Heart Ferroportin Protein Content Is Regulated by Heart Iron Concentration and Systemic Hepcidin Expression
Abstract
:1. Introduction
2. Results
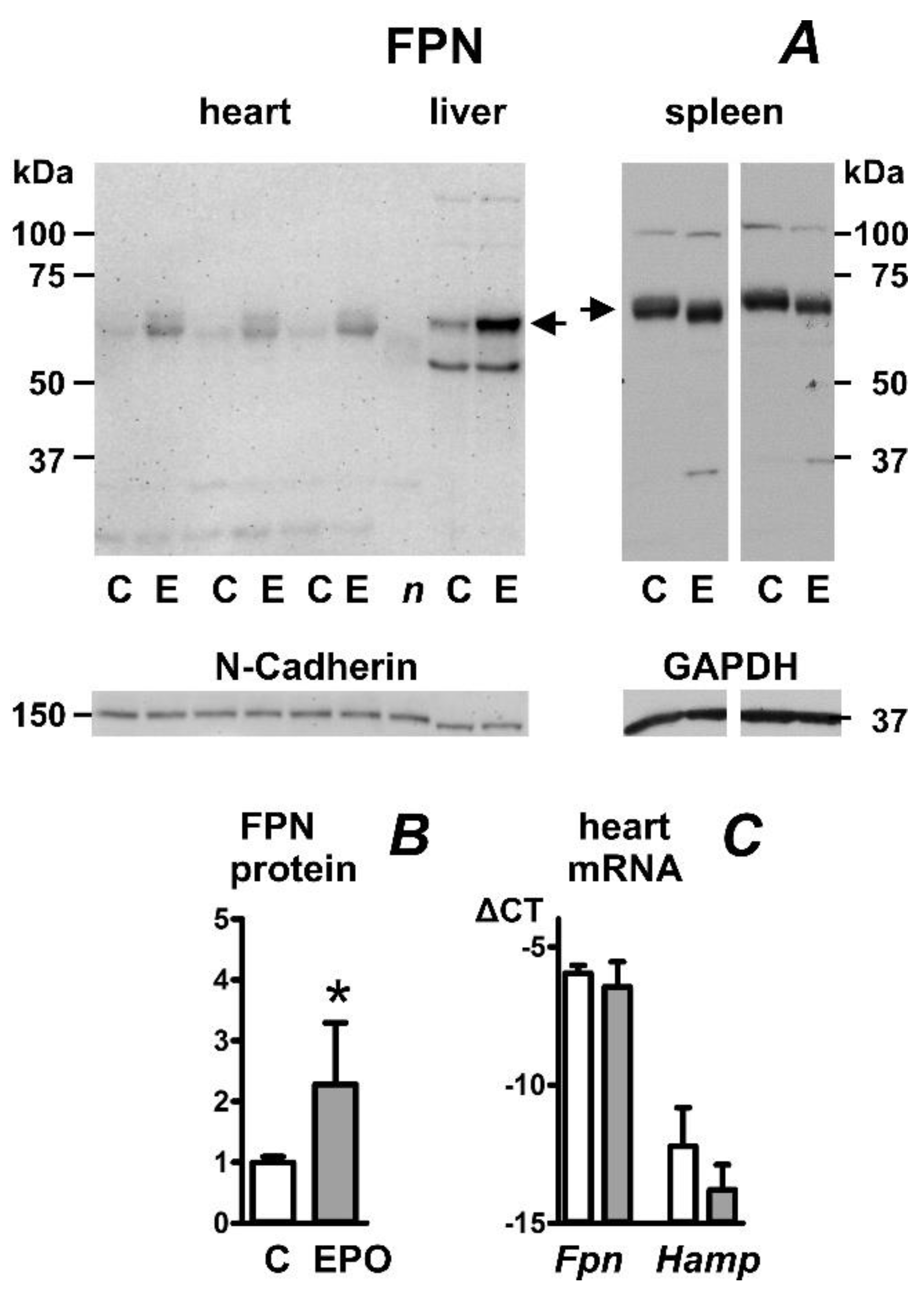
2.1. Erythropoietin Administration Increases Heart Ferroportin Protein Content in Mice
2.2. Erythropoietin Administration Increases Heart Ferroportin Protein Content in Rats
2.3. Feeding of Low-Iron Diet Decreases Ferroportin Protein Content in Rat Heart, Liver, and Spleen
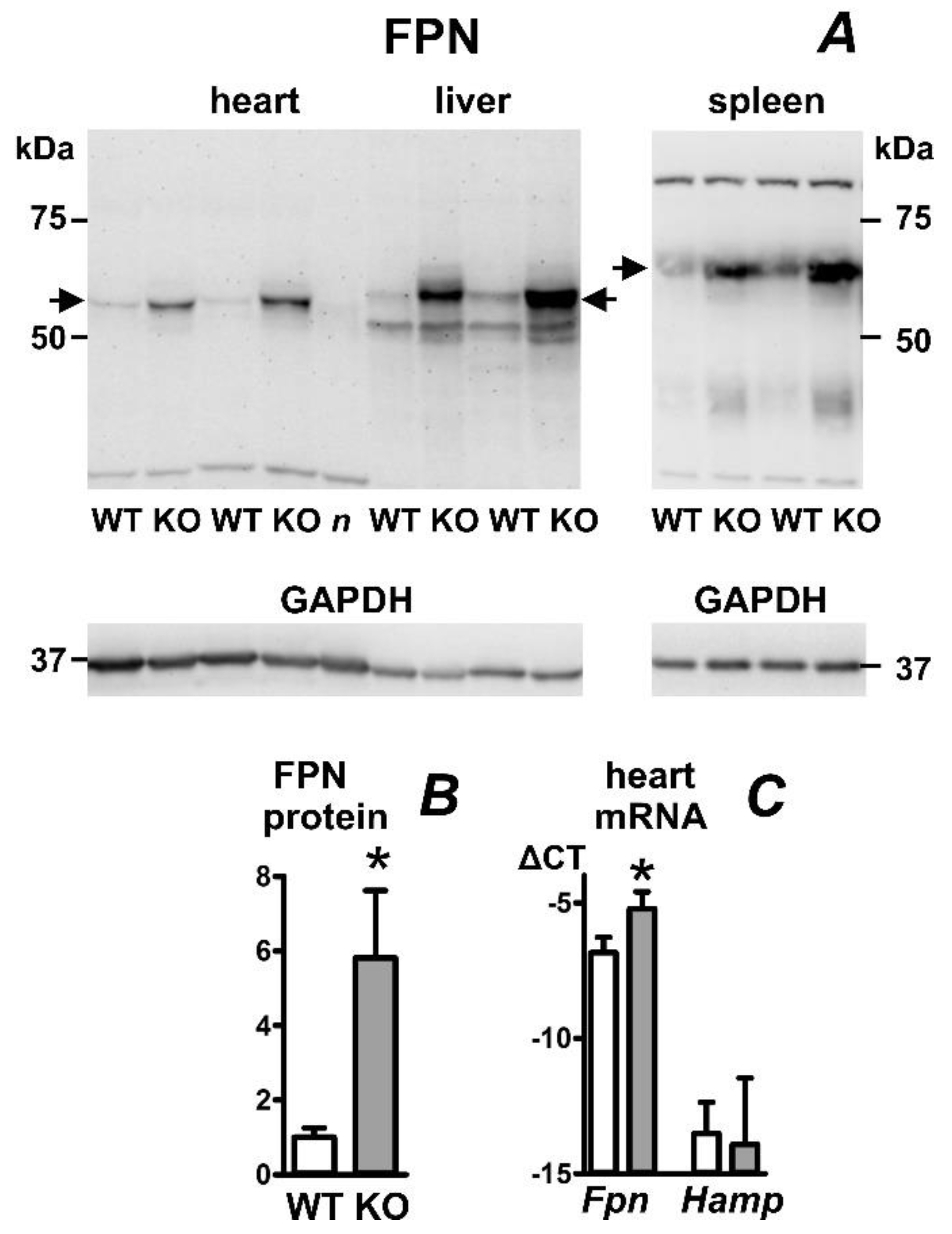
2.4. Heart Ferroportin Protein Content Is Increased in a Mouse Model of Juvenile Hemochromatosis
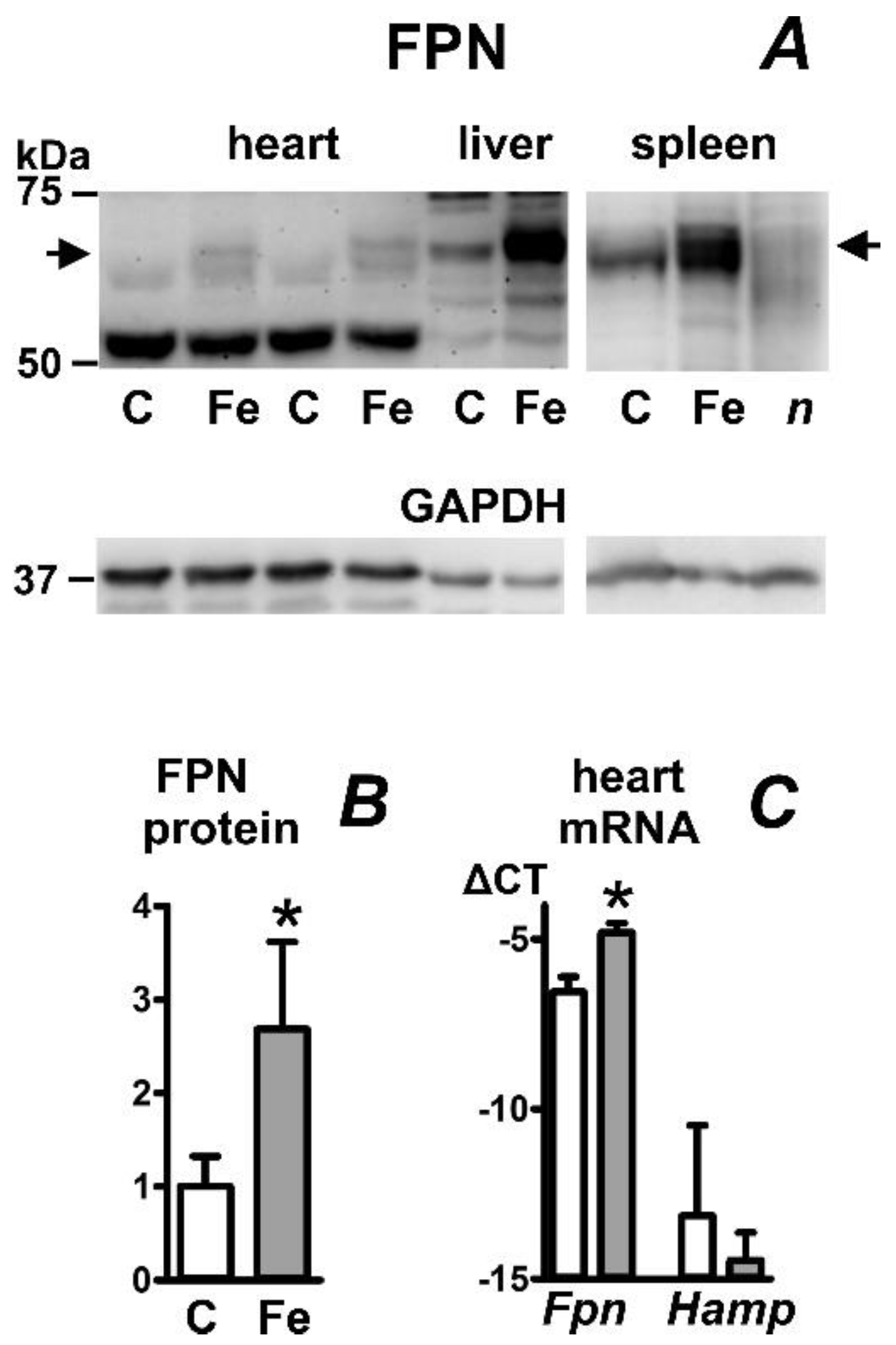
2.5. Iron Carboxymaltose Injection Increases Ferroportin Protein in Mouse Heart, Liver, and Spleen
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Ferroportin Protein Determination
4.3. Real-Time PCR
4.4. Iron Determinations
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, N.C. Forging a field: The golden age of iron biology. Blood 2008, 112, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, W.J.H.; Besser, M.; Bowden, D.J.; Kelly, D.A. Juvenile haemochromatosis. Lancet Child Adolesc. Health 2021, 5, 524–530. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Marsella, M. Iron Chelation in Thalassemia Major. Clin. Ther. 2015, 37, 2866–2877. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Pantopoulos, K. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med. 2020, 75, 100866. [Google Scholar] [CrossRef]
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015, 13, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Chen, Y.Y.; Chang, Y.Z.; Duan, X.L.; Ho, K.P.; Jiang, D.H.; Wang, K.; Qian, Z.M. Post-transcriptional expression of DMT1 in the heart of rat. J. Cell. Physiol. 2003, 196, 124–130. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Sun, H.; Trivieri, M.G.; Koch, S.E.; Dawood, F.; Ackerley, C.; Yazdanpanah, M.; Wilson, G.J.; Schwartz, A.; Liu, P.P.; et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003, 9, 1187–1194. [Google Scholar] [CrossRef]
