susceptibility. Cells 2019, 8, 33. [Google Scholar] [CrossRef]
- Peppler, W.T.; Anderson, Z.G.; MacRae, L.M.; MacPherson, R.E.K.; Wright, D.C. Habitual Physical Activity Protects against Lipopolysaccharide-Induced Inflammation in Mouse Adipose Tissue. Adipocyte 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-X.; Wang, T.; Zhou, F.; Wang, Y.; Xing, J.-W.; Zhang, S.; Gu, S.-Z.; Sang, L.-X.; Dai, C.; Wang, H.-L. Voluntary Exercise Prevents Colonic Inflammation in High-Fat Diet-Induced Obese Mice by up-Regulating PPAR-γ Activity. Biochem. Biophys. Res. Commun. 2015, 459, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Taibi, A.; Ku, M.; Lin, Z.; Gargari, G.; Kubant, A.; Lepp, D.; Power, K.A.; Guglielmetti, S.; Thompson, L.U.; Comelli, E.M. Discriminatory and Cooperative Effects within the Mouse Gut Microbiota in Response to Flaxseed and Its Oil and Lignan Components. J. Nutr. Biochem. 2021, 98, 108818. [Google Scholar] [CrossRef] [PubMed]
- Rickard, S.E.; Orcheson, L.J.; Seidl, M.M.; Luyengi, L.; Fong, H.H.; Thompson, L.U. Dose-Dependent Production of Mammalian Lignans in Rats and in Vitro from the Purified Precursor Secoisolariciresinol Diglycoside in Flaxseed. J. Nutr. 1996, 126, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Srinivasan, G.; Delgado, M.A.; Young, A.N.; Gewirtz, A.T.; Vijay-Kumar, M. Fecal Lipocalin 2, a Sensitive and Broadly Dynamic Non-Invasive Biomarker for Intestinal Inflammation. PLoS ONE 2012, 7, e44328. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar] [CrossRef]
- Buisson, A.; Vazeille, E.; Fumery, M.; Pariente, B.; Nancey, S.; Seksik, P.; Peyrin-Biroulet, L.; Allez, M.; Ballet, N.; Filippi, J.; et al. Faster and Less Invasive Tools to Identify Patients with Ileal Colonization by Adherent-Invasive E. Coli in Crohn’s Disease. United Eur. Gastroenterol. J. 2021, 9, 1007–1018. [Google Scholar] [CrossRef]
- Shim, Y.Y.; Gui, B.; Arnison, P.G.; Wang, Y.; Reaney, M.J.T. Flaxseed (Linum Usitatissimum L.) Bioactive Compounds and Peptide Nomenclature: A Review. Trends Food Sci. Technol. 2014, 38, 5–20. [Google Scholar] [CrossRef]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.-F. Adherent-Invasive Escherichia Coli in Inflammatory Bowel Disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-What Role Do Proteobacteria Play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Yu, H.-N.; Zhu, J.; Pan, W.; Shen, S.-R.; Shan, W.-G.; Das, U.N. Effects of Fish Oil with a High Content of N-3 Polyunsaturated Fatty Acids on Mouse Gut Microbiota. Arch. Med. Res. 2014, 45, 195–202. [Google Scholar] [CrossRef]
- Valle, M.; Mitchell, P.L.; Pilon, G.; St-Pierre, P.; Varin, T.; Richard, D.; Vohl, M.-C.; Jacques, H.; Delvin, E.; Levy, E.; et al. Cholecalciferol Supplementation Does Not Prevent the Development of Metabolic Syndrome or Enhance the Beneficial Effects of Omega-3 Fatty Acids in Obese Mice. J. Nutr. 2021, 151, 1175–1189. [Google Scholar] [CrossRef]
- Zhu, X.; Bi, Z.; Yang, C.; Guo, Y.; Yuan, J.; Li, L.; Guo, Y. Effects of Different Doses of Omega-3 Polyunsaturated Fatty Acids on Gut Microbiota and Immunity. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Gallè, F.; Valeriani, F.; Cattaruzza, M.S.; Gianfranceschi, G.; Liguori, R.; Antinozzi, M.; Mederer, B.; Liguori, G.; Romano Spica, V. Mediterranean Diet, Physical Activity and Gut Microbiome Composition: A Cross-Sectional Study among Healthy Young Italian Adults. Nutrients 2020, 12, 2164. [Google Scholar] [CrossRef]
