Ameliorative Effects of Lactobacillus plantarum HAC01 Lysate on 3T3-L1 Adipocyte Differentiation via AMPK Activation and MAPK Inhibition
Abstract
:1. Introduction
2. Results
2.1. L. plantarum HAC01 Lysate Inhibits the Differentiation of 3T3-L1 Cells
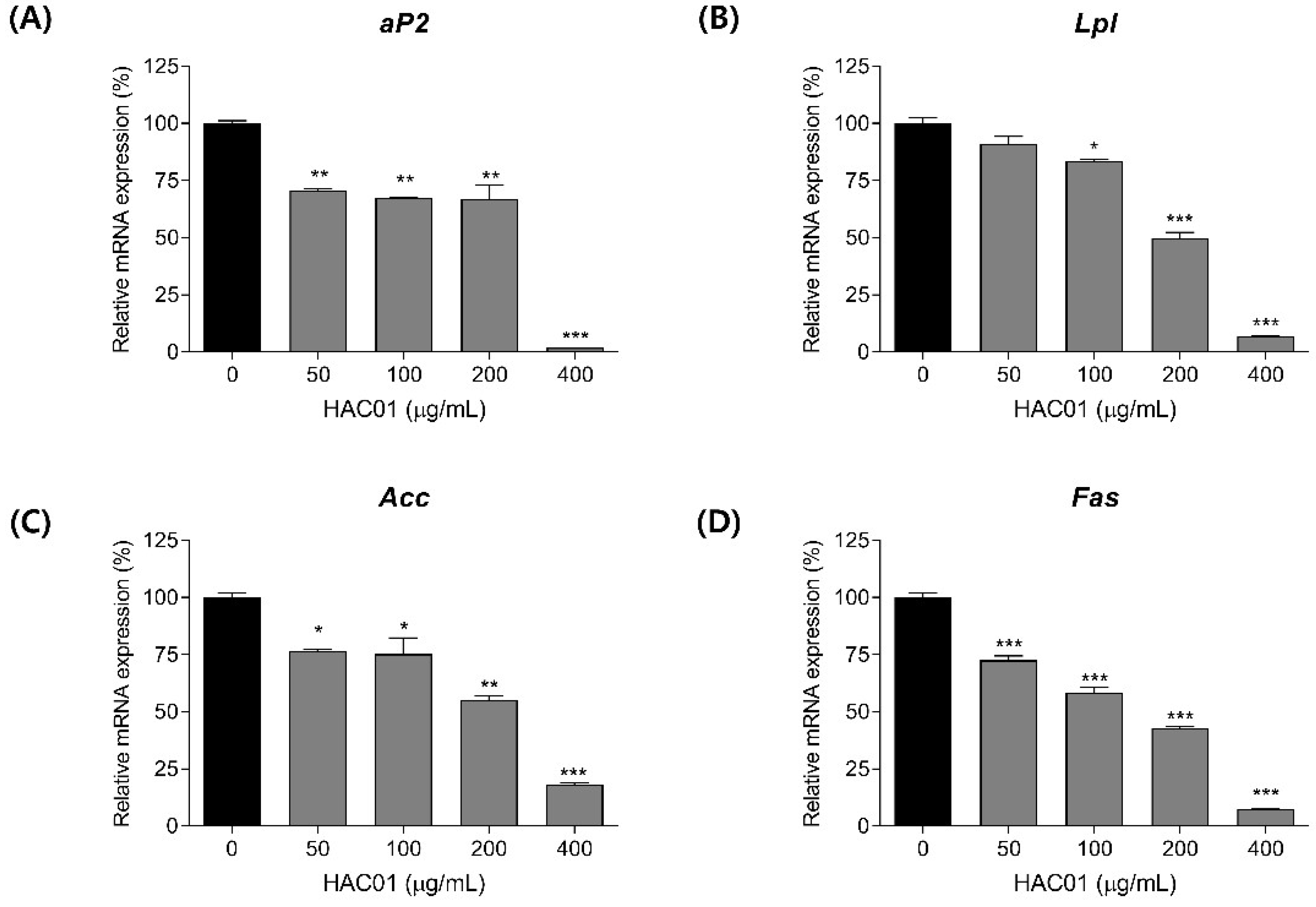
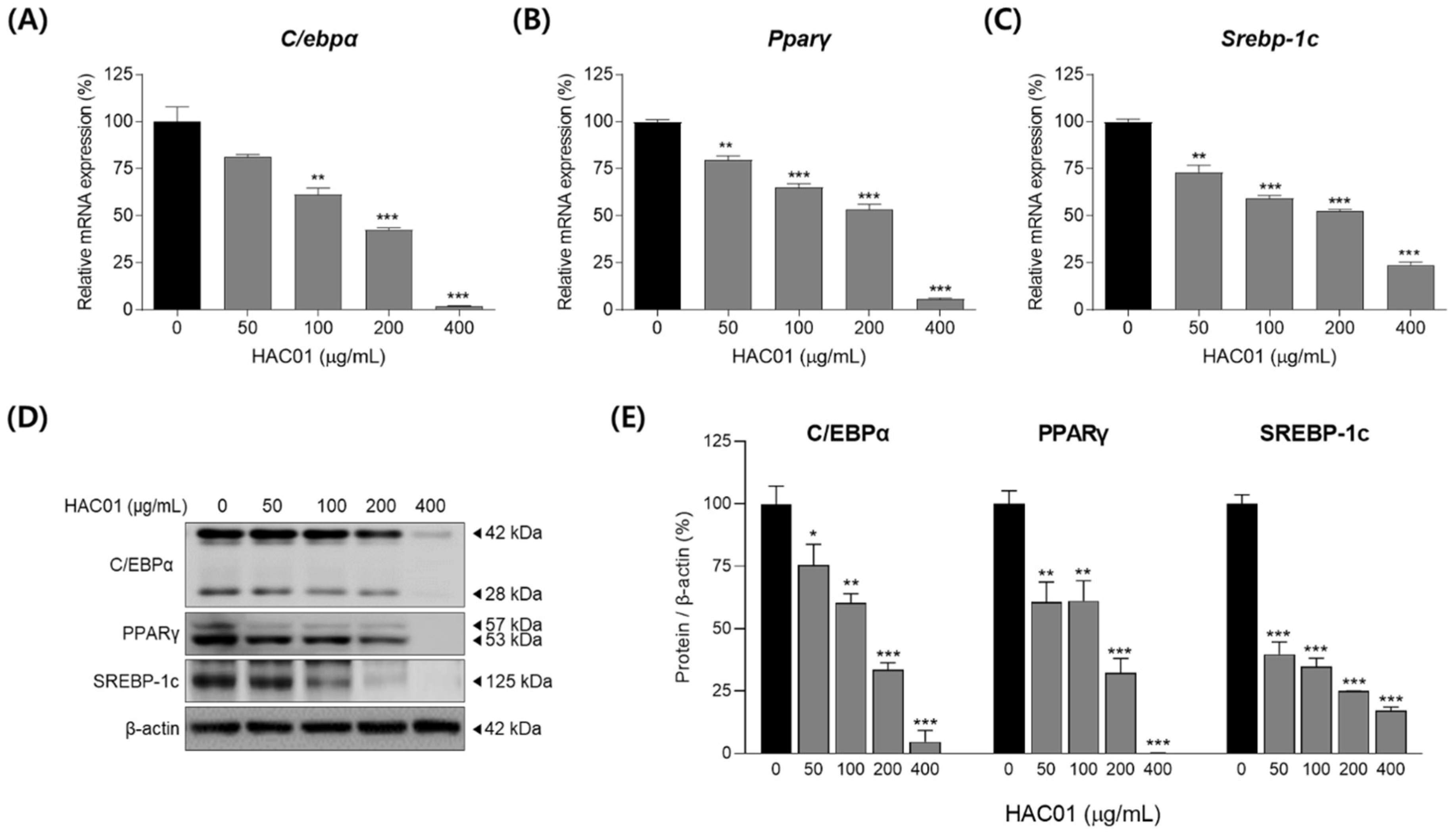
2.2. L. plantarum HAC01 Lysate Inhibits the Expression of Adipogenic and Lipogenic Factors in 3T3-L1 Pre-Adipocytes
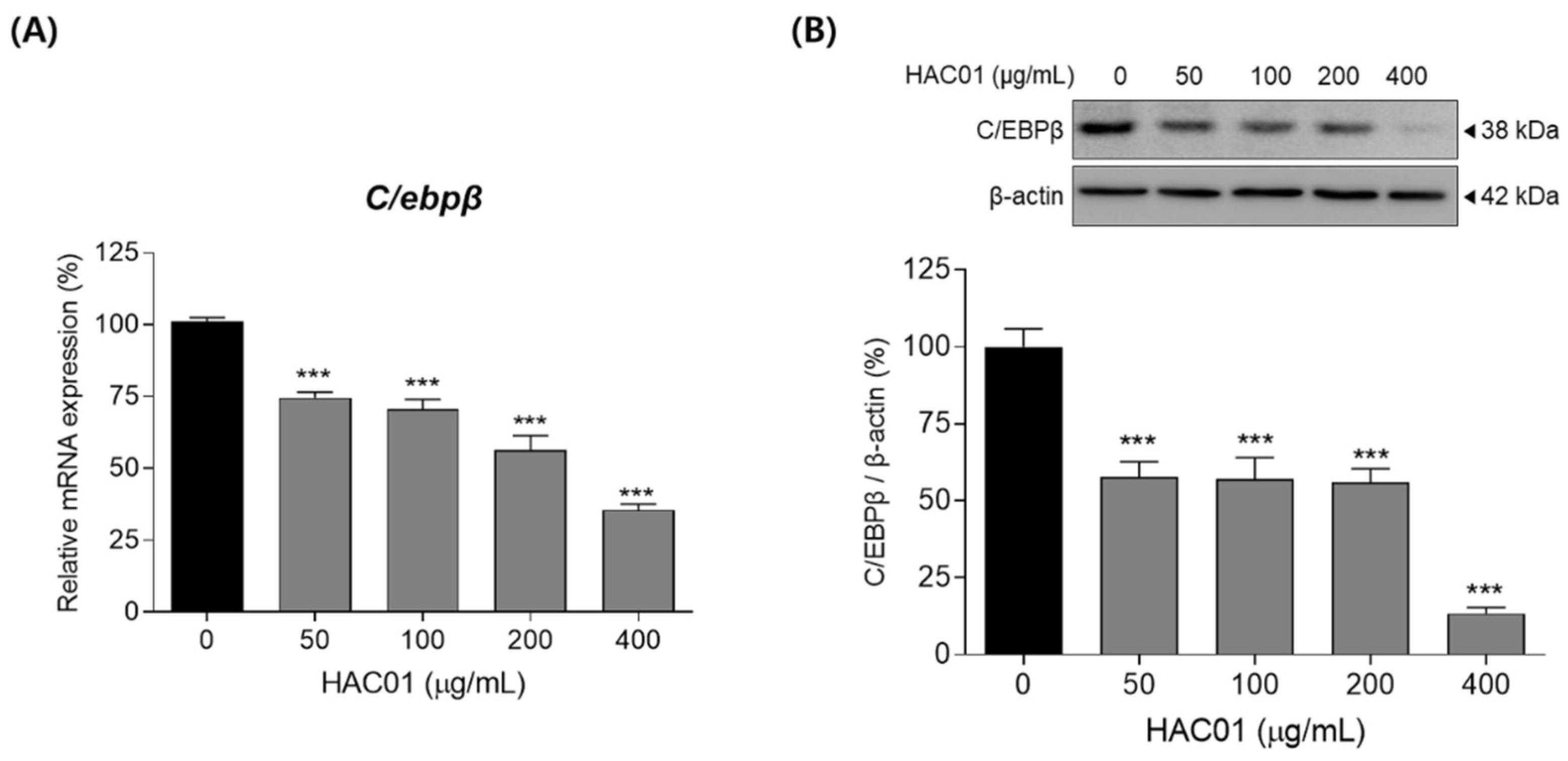
2.3. L. plantarum HAC01 Lysate Suppresses the Early Stage of Adipocyte Differentiation
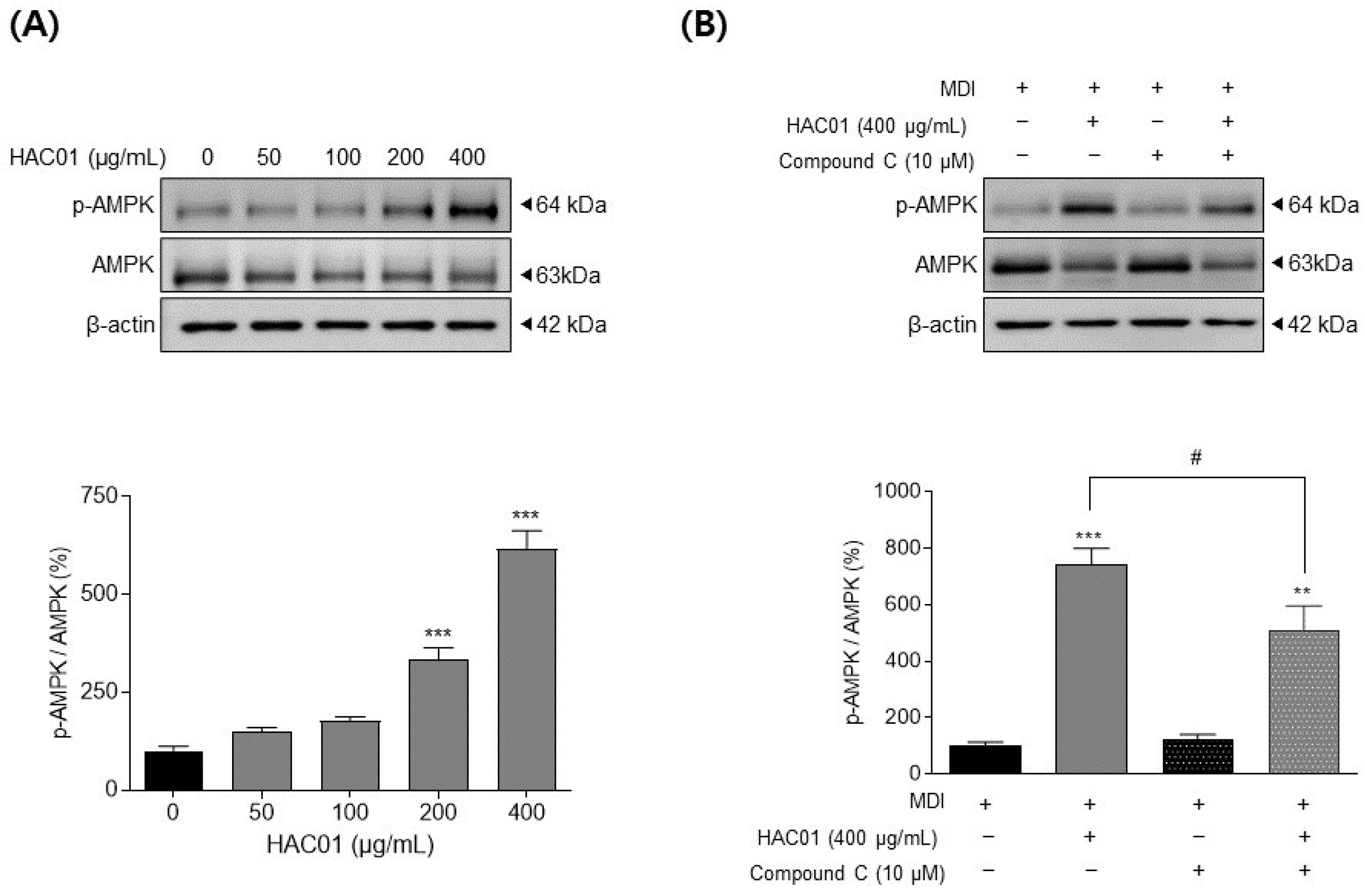
2.4. L. plantarum HAC01 Lysate Inhibits Adipocyte Differentiation via the Activation of AMPK
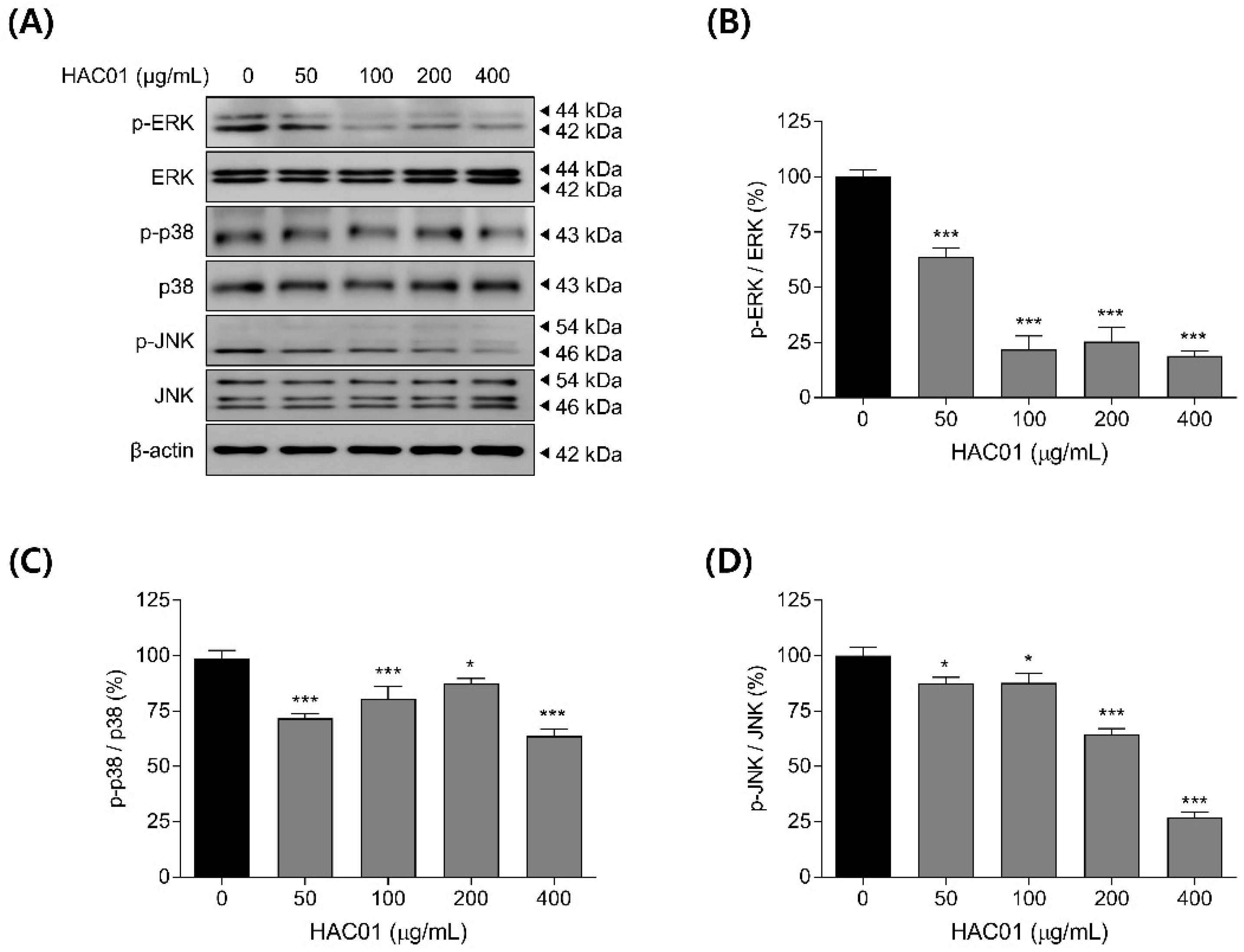
2.5. L. plantarum HAC01 Lysate Regulates the Phosphorylation of MAPK
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Differentiation
4.3. Cell Viability Assay
4.4. Oil Red O Staining
4.5. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
4.6. Western Blotting
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatr. Diabetes 2019, 20, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Kong, L.; Shan, M.; Lu, Z.; Lu, Y. Protective and ameliorating effects of probiotics against diet-induced obesity: A review. Food Res. Int. 2021, 147, 110490. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kwon, M.H.; Kim, H.K.; Lee, H.S.; Cho, J.S.; Lee, Y.I. Anti-Obesity effect of lactobacillus plantarum lb818 is associated with regulation of gut microbiota in high-fat diet-fed obese mice. J. Med. Food 2020, 23, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and postbiotics of probiotic lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Ahn, Y.-S.; Park, M.Y.; Shin, J.-H.; Kim, J.Y.; Kwon, O. Lysate of probiotic lactobacillus plantarum K8 modulate the mucosal inflammatory system in dextran sulfate sodium-induced colitic rats. Korean J. Food Sci. Anim. Resour. 2014, 34, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.-J.; Jung, A.-H.; Suh, H.J.; Choi, H.-S.; Kim, H. Lactiplantibacillus plantarum K8-based paraprobiotics prevents obesity and obesity-induced inflammatory responses in high fat diet-fed mice. Food Res. Int. 2022, 155, 111066. [Google Scholar] [CrossRef]
- Kim, H.; Lim, J.-J.; Shin, H.Y.; Suh, H.J.; Choi, H.-S. Lactobacillus plantarum K8-based paraprobiotics suppress lipid accumulation during adipogenesis by the regulation of JAK/STAT and AMPK signaling pathways. J. Funct. Foods 2021, 87, 104824. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Oh, N.; Park, J.; Kwon, M.; Seo, J.; Roh, S. Oral intake of Lactobacillus plantarum L-14 extract alleviates TLR2- and AMPK-mediated obesity-associated disorders in high-fat-diet-induced obese C57BL/6J mice. Cell Prolif. 