- Paterek, A.; Mackiewicz, U.; Mączewski, M. Iron and the heart: A paradigm shift from systemic to cardiomyocyte abnormalities. J. Cell. Physiol. 2019, 234, 21613–21629. [Google Scholar] [CrossRef]
- Abboud, S.; Haile, D.J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000, 275, 19906–19912. [Google Scholar] [CrossRef] [Green Version]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 2000, 5, 299–309. [Google Scholar] [CrossRef]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.P.; Meynard, D.; Coppin, H. Regulators of hepcidin expression. Vitam. Horm. 2019, 110, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [Green Version]
- Colucci, S.; Marques, O.; Altamura, S. 20 years of Hepcidin: How far we have come. Semin. Hematol. 2021, 58, 132–144. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; An, P.; Min, J.; Wang, F. Cardiomyocyte-specific deletion of ferroportin using MCK-Cre has no apparent effect on cardiac iron homeostasis. Int. J. Cardiol. 2015, 201, 90–92. [Google Scholar] [CrossRef]
- Lakhal-Littleton, S.; Wolna, M.; Carr, C.A.; Miller, J.J.; Christian, H.C.; Ball, V.; Santos, A.; Diaz, R.; Biggs, D.; Stillion, R.; et al. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc. Natl. Acad. Sci. USA 2015, 112, 3164–3169. [Google Scholar] [CrossRef] [Green Version]
- Lakhal-Littleton, S.; Wolna, M.; Chung, Y.J.; Christian, H.C.; Heather, L.C.; Brescia, M.; Ball, V.; Diaz, R.; Santos, A.; Biggs, D.; et al. An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. Elife 2016, 5, e19804. [Google Scholar] [CrossRef]
