Extracellular Vesicles in Corneal Fibrosis/Scarring
Abstract
:1. Introduction
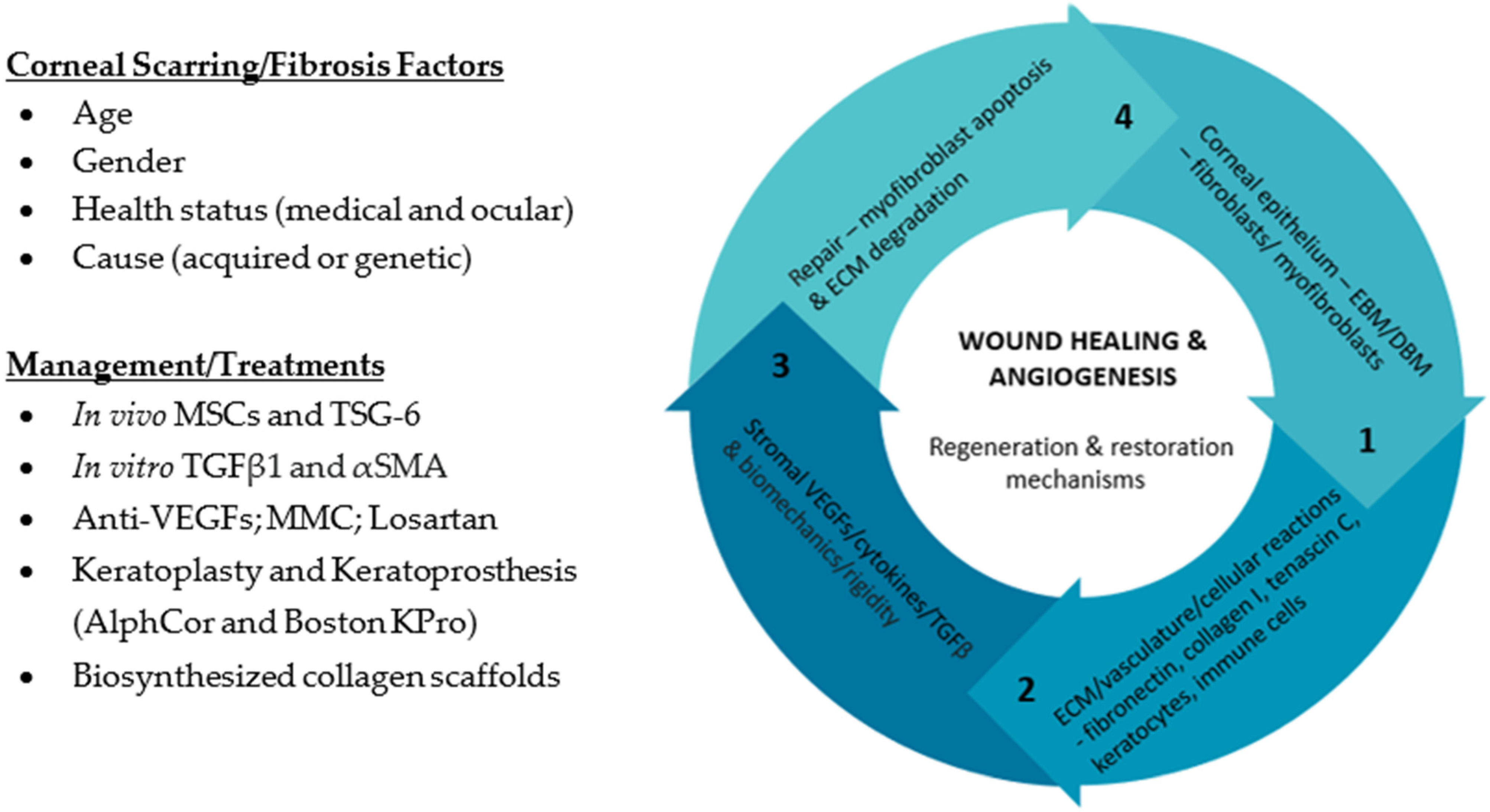
2. What Is the Clinical Relevance of Corneal Scarring/Fibrosis?
3. How Does Corneal Stromal Cells Contribute to Corneal Fibrosis/Scarring?
3.1. Human Corneal Keratocytes (HCKs)
3.2. Human Corneal Fibroblasts (HCFs)
3.3. Human Corneal Myofibroblasts (HCMs)
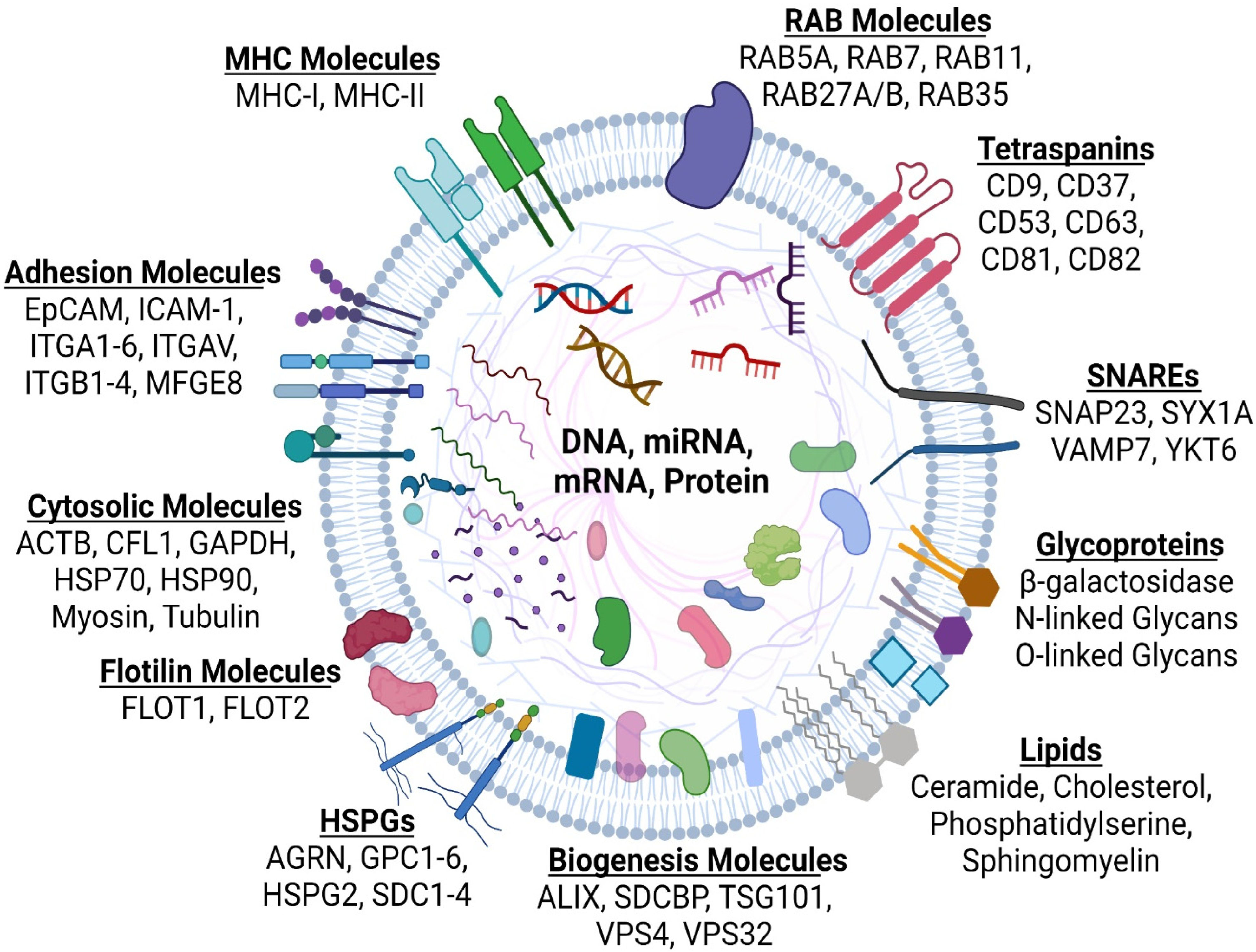
4. What Are Extracellular Vesicles (EVs)?
4.1. Microvesicles
4.2. Exosomes
4.3. Exomeres and Supermeres
5. What Roles Do Extracellular Vesicles Play in Corneal Fibrosis/Scarring?
6. Can We Utilize Extracellular Vesicles in Treatments Regiments in Corneal Fibrosis/Scarring?
7. Summary Statement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fini, M.E.; Stramer, B. How the Cornea Heals: Cornea-specific repair mechanisms affecting surgical outcomes. Cornea 2005, 24, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Lin, M.P.; Ford, B.M.R.; Santhiago, M.R.; Singh, V.; Grossman, G.H.; Agrawal, M.V.; Roy, A.S.; Butler, M.R.S.; Dupps, W.J.; et al. Biological and Biomechanical Responses to Traditional Epithelium-Off and Transepithelial Riboflavin-UVA CXL Techniques in Rabbits. J. Refract. Surg. 2013, 29, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M. Corneal collagen—Its role in maintaining corneal shape and transparency. Biophys. Rev. 2009, 1, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadea, L.; Maraone, G.; Verboschi, F.; Vingolo, E.M.; Tognetto, D. Effect of corneal light scatter on vision: A review of the literature. Int. J. Ophthalmol. 2016, 9, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Hutcheon, A.E.; Choi, R.; Hong, J.; Lee, J.; Mohan, R.R.; Ambrósio, R.; Zieske, J.; Wilson, S. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 2003, 76, 71–87. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.L.; El Haj, A.J.; Yang, Y. Control of Scar Tissue Formation in the Cornea: Strategies in Clinical and Corneal Tissue Engineering. J. Funct. Biomater. 2012, 3, 642–687. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.E. Corneal myofibroblasts and fibrosis. Exp. Eye Res. 2020, 201, 108272. [Google Scholar] [CrossRef]
- Sigismund, S.; Confalonieri, S.; Ciliberto, A.; Polo, S.; Scita, G.; Di Fiore, P.P. Endocytosis and signaling: Cell logistics shape the eukaryotic cell plan. Physiol. Rev. 2012, 92, 273–366. [Google Scholar] [CrossRef] [Green Version]
- Han, K.-Y.; Dugas-Ford, J.; Seiki, M.; Chang, J.-H.; Azar, D.T. Evidence for the involvement of MMP14 in MMP2 processing and recruitment in exosomes of corneal fibroblasts. Investig. Opthalmol. Vis. Sci. 2014, 56, 5323–5329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.-Y.; Tran, J.A.; Chang, J.-H.; Azar, D.T.; Zieske, J. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Sci. Rep. 2017, 7, srep40548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci. Rep. 2018, 8, 15173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, R.C.; Katzmann, D.J. Biogenesis and Function of Multivesicular Bodies. Annu. Rev. Cell Dev. Biol. 2007, 23, 519–547. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Webber, J.; Yeung, V.; Clayton, A. Extracellular vesicles as modulators of the cancer microenvironment. Semin. Cell Dev. Biol. 2015, 40, 27–34. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [Green Version]
- McKay, T.B.; Hutcheon, A.E.K.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells 2020, 9, 1080. [Google Scholar] [CrossRef]
- Zieske, J.D.; Hutcheon, A.E.K.; Guo, X. Extracellular Vesicles and Cell–Cell Communication in the Cornea. Anat. Rec. 2020, 303, 1727–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, T.B.; Yeung, V.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D.; Ciolino, J.B. Extracellular Vesicles in the Cornea: Insights from Other Tissues. Anal. Cell. Pathol. 2021, 2021, e9983900. [Google Scholar] [CrossRef] [PubMed]
- Yeung, V.; Zhang, T.C.; Yuan, L.; Parekh, M.; Cortinas, J.A.; Delavogia, E.; Hutcheon, A.E.K.; Guo, X.; Ciolino, J.B. Extracellular Vesicles Secreted by Corneal Myofibroblasts Promote Corneal Epithelial Cell Migration. Int. J. Mol. Sci. 2022, 23, 3136. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Krishna, P.V.; Stratis, A.K.; Gopinathan, U. The value of corneal transplantation in reducing blindness. Eye 2005, 19, 1106–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Girolamo, N.; Chui, J.; Coroneo, M.T.; Wakefield, D. Pathogenesis of pterygia: Role of cytokines, growth factors, and matrix metalloproteinases. Prog. Retin. Eye Res. 2004, 23, 195–228. [Google Scholar] [CrossRef]