2021, 54, e13039. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, A.; Nakanishi, M.; Okuyama, S.; Furukawa, Y.; Nakajima, M. Heptamethoxyflavone inhibits adipogenesis via enhancing PKA signaling. Eur. J. Pharmacol. 2019, 865, 172758. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Seo, H.A.; Go, Y.; Oh, C.J.; Jeoung, N.H.; Park, K.G.; Lee, I.K. Dimethylfumarate suppresses adipogenic differentiation in 3T3-L1 preadipocytes through inhibition of STAT3 activity. PLoS ONE 2013, 8, e61411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, C.; Wu, J.; Liu, W.; Li, D.; Xu, J. Harmane ameliorates obesity though inhibiting lipid accumulation and inducing adipocyte browning. RSC. Adv. 2020, 10, 4397–4403. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Kong, C.S. Artemisia princeps Inhibits Adipogenic Differentiation of 3T3-L1 Pre-Adipocytes via Downregulation of PPARgamma and MAPK Pathways. Prev. Nutr. Food Sci. 2019, 24, 299–307. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, D.; Park, G.S.; Ko, S.H.; Park, J.; Lee, Y.K.; Kang, J. Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct. 2021, 12, 6363–6373. [Google Scholar] [CrossRef]
- Park, S.; Ji, Y.; Jung, H.Y.; Park, H.; Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef]