- Lakhal-Littleton, S. Mechanisms of cardiac iron homeostasis and their importance to heart function. Free Radic. Biol. Med. 2019, 133, 234–237. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Zhang, D.L.; Hughes, R.M.; Ollivierre-Wilson, H.; Ghosh, M.C.; Rouault, T.A. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009, 9, 461–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theurl, I.; Ludwiczek, S.; Eller, P.; Seifert, M.; Artner, E.; Brunner, P.; Weiss, G. Pathways for the regulation of body iron homeostasis in response to experimental iron overload. J. Hepatol. 2005, 43, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.R.; Clarke, S.L.; Ho, Y.H.; Bruss, M.D.; Vasanthakumar, A.; Anderson, S.A.; Eisenstein, R.S. Differential translational control of 5’ IRE-containing mRNA in response to dietary iron deficiency and acute iron overload. Metallomics 2020, 12, 2186–2198. [Google Scholar] [CrossRef] [PubMed]
- Delaby, C.; Pilard, N.; Puy, H.; Canonne-Hergaux, F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: Early mRNA induction by haem, followed by iron-dependent protein expression. Biochem. J. 2008, 411, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canonne-Hergaux, F.; Donovan, A.; Delaby, C.; Wang, H.J.; Gros, P. Comparative studies of duodenal and macrophage ferroportin proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G156–G163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.S.; Anderson, S.A.; Meyers, K.R.; Hernandez, C.; Eisenstein, R.S.; Enns, C.A. Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin. J. Biol. Chem. 2007, 282, 12547–12556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederkofler, V.; Salie, R.; Arber, S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J. Clin. Investig. 2005, 115, 2180–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsarou, A.; Gkouvatsos, K.; Fillebeen, C.; Pantopoulos, K. Tissue-Specific Regulation of Ferroportin in Wild-Type and Hjv-/- Mice Following Dietary Iron Manipulations. Hepatol. Commun. 