- Biber, H. Ueber Einige Seltene Hornhauterkrankungen: Die Oberflaechlichegittrige Keratitis. Ph.D. Thesis, A Diggelmann, Zürich, Switzerland, 1890. [Google Scholar]
- Groenouw, A. Knoetchenfoermige Hornhauttruebungen (Noduli corneae). Arch. Augenheilkd. 1890, 21, 281–289. [Google Scholar]
- Huang, Y.-X.; Li, Q.-H. An active artificial cornea with the function of inducing new corneal tissue generation in vivo—A new approach to corneal tissue engineering. Biomed. Mater. 2007, 2, S121–S125. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.S.; Møller, H.U.; Aldave, A.J.; Seitz, B.; Bredrup, C.; Kivelä, T.; Munier, F.L.; Rapuano, C.J.; Nischal, K.K.; Kim, E.K.; et al. IC3D Classification of Corneal Dystrophies—Edition 2. Cornea 2015, 34, 117–159. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Investig. 2007, 117, 576–586. [Google Scholar] [CrossRef]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S. Coordinated Modulation of Corneal Scarring by the Epithelial Basement Membrane and Descemet’s Basement Membrane. J. Refract. Surg. 2019, 35, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.M.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The Corneal Epithelial Basement Membrane: Structure, Function, and Disease. Investig. Opthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.; Hsieh, T.-L.; Ji, A.T.-Q.; Hsu, W.-T.; Liu, C.-Y.; Lee, O.K.-S.; Ho, J.H.-C. Stromal Tissue Rigidity Promotes Mesenchymal Stem Cell-Mediated Corneal Wound Healing Through the Transforming Growth Factor β Signaling Pathway. Stem Cells 2016, 34, 2525–2535. [Google Scholar] [CrossRef] [Green Version]
- Raghunathan, V.K.; Thomasy, S.M.; Strøm, P.; Yañez-Soto, B.; Garland, S.P.; Sermeno, J.; Reilly, C.M.; Murphy, C.J. Tissue and cellular biomechanics during corneal wound injury and repair. Acta Biomater. 2017, 58, 291–301. [Google Scholar] [CrossRef]
- Esquenazi, S.; He, J.; Li, N.; Bazan, H.E.P. Immunofluorescence of Rabbit Corneas After Collagen Cross-Linking Treatment With Riboflavin and Ultraviolet A. Cornea 2010, 29, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Skuta, G.; Cantor, L.; Weiss, J. Refractive Surgery, Section 13 Basic and Clinical Science Course; American Academy of Opthamology: San Fransisco, CA, USA, 2011. [Google Scholar]
- Saika, S.; Yamanaka, O.; Sumioka, T.; Miyamoto, T.; Miyazaki, K.-I.; Okada, Y.; Kitano, A.; Shirai, K.; Tanaka, S.-I.; Ikeda, K. Fibrotic disorders in the eye: Targets of gene therapy. Prog. Retin. Eye Res. 2008, 27, 177–196. [Google Scholar] [CrossRef]
- McKleroy, W.; Lee, T.-H.; Atabai, K. Always cleave up your mess: Targeting collagen degradation to treat tissue fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L709–L721. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.E.; Medeiros, C.S.; Santhiago, M.R. Pathophysiology of Corneal Scarring in Persistent Epithelial Defects After PRK and Other Corneal Injuries. J. Refract. Surg. 2018, 34, 59–64. [Google Scholar] [CrossRef]
- Hindman, H.B.; DeMagistris, M.; Callan, C.; McDaniel, T.; Bubel, T.; Huxlin, K.R. Impact of topical anti-fibrotics on corneal nerve regeneration in vivo. Exp. Eye Res. 2019, 181, 49–60. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Marino, G.K.; Lassance, L.; Shanmugapriya, T.; Santhiago, M.R.; Wilson, S. The Impact of Photorefractive Keratectomy and Mitomycin C on Corneal Nerves and Their Regeneration. J. Refract. Surg. 2018, 34, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Taneri, S.; Koch, J.M.; Melki, S.; Azar, D.T. Mitomycin-C assisted photorefractive keratectomy in the treatment of buttonholed laser in situ keratomileusis flaps associated with epithelial ingrowth. J. Cataract Refract. Surg. 2005, 31, 2026–2030. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, M.; Kirwan, C. Laser epithelial keratomileusis in 2010—A review. Clin. Exp. Ophthalmol. 2010, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Katzman, L.R.; Jeng, B.H. Management strategies for persistent epithelial defects of the cornea. Saudi J. Ophthalmol. 2014, 28, 168–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, G.K.; Santhiago, M.R.; Santhanam, A.; Lassance, L.; Thangavadivel, S.; Medeiros, C.S.; Bose, K.; Tam, K.P.; Wilson, S.E. Epithelial basement membrane injury and regeneration modulates corneal fibrosis after pseudomonas corneal ulcers in rabbits. Exp. Eye Res. 2017, 161, 101–105. [Google Scholar] [CrossRef]