- Carreño, D.; Toral, P.G.; Pinloche, E.; Belenguer, A.; Yáñez-Ruiz, D.R.; Hervás, G.; McEwan, N.R.; Newbold, C.J.; Frutos, P. Rumen Bacterial Community Responses to DPA, EPA and DHA in Cattle and Sheep: A Comparative in Vitro Study. Sci. Rep. 2019, 9, 11857. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Park, B.K.; Shin, J.S.; Choi, S.H.; Smith, S.B.; Yan, C.G. Effects of Dietary Linseed Oil and Propionate Precursors on Ruminal Microbial Community, Composition, and Diversity in Yanbian Yellow Cattle. PLoS ONE 2015, 10, e0126473. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Arqués, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Muñoz, R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Kläring, K.; Heinzmann, S.S.; Platz, S.; Scholz, B.; Engel, K.-H.; Schmitt-Kopplin, P.; Haller, D.; Rohn, S.; Skurk, T.; et al. Gut Metabolites and Bacterial Community Networks during a Pilot Intervention Study with Flaxseeds in Healthy Adult Men. Mol. Nutr. Food Res. 2015, 59, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.F.; Zang, Y. A Review of Lignan Metabolism, Milk Enterolactone Concentration, and Antioxidant Status of Dairy Cows Fed Flaxseed. Molecules 2018, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Kim, E.; Levy, L.; Davidson, L.A.; Goldsby, J.S.; Miles, F.L.; Navarro, S.L.; Randolph, T.W.; Zhao, N.; Ivanov, I.; et al. Colonic Mucosal and Exfoliome Transcriptomic Profiling and Fecal Microbiome Response to a Flaxseed Lignan Extract Intervention in Humans. Am. J. Clin. Nutr. 2019, 110, 377–390. [Google Scholar] [CrossRef]
- Clavel, T.; Lippman, R.; Gavini, F.; Doré, J.; Blaut, M. Clostridium Saccharogumia Sp. Nov. and Lactonifactor Longoviformis Gen. Nov., Sp. Nov., Two Novel Human Faecal Bacteria Involved in the Conversion of the Dietary Phytoestrogen Secoisolariciresinol Diglucoside. Syst. Appl. Microbiol. 2007, 30, 16–26. [Google Scholar] [CrossRef]
- Schogor, A.L.B.; Huws, S.A.; Santos, G.T.D.; Scollan, N.D.; Hauck, B.D.; Winters, A.L.; Kim, E.J.; Petit, H.V. Ruminal Prevotella Spp. May Play an Important Role in the Conversion of Plant Lignans into Human Health Beneficial Antioxidants. PLoS ONE 2014, 9, e87949. [Google Scholar] [CrossRef]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic Characterization of the Beta-Glucuronidase Enzyme from a Human Intestinal Bacterium, Ruminococcus Gnavus. Microbiology 2005, 151, 2323–2330. [Google Scholar] [CrossRef]
- Fuentealba, C.; Figuerola, F.; Estévez, A.M.; Bastías, J.M.; Muñoz, O. Bioaccessibility of Lignans from Flaxseed (Linum Usitatissimum L.) Determined by Single-Batch in Vitro Simulation of the Digestive Process. J. Sci. Food Agric. 2014, 94, 1729–1738. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus Gnavus, a Member of the Human Gut Microbiome Associated with Crohn’s Disease, Produces an Inflammatory Polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef] [PubMed]
- Plissonneau, C.; Capel, F.; Chassaing, B.; Dupuit, M.; Maillard, F.; Wawrzyniak, I.; Combaret, L.; Dutheil, F.; Etienne, M.; Mairesse, G.; et al. High-Intensity Interval Training and α-Linolenic Acid Supplementation Improve DHA Conversion and Increase the Abundance of Gut Mucosa-Associated Oscillospira Bacteria. Nutrients 2021, 13, 788. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community Characteristics of the Gut Microbiomes of Competitive Cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Berg Miller, M.E.; Pence, B.D.; Whitlock, K.; Nehra, V.; Gaskins, H.R.; White, B.A.; Fryer, J.D.; Woods, J.A. Voluntary and Forced Exercise Differentially Alters the Gut Microbiome in C57BL/6J Mice. J. Appl. Physiol. 2015, 118, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Samaan, M.C.; Marcinko, K.; Sikkema, S.; Fullerton, M.D.; Ziafazeli, T.; Khan, M.I.; Steinberg, G.R. Endurance Interval Training in Obese Mice Reduces Muscle Inflammation and Macrophage Content Independently of Weight Loss. Physiol. Rep. 2014, 2, e12012. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An Improved Greengenes Taxonomy with Explicit Ranks for Ecological and Evolutionary Analyses of Bacteria and Archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]