2021, 5, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Viatte, L.; Bennoun, M.; Beaumont, C.; Kahn, A.; Vaulont, S. Hepcidin, a new iron regulatory peptide. Blood Cells Mol. Dis. 2002, 29, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, M.; Krijt, J.; Sulc, K.; Necas, E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol. Res. 2006, 55, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Pak, M.; Lopez, M.A.; Gabayan, V.; Ganz, T.; Rivera, S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood 2006, 108, 3730–3735. [Google Scholar] [CrossRef] [PubMed]
- Petrak, J.; Havlenova, T.; Krijt, M.; Behounek, M.; Franekova, J.; Cervenka, L.; Pluhacek, T.; Vyoral, D.; Melenovsky, V. Myocardial iron homeostasis and hepcidin expression in a rat model of heart failure at different levels of dietary iron intake. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, S.; Wang, Y.; Galy, B.; Korf-Klingebiel, M.; Hirsch, V.; Baru, A.M.; Rostami, F.; Reboll, M.R.; Heineke, J.; Flögel, U.; et al. Iron-regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur. Heart J. 2017, 38, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canonne-Hergaux, F.; Gruenheid, S.; Ponka, P.; Gros, P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood 1999, 93, 4406–4417. [Google Scholar] [CrossRef]
- Fujikura, Y.; Krijt, J.; Povýšil, C.; Mělková, Z.; Přikryl, P.; Vokurka, M.; Nečas, E. Iron Overload Causes Alterations of E-Cadherin in the Liver. Folia Biol. 2016, 62, 95–102. [Google Scholar]
- Frýdlová, J.; Rychtarčíková, Z.; Gurieva, I.; Vokurka, M.; Truksa, J.; Krijt, J. Effect of erythropoietin administration on proteins participating in iron homeostasis in Tmprss6-mutated mask mice. PLoS ONE 2017, 12, e0186844. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, Y. Transmembrane protein western blotting: Impact of sample preparation on detection of SLC11A2 (DMT1) and SLC40A1 (ferroportin). PLoS ONE 2020, 15, e0235563. [Google Scholar] [CrossRef]
- Torrance, J.D.; Bothwell, T.H. Tissue Iron Stores. In Methods in Hematology; Cook, J.D., Ed.; Churchill Livingstone: New York, NY, USA, 1980; pp. 90–115. [Google Scholar]