- Mittal, S.K.; Omoto, M.; Amouzegar, A.; Sahu, A.; Rezazadeh, A.; Katikireddy, K.R.; Shah, D.I.; Sahu, S.K.; Chauhan, S.K. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Rep. 2016, 7, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Roddy, G.W.; Oh, J.Y.; Lee, R.H.; Bartosh, T.J.; Ylostalo, J.; Coble, K.; Rosa, R.H., Jr.; Prockop, D.J. Action at a Distance: Systemically Administered Adult Stem/Progenitor Cells (MSCs) Reduce Inflammatory Damage to the Cornea Without Engraftment and Primarily by Secretion of TNF-α Stimulated Gene/Protein 6. Stem Cells 2011, 29, 1572–1579. [Google Scholar] [CrossRef]
- Sriram, S.; Tran, J.A.; Guo, X.; Hutcheon, A.E.K.; Lei, H.; Kazlauskas, A.; Zieske, J.D. PDGFRα Is a Key Regulator of T1 and T3′s Differential Effect on SMA Expression in Human Corneal Fibroblasts. Investig. Opthalmol. Vis. Sci. 2017, 58, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaj, A.S.; Appukuttan, B.; Wilmarth, P.A.; Pan, Y.; Stempel, A.J.; Chipps, T.J.; Benedetti, E.E.; Zamora, D.O.; Choi, D.; David, L.L.; et al. Role of the retinal vascular endothelial cell in ocular disease. Prog. Retin. Eye Res. 2013, 32, 102–180. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.K.; Zhang, X.; Uehara, H.; Young, J.R.; Archer, B.; Ambati, B. Vascular Endothelial Growth Factor Receptor 1 Morpholino Increases Graft Survival in a Murine Penetrating Keratoplasty Model. Investig. Opthalmol. Vis. Sci. 2012, 53, 8458–8471. [Google Scholar] [CrossRef]
- Majmudar, P.A.; Schallhorn, S.C.; Cason, J.B.; Donaldson, K.E.; Kymionis, G.D.; Shtein, R.M.; Verity, S.M.; Farjo, A.A. Mitomycin-C in Corneal Surface Excimer Laser Ablation Techniques. Ophthalmology 2015, 122, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L.P.; Hilgert, G.S.; Shiju, T.M.; Murillo, S.E.; Santhiago, M.R.; Wilson, S.E. Topical losartan inhibits corneal scarring fibrosis and collagen type IV deposition after Descemet’s membrane-endothelial excision in rabbits. Exp. Eye Res. 2022, 216, 108940. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.F.; Ayres, B.D.; Hammersmith, K.M.; Rapuano, C.J.; Laibson, P.R.; Myers, J.S.; Jin, Y.-P.; Cohen, E.J. Boston Keratoprosthesis Outcomes and Complications. Cornea 2009, 28, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018, 22, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Mallone, F.; Costi, R.; Marenco, M.; Plateroti, R.; Minni, A.; Attanasio, G.; Artico, M.; Lambiase, A. Understanding Drivers of Ocular Fibrosis: Current and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 11748. [Google Scholar] [CrossRef]
- Willoughby, C.; Ponzin, D.; Ferrari, S.; Lobo, A.; Landau, K.; Omidi, Y. Anatomy and physiology of the human eye: Effects of mucopolysaccharidoses disease on structure and function—A review. Clin. Exp. Ophthalmol. 2010, 38, 2–11. [Google Scholar] [CrossRef]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef] [Green Version]
- Joseph, A.; Hossain, P.; Jham, S.; Jones, R.E.; Tighe, P.; McIntosh, R.S.; Dua, H.S. Expression of CD34 and L-Selectin on Human Corneal Keratocytes. Investig. Opthalmol. Vis. Sci. 2003, 44, 4689–4692. [Google Scholar] [CrossRef] [Green Version]
- Poole, C.; Brookes, N.; Clover, G. Keratocyte networks visualised in the living cornea using vital dyes. J. Cell Sci. 1993, 106, 685–691. [Google Scholar] [CrossRef]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef] [Green Version]
- Yeung, V.; Sriram, S.; Tran, J.A.; Guo, X.; Hutcheon, A.E.K.; Zieske, J.D.; Karamichos, D.; Ciolino, J.B. FAK Inhibition Attenuates Corneal Fibroblast Differentiation In Vitro. Biomolecules 2021, 11, 1682. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Ishida, W.; Fukushima, A.; Nishida, T. Corneal Fibroblasts as Sentinel Cells and Local Immune Modulators in Infectious Keratitis. Int. J. Mol. Sci. 2017, 18, 1831. [Google Scholar] [CrossRef] [PubMed]
- Akpek, E.K.; Gottsch, J.D. Immune defense at the ocular surface. Eye 2003, 17, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Foulsham, W.; Coco, G.; Amouzegar, A.; Chauhan, S.K.; Dana, R. When Clarity Is Crucial: Regulating Ocular Surface Immunity. Trends Immunol. 2018, 39, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saika, S. TGFβ pathobiology in the eye. Lab. Investig. 2005, 86, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Stein, J.M.; Luzio, J.P. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem. J. 1991, 274, 381–386. [Google Scholar] [CrossRef]