- Oh, M.R.; Jang, H.Y.; Lee, S.Y.; Jung, S.J.; Chae, S.W.; Lee, S.O.; Park, B.H. Lactobacillus plantarum HAC01 supplementation improves glycemic control in prediabetic subjects: A randomized, double-blind, placebo-controlled trial. Nutrients 2021, 13, 2337. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Zakostelska, Z.; Kverka, M.; Klimesova, K.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Hornova, M.; Srutkova, D.; Hudcovic, T.; Ridl, J.; et al. Lysate of probiotic lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE 2011, 6, e27961. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yu, B.; Zhou, J.; Zhang, J.; Zhang, R.; Xie, J.; Wang, Q.; Zhao, S. Lysates of lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci. Rep. 2019, 9, 20128. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.; Hamouda, R.A.; Mahrous, H.; Salem, M.L.; Hamza, H.A.; Elhafez, E. In vitro treatment with intact cells or cell lysates of lactobacillus and spirulina induced lowering effects on induced hypercholesteremia. Int. J. Pharmacol. 2015, 11, 638–643. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, E.J.; Park, G.S.; Ko, S.H.; Park, J.; Lee, Y.K.; Kim, J.Y.; Lee, D.; Kang, J.; Lee, H.J. Lactiplantibacillusplantarum ATG-K2 exerts an anti-obesity effect in high-fat diet-induced obese mice by modulating the gut microbiome. Int. J. Mol. Sci. 2021, 22, 12665. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Migueliz, I.; Romo-Hualde, A.; Lopez-Yoldi, M.; Martinez, J.A.; Vizmanos, J.L.; Milagro, F.I.; Gonzalez-Navarro, C.J. Phenolic compounds inhibit 3T3-L1 adipogenesis depending on the stage of differentiation and their binding affinity to ppargamma. Molecules 2019, 24, 1045. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Chuang, W.T.; Lin, A.H.; Tsai, C.W.; Huang, C.S.; Chen, Y.T.; Chen, H.W.; Lii, C.K. Andrographolide inhibits adipogenesis of 3T3-L1 cells by suppressing C/EBPbeta expression and activation. Toxicol. Appl. Pharmacol. 2016, 307, 115–122. [Google Scholar] [CrossRef]
- Maki, C.; Funakoshi-Tago, M.; Aoyagi, R.; Ueda, F.; Kimura, M.; Kobata, K.; Tago, K.; Tamura, H. Coffee extract inhibits adipogenesis in 3T3-L1 preadipocyes by interrupting insulin signaling through the downregulation of IRS1. PLoS ONE 2017, 12, e0173264. [Google Scholar] [CrossRef] [Green Version]
- Han, M.H.; Kim, H.J.; Jeong, J.W.; Park, C.; Kim, B.W.; Choi, Y.H. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of vitis coignetiae pulliat is associated with the activation of AMPK signaling pathway. Toxicol. Res. 2018, 34, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Sharma, A.; Lee, H.J. Anti-adipogenic effects of delphinidin-3-O-beta-glucoside in 3T3-L1 preadipocytes and primary white adipocytes. Molecules 2019, 24, 1848. [Google Scholar] [CrossRef] [Green Version]