| HFD (n = 16) | HFD-LS-O (n = 16) | HFD-LS-E (n = 16) | p | |
|---|---|---|---|---|
| Acetate | 10.6 ± 4.7 | 11.2 ± 5.9 | 13.5 ± 4.4 | 0.11 |
| Butyrate | 0.18 ± 0.3 | 0.16 ± 0.2 | 0.35 ± 0.3 | 0.13 |
| Propionate | 2.22 ± 1.2 | 2.1 ± 0.7 | 2.7 ± 0.7 | 0.07 |
| Total SCFAs | 12.8 ± 5.8 | 13.3 ± 6.5 | 16.2 ±4.8 | 0.09 |
| HFD (n = 16) | HFD-LS-O (n = 16) | HFD-LS-E (n = 16) | |
|---|---|---|---|
| Proteins (%kcal) | 19.0 | 19.0 | 19.5 |
| Carbohydrates (%kcal) | 40.6 | 40.6 | 40.1 |
| Lipids (%kcal) | 40.4 | 40.4 | 40.4 |
| SFAs (% of lipid fraction) | 40.0 | 40.0 | 36.7 |
| MUFAs (%) | 44.1 | 44.3 | 41.0 |
| PUFAs (%) | 15.8 | 15.8 | 20.6 |
| LA (C18:2 n-6) (%) | 1.0 | 3.6 | 5.2 |
| ALA (C18:3 n-3) (%) | 14.8 | 12.1 | 15.4 |
| kcal/100 g | 468.4 | 468.4 | 460.4 |
| n-6/n-3 PUFA ratio | 14.8 | 3.3 | 3.0 |
| HFD (n = 16) | HFD-LS-O (n = 16) | HFD-LS-E (n = 16) | ||||
|---|---|---|---|---|---|---|
| w/w (%) | kcal/100 g | w/w (%) | kcal/100 g | w/w (%) | kcal/100 g | |
| Casein | 22 | 88 | 22 | 88 | 21 | 84 |
| L-Cystine | 0.3 | 1.2 | 0.3 | 1.2 | 0.3 | 1.2 |
| Sunflower oil | 2 | 18 | 0.99 | 8.91 | 2 | 18 |
| Lard | 19 | 171 | 19 | 171 | 17 | 153 |
| Linseed oil | 1.01 | 9.09 | ||||
| Extruded linseed | 6 | 15.12 | ||||
| Starch | 17 | 68 | 17 | 68 | 15 | 60 |
| Maltodextrin | 7 | 28 | 7 | 28 | 7 | 28 |
| Sucrose | 22.55 | 90.2 | 22.55 | 90.2 | 22 | 88 |
| Cellulose | 5 | 5 | 4.55 | |||
| AIN93M | 4 | 4 | 4 | |||
| AIN93Vx | 1 | 4 | 1 | 4 | 1 | 4 |
| Choline | 0.15 | 0.15 | 0.15 | |||
| Total | 100 | 468.4 | 100 | 468.4 | 100 | 460.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plissonneau, C.; Sivignon, A.; Chassaing, B.; Capel, F.; Martin, V.; Etienne, M.; Wawrzyniak, I.; Chausse, P.; Dutheil, F.; Mairesse, G.; et al. Beneficial Effects of Linseed Supplementation on Gut Mucosa-Associated Microbiota in a Physically Active Mouse Model of Crohn’s Disease. Int. J. Mol. Sci. 2022, 23, 5891. https://doi.org/10.3390/ijms23115891
Plissonneau C, Sivignon A, Chassaing B, Capel F, Martin V, Etienne M, Wawrzyniak I, Chausse P, Dutheil F, Mairesse G, et al. Beneficial Effects of Linseed Supplementation on Gut Mucosa-Associated Microbiota in a Physically Active Mouse Model of Crohn’s Disease. International Journal of Molecular Sciences. 2022; 23(11):5891. https://doi.org/10.3390/ijms23115891
Chicago/Turabian StylePlissonneau, Claire, Adeline Sivignon, Benoit Chassaing, Frederic Capel, Vincent Martin, Monique Etienne, Ivan Wawrzyniak, Pierre Chausse, Frederic Dutheil, Guillaume Mairesse, and et al. 2022. "Beneficial Effects of Linseed Supplementation on Gut Mucosa-Associated Microbiota in a Physically Active Mouse Model of Crohn’s Disease" International Journal of Molecular Sciences 23, no. 11: 5891. https://doi.org/10.3390/ijms23115891
APA StylePlissonneau, C., Sivignon, A., Chassaing, B., Capel, F., Martin, V., Etienne, M., Wawrzyniak, I., Chausse, P., Dutheil, F., Mairesse, G., Chesneau, G., Boisseau, N., & Barnich, N. (2022). Beneficial Effects of Linseed Supplementation on Gut Mucosa-Associated Microbiota in a Physically Active Mouse Model of Crohn’s Disease. International Journal of Molecular Sciences, 23(11), 5891. https://doi.org/10.3390/ijms23115891