- Sims, P.J.; Faioni, E.M.; Wiedmer, T.; Shattil, S.J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 1988, 263, 18205–18212. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Meehan, B.; Rak, J.; Di Vizio, D. Oncosomes—Large and small: What are they, where they came from? J. Extracell. Vesicles 2016, 5, 33109. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Satta, N.; Toti, F.; Feugeas, O.; Bohbot, A.; Dachary-Prigent, J.; Eschwège, V.; Hedman, H.; Freyssinet, J.M. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J. Immunol. 1994, 153, 3245–3255. [Google Scholar] [PubMed]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2016, 8, 220–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Bevers, E.M.; Williamson, P.L. Getting to the Outer Leaflet: Physiology of Phosphatidylserine Exposure at the Plasma Membrane. Physiol. Rev. 2016, 96, 605–645. [Google Scholar] [CrossRef]
- Ståhl, A.-L.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2017, 34, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.D.T.; Alves, L.R. Extracellular Vesicles in Viral Infections: Two Sides of the Same Coin? Front. Cell. Infect. Microbiol. 2020, 10, 593170. [Google Scholar] [CrossRef]
- Piccin, A.; Murphy, W.G.; Smith, O.P. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev. 2007, 21, 157–171. [Google Scholar] [CrossRef]
- del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; Lópezet, J.A. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef]
- Shen, B.; Fang, Y.; Wu, N.; Gould, S.J. Biogenesis of the Posterior Pole Is Mediated by the Exosome/Microvesicle Protein-sorting Pathway. J. Biol. Chem. 2011, 286, 44162–44176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-P.; Lin, Z.-X.; Jiang, X.-Y.; Yu, X.-Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021, 17, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enkavi, G.; Javanainen, M.; Kulig, W.; Róg, T.; Vattulainen, I. Multiscale Simulations of Biological Membranes: The Challenge To Understand Biological Phenomena in a Living Substance. Chem. Rev. 2019, 119, 5607–5774. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Shephard, A.P.; Yeung, V.; Clayton, A.; Webber, J.P. Prostate cancer exosomes as modulators of the tumor microenvironment. J. Cancer Metastasis Treat. 2017, 3, 288. [Google Scholar] [CrossRef]
- Yeung, V.; Webber, J.P.; Dunlop, E.A.; Morgan, H.; Hutton, J.; Gurney, M.; Jones, E.; Falcon-Perez, J.; Tabi, Z.; Errington, R.; et al. Rab35-dependent extracellular nanovesicles are required for induction of tumour supporting stroma. Nanoscale 2018, 10, 8547–8559. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2017, 39, 501–513. [Google Scholar] [CrossRef]
- Yeung, V.; Willis, G.R.; Taglauer, E.; Mitsialis, S.A.; Kourembanas, S. Paving the Road for Mesenchymal Stem Cell-Derived Exosome Therapy in Bronchopulmonary Dysplasia and Pulmonary Hypertension. Stem Cell-Based Ther. Lung Dis. 2019, 131–152. [Google Scholar] [CrossRef]
- Odintsova, E.; van Niel, G.; Conjeaud, H.; Raposo, G.; Iwamoto, R.; Mekada, E.; Berditchevski, F. Metastasis Suppressor Tetraspanin CD82/KAI1 Regulates Ubiquitylation of Epidermal Growth Factor Receptor. J. Biol. Chem. 2013, 288, 26323–26334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Buschow, S.I.; Nolte-‘t Hoen, E.N.M.N.; Van Niel, G.; Pols, M.S.; ten Broeke, T.T.; Lauwen, M.; Ossendorp, F.; Melief, C.J.M.; Raposo, G.; Wubbolts, R.; et al. MHC II in dendritic cells is targeted to lysosomes or t cell-induced exosomes via distinct multivesicular body pathways. Traffic 2009, 10, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome release of β-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, S.N.; Conlon, M.M.; Rider, M.A.; Brownstein, N.; Meckes, D.G. Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J. Extracell. Vesicles 2016, 5, 31295. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Hays, F.A.; Tomasek, J.J.; Benyajati, S.; Zhang, X.A. Tetraspanin CD82 interaction with cholesterol promotes extracellular vesicle–mediated release of ezrin to inhibit tumour cell movement. J. Extracell. Vesicles 2019, 9, 1692417. [Google Scholar] [CrossRef]