- Yim, M.J.; Hosokawa, M.; Mizushina, Y.; Yoshida, H.; Saito, Y.; Miyashita, K. Suppressive effects of amarouciaxanthin a on 3T3-L1 adipocyte differentiation through down-regulation of PPARgamma and C/EBPalpha mRNA expression. J. Agric. Food Chem. 2011, 59, 1646–1652. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferre, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Lingesh, A.; Paul, D.; Naidu, V.; Satheeshkumar, N. AMPK activating and anti adipogenic potential of Hibiscus rosa sinensis flower in 3T3-L1 cells. J. Ethnopharmacol. 2019, 233, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Mayer, F.V.; Sanders, M.J.; Gamblin, S.J. AMP-activated protein kinase: Nature’s energy sensor. Nat. Chem. Biol. 2011, 7, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nagasawa, Y.; Fujimori, K. Glycyrrhizic acid suppresses early stage of adipogenesis through repression of MEK/ERK-mediated C/EBPbeta and C/EBPdelta expression in 3T3-L1 cells. Chem.-Biol. Interact. 2021, 346, 109595. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Lee, H.S.; Han, H.K.; Choi, C.I. Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2021, 22, 11409. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Rhee, D.K.; Kim, B.O.; Pyo, S. Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways. Biomed. Pharmacother. 