| Group | Heart Iron (µg/g) | Liver Iron (µg/g) | Spleen Iron (µg/g) | Liver Hamp (ΔCT) |
|---|---|---|---|---|
| Mouse, control | 56.8 ± 5.6 | 79.5 ± 17.6 | 293.4 ± 55.9 | −0.22 ± 0.62 |
| Mouse, EPO | 51.6 ± 0.4 | 54.6 ± 5.6 | 133.7 ± 50.1 * | −5.42 ± 2.90 * |
| Rat, control | 42.7 ± 0.8 | 243.8 ± 21.9 | 729.7 ± 13.8 | 1.14 ± 0.35 |
| Rat, EPO | 47.1 ± 3.0 | 213.2 ± 74.9 | 195.0 ± 27.6 * | −6.45 ± 2.58 * |
| Rat, control diet | 42.3 ± 5.4 | 363.3 ± 103.2 | 855.3 ± 105.8 | 0.83 ± 0.57 |
| Rat, low Fe diet | 24.2 ± 4.0 * | 28.7 ± 4.1 * | 31.3 ± 2.6 * | −14.49 ± 1.16 |
| Mouse, Hjv+/+ | 58.5 ± 13.1 | 96.7 ± 33.0 | 1032.3 ± 423.0 | 0.54 ± 0.69 |
| Mouse, Hjv−/− | 463 ± 8.6 * | 1952.1 ± 369.0 * | 402.3 ± 126.4 | −8.10 ± 2.30 * |
| Mouse, control | 50.5 ± 1.5 | 73.5 ± 2.5 | 498.0 ± 124.0 | 0.49 ± 1.11 |
| Mouse, Fe injection | 316.1 ± 19.9 * | 3020.5 ± 189.5 * | 8249.3 ± 541.3 * | 4.14 ± 0.54 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezovsky, B.; Frýdlová, J.; Gurieva, I.; Rogalsky, D.W.; Vokurka, M.; Krijt, J. Heart Ferroportin Protein Content Is Regulated by Heart Iron Concentration and Systemic Hepcidin Expression. Int. J. Mol. Sci. 2022, 23, 5899. https://doi.org/10.3390/ijms23115899
Berezovsky B, Frýdlová J, Gurieva I, Rogalsky DW, Vokurka M, Krijt J. Heart Ferroportin Protein Content Is Regulated by Heart Iron Concentration and Systemic Hepcidin Expression. International Journal of Molecular Sciences. 2022; 23(11):5899. https://doi.org/10.3390/ijms23115899
Chicago/Turabian StyleBerezovsky, Betty, Jana Frýdlová, Iuliia Gurieva, Daniel W. Rogalsky, Martin Vokurka, and Jan Krijt. 2022. "Heart Ferroportin Protein Content Is Regulated by Heart Iron Concentration and Systemic Hepcidin Expression" International Journal of Molecular Sciences 23, no. 11: 5899. https://doi.org/10.3390/ijms23115899