- Imjeti, N.S.; Menck, K.; Egea-Jimenez, A.L.; Lecointre, C.; Lembo, F.; Bouguenina, H.; Badache, A.; Ghossoub, R.; David, G.; Roche, S.; et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2017, 114, 12495–12500. [Google Scholar] [CrossRef] [Green Version]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; DeGeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Fader, C.M.; Sánchez, D.G.; Mestre, M.B.; Colombo, M.I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: Two v-SNARE proteins involved in specific steps of the au-topha-gy/multivesicular body pathways. Biochim. Biophys. Acta 2009, 1793, 1901–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.-S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Li, Y.; Zhang, H.; Hu, J.; Liao, J.; Su, Y.; Li, Q.; Chen, B.; Li, C.; Wang, Z.; et al. exoRBase 2.0: An atlas of mRNA, lncRNA and circRNA in extracellular vesicles from human biofluids. Nucleic Acids Res. 2021, 50, D118–D128. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [Green Version]
- Egea-Jimenez, A.L.; Zimmermann, P. Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles. J. Lipid Res. 2018, 59, 1554–1560. [Google Scholar] [CrossRef] [Green Version]
- Paolillo, M.; Schinelli, S. Integrins and Exosomes, a Dangerous Liaison in Cancer Progression. Cancers 2017, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- DeRita, R.M.; Sayeed, A.; Garcia, V.; Krishn, S.R.; Shields, C.D.; Sarker, S.; Friedman, A.; McCue, P.; Molugu, S.K.; Rodeck, U.; et al. Tumor-Derived Extracellular Vesicles Require β1 Integrins to Promote Anchorage-Independent Growth. iScience 2019, 14, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, S.; Santos, S.G.; Campbell, E.C.; Nimmo, A.M.S.; Botting, C.; Prescott, A.; Antoniou, A.N.; Powis, S.J. Novel MHC Class I Structures on Exosomes. J. Immunol. 2009, 183, 1884–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebrasseur, N. MHC and antigen in exosomes. J. Cell Biol. 2007, 179, 4. [Google Scholar] [CrossRef] [Green Version]
- Hakulinen, J.; Sankkila, L.; Sugiyama, N.; Lehti, K.; Keski-Oja, J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J. Cell. Biochem. 2008, 105, 1211–1218. [Google Scholar] [CrossRef]
- Han, K.-Y.; Chang, J.-H.; Azar, D.T. MMP14-Containing Exosomes Cleave VEGFR1 and Promote VEGFA-Induced Migration and Proliferation of Vascular Endothelial Cells. Investig. Opthalmol. Vis. Sci. 2019, 60, 2321–2329. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.; Yang, Y.-P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell Biol. 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- Van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Zieske, J.; Higashijima, S.C.; Spurr-Michaud, S.J.; Gipson, I.K. Biosynthetic responses of the rabbit cornea to a keratectomy wound. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1668–1677. [Google Scholar]
- Matsubara, M.; Zieske, J.; Fini, M.E. Mechanism of basement membrane dissolution preceding corneal ulceration. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3221–3237. [Google Scholar]
- Ramos, T.; Parekh, M.; Kaye, S.B.; Ahmad, S. Epithelial Cell-Derived Extracellular Vesicles Trigger the Differentiation of Two Epithelial Cell Lines. Int. J. Mol. Sci. 2022, 23, 1718. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Lee, P.; Lu, C.; Liu, Y.; Wang, S.; Liu, C.; Chang, Y.; Chen, Y.; Su, C.; Li, C.; et al. Thrombospondin 1-induced exosomal proteins attenuate hypoxia-induced paraptosis in corneal epithelial cells and promote wound healing. FASEB J. 2020, 35, e21200. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.; Guschina, I.A.; Yeung, V.; Clayton, A.; Draman, M.S.; Von Ruhland, C.; Ludgate, M.; James, P.E.; Rees, D.A. Characterisation of adipocyte-derived extracellular vesicles released pre- and post-adipogenesis. J. Extracell. Vesicles 2015, 4, 29159. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.M.; Crisostomo, P.; Lahm, T.; Markel, T.; Herring, C.; Wang, M.; Meldrum, K.K.; Lillemoe, K.D.; Meldrum, D.R. Mesenchymal Stem Cells Attenuate Hypoxic Pulmonary Vasoconstriction by a Paracrine Mechanism. J. Surg. Res. 2007, 143, 281–285. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Reis, M.; Yeung, V.; Liu, X.; Ericsson, M.; Andrews, N.A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stromal cell-derived small extracellular vesicles restore lung architecture and improve exercise capacity in a model of neonatal hyperoxia-induced lung injury. J. Extracell. Vesicles 2020, 9, 1790874. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, A.; Willis, G.R.; Yeung, V.; Reis, M.; Liu, X.; Mitsialis, S.A.; Kourembanas, S. Therapeutic Effects of Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles in Oxygen-Induced Multi-Organ Disease: A Developmental Perspective. Front. Cell Dev. Biol. 2021, 9, 647025. [Google Scholar] [CrossRef]