2018, 102, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Lim, S.W.; Ki, H.H.; Nepali, S.; Lee, Y.M.; Kim, D.K. Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. Int. J. Mol. Med. 2014, 34, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Park, E.-J.; Lee, H.-J. Ameliorative Effects of Lactobacillus plantarum HAC01 Lysate on 3T3-L1 Adipocyte Differentiation via AMPK Activation and MAPK Inhibition. Int. J. Mol. Sci. 2022, 23, 5901. https://doi.org/10.3390/ijms23115901
Kim J-Y, Park E-J, Lee H-J. Ameliorative Effects of Lactobacillus plantarum HAC01 Lysate on 3T3-L1 Adipocyte Differentiation via AMPK Activation and MAPK Inhibition. International Journal of Molecular Sciences. 2022; 23(11):5901. https://doi.org/10.3390/ijms23115901
Chicago/Turabian StyleKim, Jong-Yeon, Eun-Jung Park, and Hae-Jeung Lee. 2022. "Ameliorative Effects of Lactobacillus plantarum HAC01 Lysate on 3T3-L1 Adipocyte Differentiation via AMPK Activation and MAPK Inhibition" International Journal of Molecular Sciences 23, no. 11: 5901. https://doi.org/10.3390/ijms23115901
APA StyleKim, J.-Y., Park, E.-J., & Lee, H.-J. (2022). Ameliorative Effects of Lactobacillus plantarum HAC01 Lysate on 3T3-L1 Adipocyte Differentiation via AMPK Activation and MAPK Inhibition. International Journal of Molecular Sciences, 23(11), 5901. https://doi.org/10.3390/ijms23115901