- Reis, M.; Willis, G.R.; Fernandez-Gonzalez, A.; Yeung, V.; Taglauer, E.; Magaletta, M.; Parsons, T.; Derr, A.; Liu, X.; Maehr, R.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Restore Thymic Architecture and T Cell Function Disrupted by Neonatal Hyperoxia. Front. Immunol. 2021, 12, 640595. [Google Scholar] [CrossRef]
- Taglauer, E.S.; Fernandez-Gonzalez, A.; Willis, G.R.; Reis, M.; Yeung, V.; Liu, X.; Prince, L.S.; Mitsialis, S.A.; Kourembanas, S. Antenatal Mesenchymal Stromal Cell Extracellular Vesicle Therapy Prevents Preeclamptic Lung Injury in Mice. Am. J. Respir. Cell Mol. Biol. 2022, 66, 86–95. [Google Scholar] [CrossRef]
- Taglauer, E.S.; Fernandez-Gonzalez, A.; Willis, G.R.; Reis, M.; Yeung, V.; Liu, X.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stromal cell-derived extracellular vesicle therapy prevents preeclamptic physiology through intrauterine immunomodulation†. Biol. Reprod. 2020, 104, 457–467. [Google Scholar] [CrossRef]
- Willis, G.R.; Reis, M.; Gheinani, A.H.; Fernandez-Gonzalez, A.; Taglauer, E.S.; Yeung, V.; Liu, X.; Ericsson, M.; Haas, E.; Mitsialis, S.A.; et al. Extracellular Vesicles Protect the Neonatal Lung from Hyperoxic Injury through the Epigenetic and Transcriptomic Reprogramming of Myeloid Cells. Am. J. Respir. Crit. Care Med. 2021, 204, 1418–1432. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; El Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2008, 1, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curley, G.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 2011, 67, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Holtzman, J.; Lee, H. Emerging role of extracellular vesicles in the respiratory system. Exp. Mol. Med. 2020, 52, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, P.; Xu, J.; Liu, Y.; Li, H.; Wang, L.; Di, G. hADSCs derived extracellular vesicles inhibit NLRP3inflammasome activation and dry eye. Sci. Rep. 2020, 10, 14521. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yin, J.; Hao, L.; Liu, X.; Shi, Q.; Diao, Y.; Yu, G.; Liu, L.; Chen, J.; Zhong, J. Exosomes From Human Umbilical Cord Mesenchymal Stem Cells Treat Corneal Injury via Autophagy Activation. Front. Bioeng. Biotechnol. 2022, 10, 879192. [Google Scholar] [CrossRef]
- Shen, T.; Zheng, Q.-Q.; Shen, J.; Li, Q.-S.; Song, X.-H.; Luo, H.-B.; Hong, C.-Y.; Yao, K. Effects of Adipose-derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin. Med. J. 2018, 131, 704–712. [Google Scholar] [CrossRef]
- Escandon, P.; Liu, A.; Nicholas, S.E.; Khan, A.; Riaz, K.M.; Karamichos, D. Unravelling Novel Roles of Salivary Exosomes in the Regulation of Human Corneal Stromal Cell Migration and Wound Healing. Int. J. Mol. Sci. 2022, 23, 4330. [Google Scholar] [CrossRef]
- Liu, M.M.; Tuo, J.; Chan, C.-C. Gene therapy for ocular diseases. Br. J. Ophthalmol. 2010, 95, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhang, X.; Tang, Y.; Li, S.; Chen, J. Progress on ocular siRNA gene-silencing therapy and drug delivery systems. Fundam. Clin. Pharmacol. 2020, 35, 4–24. [Google Scholar] [CrossRef]
- Yu, Z.; Efstathiou, N.E.; Correa, V.S.M.C.; Chen, X.; Ishihara, K.; Iesato, Y.; Narimatsu, T.; Ntentakis, D.; Chen, Y.; Vavvas, D.G. Receptor interacting protein 3 kinase, not 1 kinase, through MLKL-mediated necroptosis is involved in UVA-induced corneal endothelium cell death. Cell Death Discov. 2021, 7, 366. [Google Scholar] [CrossRef] [PubMed]
- Awwad, S.; Ahmed, A.H.A.M.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, W.; Vavvas, D.G.; Zhang, F.; She, H.; Zhou, H.; Li, L.; Huang, Y.; Ntentakis, D.P.; Shi, X. Incidence of Endophthalmitis after Intravitreal Anti-Vascular Endothelial Growth Factor Injections in an Operating Room in China. J. Ophthalmol. 2020, 2020, 5163484. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-J.; Hsieh, C.-M.; Nepali, K.; Liou, J.-P. Ocular Disease Therapeutics: Design and Delivery of Drugs for Diseases of the Eye. J. Med. Chem. 2020, 63, 10533–10593. [Google Scholar] [CrossRef] [PubMed]
- Mcmonnies, C.W. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin. Exp. Optom. 2015, 98, 122–125. [Google Scholar] [CrossRef]
- Ntentaki, A.M.; Delavogia, E.; Kalomoiris, L.; Venieri, D.; Arkadopoulos, N.; Kalogerakis, N.; Ntentakis, P.D.P.; Bs, D.V.; Kalogerakis, P.N. Dissolved oxygen technologies as a novel strategy for non-healing wounds: A critical review. Wound Repair Regen. 2021, 29, 1062–1079. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, X.; Lv, Y.; Zheng, C.; Dong, Y.; Dou, G.; Zhu, B.; Liu, A.; Wang, W.; Zhou, J.; et al. Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res. Ther. 2020, 11, 507. [Google Scholar] [CrossRef]
- Shi, R.; Jin, Y.; Hu, W.; Lian, W.; Cao, C.; Han, S.; Zhao, S.; Yuan, H.; Yang, X.; Shi, J.; et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am. J. Physiol. Cell Physiol. 2020, 318, C848–C856. [Google Scholar] [CrossRef]
- Carnino, J.M.; Lee, H.; Jin, Y. Isolation and characterization of extracellular vesicles from Broncho-alveolar lavage fluid: A review and comparison of different methods. Respir. Res. 2019, 20, 240. [Google Scholar] [CrossRef] [Green Version]
- Rahbarghazi, R.; Jabbari, N.; Sani, N.A.; Asghari, R.; Salimi, L.; Kalashani, S.A.; Feghhi, M.; Etemadi, T.; Akbariazar, E.; Mahmoudi, M.; et al. Tumor-derived extracellular vesicles: Reliable tools for Cancer diagnosis and clinical applications. Cell Commun. Signal. 2019, 17, 73. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.R.; Oropallo, A.R.; Grande, D.A.; Kirsner, R.S.; Badiavas, E.V. Extracellular Vesicles as Therapeutic Tools for the Treatment of Chronic Wounds. Pharmaceutics 2021, 13, 1543. [Google Scholar] [CrossRef] [PubMed]
- Pratheesh, M.D.; Gade, N.E.; Nath, A.; Dubey, P.K.; Sivanarayanan, T.B.; Madhu, D.N.; Sreekumar, T.R.; Amarpal; Saikumar, G.; Sharma, G.T. Evaluation of persistence and distribution of intra-dermally administered PKH26 labelled goat bone marrow derived mesenchymal stem cells in cutaneous wound healing model. Cytotechnology 2017, 69, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Cheng, S.; Xi, Z.; Chen, G.; Liu, K.; Ma, R.; Zhou, C. Extracellular vesicle-carried microRNA-27b derived from mesenchymal stem cells accelerates cutaneous wound healing via E3 ubiquitin ligase ITCH. J. Cell. Mol. Med. 2020, 24, 11254–11271. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, Y.; Shi, L.; Li, B.; Li, J.; Wei, Z.; Lv, H.; Wu, L.; Zhang, H.; Yang, B.; et al. Magnetic targeting enhances the cutaneous wound-healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J. Nanobiotechnol. 2020, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Shojaati, G.; Khandaker, I.; Funderburgh, M.L.; Mann, M.M.; Basu, R.; Stolz, D.B.; Geary, M.L.; Dos Santos, A.; Deng, S.X.; Funderburgh, J.L. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl. Med. 2019, 8, 1192–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes Derived From Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Investig. Opthalmol. Vis. Sci. 2019, 60, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, S.X.; Dos Santos, A.; Gee, S. Therapeutic Potential of Extracellular Vesicles for the Treatment of Corneal Injuries and Scars. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef]
- Bosch, S.; De Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.-M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef] [Green Version]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef]
- Frank, J.; Richter, M.; De Rossi, C.; Lehr, C.-M.; Fuhrmann, K.; Fuhrmann, G. Extracellular vesicles protect glucuronidase model enzymes during freeze-drying. Sci. Rep. 2018, 8, 12377. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.; Adamiak, M.; Mathiyalagan, P.; Kenneweg, F.; Kafert-Kasting, S.; Thum, T. Therapeutic and Diagnostic Translation of Extracellular Vesicles in Cardiovascular Diseases. Circulation 2021, 143, 1426–1449. [Google Scholar] [CrossRef]

| Type | Cause | Signs | Symptoms | Duration | Management |
|---|---|---|---|---|---|
| Epithelial (Basement Membrane) | Degenerative; trauma | Abnormal basal epithelial cell adhesion | Asymptomatic; Pain; Vision Impairment; Monocular Diplopia (Ghost Images) | Fluctuates | Saline; Ointment; Antibiotic/Antifungal |
| Endothelial (Fuchs) | Mostly without known inheritance. Proposed to have autosomal dominant mutations | Guttae; Edema; Pigment dusting; Bullous keratopathy; Vascularization | Fluctuating vision with progressive loss; Pain; Photophobia; Epiphora; Corneal haze and curvature change | Progressive | Saline; Ointment; Contact lenses; No Cure |
| Trauma | Accidents; Injuries; Burns | Cornea rupture; Endophthalmitis; Hyphaemas; Ulcers | Pain; Vision impairment may result in blindness | Fluctuates | Antibiotic/Antifungal; Corneal transplant and keratoplasty |
| Drugs/Infection (Stevens–Johnson Syndrome) | Drugs (NSAIDs; sulphonamides); Infection (HSV) | Bullous; Epidermal necrolysis | Vision impairment may result in blindness | Fluctuates; Progressive | Cease drug source; Immunosuppression; Corneal transplant and keratoplasty |
| Infection (Trachoma) | Bacterial (Chlamydia) | Entropion; Trichiasis; Vascularization | Vision impairment may result in blindness | Fluctuates; Progressive | Antibiotics; Corneal transplant and keratoplasty |
| Infection (Leprosy) | Bacteria | Cataract; Keratitis; Ulcers; Uveitis; Vascularization | Vision impairment may result in blindness | Progressive | Combination drug therapy |
| References | Key Study Findings | Biological Model | Vesicle Source | Vesicle Methods |
|---|---|---|---|---|
| Han et al., 2017 [12] | Human/mouse corneal epithelial EVs induced endothelial cell proliferation and ex vivo aortic ring sprouting. | In vitro: corneal wound healing /neovascularization. | Human/mouse corneal epithelial cell line. | Total exosome isolation reagent + differential ultra- centrifugation |
| Samaeekia et al., 2018 [19] | Human corneal MSC-EVs can accelerate epithelial cell migration and proliferation in vitro and wound healing in vivo. | In vitro: corneal epithelial wound healing. In vivo: corneal debridement mouse model. | Human corneal MSCs derived from human cadaver corneas. | Differential ultra- centrifugation |
| McKay et al., 2020 [20] | Human corneal epithelial-EVs triggers corneal fibroblast to myofibroblast differentiation. | In vitro: interplay between epithelial EVs and corneal stroma. | Human corneal epithelial cell line. | Total exosome isolation reagent + differential ultracentrifugation |
| Lai et al., 2021 [125] | Human corneal epithelial-EVs treated with thrombospondin-1 (TSP-1) protected hypoxia-induced paraptosis in epithelial cells and promoted wound healing. | In vitro: interplay between corneal epithelial EVs (with TSP-1) and hypoxia-induced epithelial cells. | Human corneal epithelial cell line. | Differential ultracentrifugation |
| Yeung et al., 2022 [23] | Human corneal myofibroblast EVs promote epithelial cell migration, proliferation, and motility. | In vitro: interplay with corneal stromal EVs and epithelial cells. | Human primary corneal fibroblast /myofibroblast. | Differential ultracentrifugation |
| Ramos et al., 2022 [124] | Human corneal epithelial EVs influences transdifferentiation of human conjunctival/epithelial cells | In vitro: interplay with epithelial EVs and conjunctival/epithelial cell. | Human corneal epithelial cell line. | Differential ultracentrifugation |
| Ma et al., 2022 [138] | Umbilical cord MSC-EVs in combination with an autophagy activator alleviated corneal epithelial defects and stromal opacity in vivo. | In vitro: interplay of MSC-EVs on epithelial cells. In vivo: corneal debridement mouse model. | Human primary umbilical cord derived MSCs. | Differential ultracentrifugation |
| Escandon et al., 2022 [140] | Human salivary EVs modulated human corneal stromal cell migration and wound healing. | In vitro: interplay of salivary EVs on primary corneal stromal cells. | Human saliva from health donors. | Human biofluids characterized by ExoView® R100 platform |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeung, V.; Boychev, N.; Farhat, W.; Ntentakis, D.P.; Hutcheon, A.E.K.; Ross, A.E.; Ciolino, J.B. Extracellular Vesicles in Corneal Fibrosis/Scarring. Int. J. Mol. Sci. 2022, 23, 5921. https://doi.org/10.3390/ijms23115921
Yeung V, Boychev N, Farhat W, Ntentakis DP, Hutcheon AEK, Ross AE, Ciolino JB. Extracellular Vesicles in Corneal Fibrosis/Scarring. International Journal of Molecular Sciences. 2022; 23(11):5921. https://doi.org/10.3390/ijms23115921
Chicago/Turabian StyleYeung, Vincent, Nikolay Boychev, Wissam Farhat, Dimitrios P. Ntentakis, Audrey E. K. Hutcheon, Amy E. Ross, and Joseph B. Ciolino. 2022. "Extracellular Vesicles in Corneal Fibrosis/Scarring" International Journal of Molecular Sciences 23, no. 11: 5921. https://doi.org/10.3390/ijms23115921






