Structural Rearrangements of Carbonic Anhydrase Entrapped in Sol-Gel Magnetite Determined by ATR–FTIR Spectroscopy
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAB | Carbonic anhydrase from bovine erythrocytes |
| NPs | nanparticles |
| DCS | dynamic scanning calorimetry |
| FTIR | Fourier-transform infrared spectroscopy |
| ATR | Attenuated total reflection |
| pNPA | para-nitrophenylacetate |
References
- Cipolatti, E.P.; Silva, M.J.A.; Klein, M.; Feddern, V.; Feltes, M.M.C.; Oliveira, J.V.; Ninow, J.L.; De Oliveira, D. Current status and trends in enzymatic nanoimmobilization. J. Mol. Catal. Enzym. 2014, 99, 56–67. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Pandey, L.M.; Chandra, P. Nanoengineered material based biosensing electrodes for enzymatic biofuel cells applications. Mater. Sci. Energy Technol. 2018, 1, 38–48. [Google Scholar] [CrossRef]
- Willner, I.; Basnar, B.; Willner, B. Nanoparticle–enzyme hybrid systems for nanobiotechnology. FEBS J. 2007, 274, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, M.; Sohrabi, M.J.; Hosseinali, S.H.; Hasan, A.; Kani, P.H.; Talaei, A.J.; Karim, A.Y.; Nanakali, N.M.Q.; Salihi, A.; Aziz, F.M.; et al. Enzyme immobilization onto the nanomaterials: Application in enzyme stability and prodrug-activated cancer therapy. Int. J. Biol. Macromol. 2020, 143, 665–676. [Google Scholar] [CrossRef]
- Kolchanov, D.S.; Slabov, V.; Keller, K.; Sergeeva, E.; Zhukov, M.V.; Drozdov, A.S.; Vinogradov, A.V. Sol-gel magnetite inks for inkjet printing. J. Mater. Chem. C 2019, 7, 6426–6432. [Google Scholar] [CrossRef]
- Cottone, G.; Giuffrida, S.; Bettati, S.; Bruno, S.; Campanini, B.; Marchetti, M.; Abbruzzetti, S.; Viappiani, C.; Cupane, A.; Mozzarelli, A.; et al. More than a confinement:“Soft” and “hard” enzyme entrapment modulates biological catalyst function. Catalysts 2019, 9, 1024. [Google Scholar] [CrossRef] [Green Version]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol-gel-based advanced porous silica materials for biomedical applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Goncalves, M.C. Sol-gel silica nanoparticles in medicine: A natural choice. Design, synthesis and products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef] [Green Version]
- Avnir, D.; Braun, S.; Lev, O.; Ottolenghi, M. Enzymes and other proteins entrapped in sol-gel materials. Chem. Mater. 1994, 6, 1605–1614. [Google Scholar] [CrossRef]
- Braun, S.; Rappoport, S.; Zusman, R.; Avnir, D.; Ottolenghi, M. Biochemically active sol-gel glasses: The trapping of enzymes. Mater. Lett. 1990, 10, 1–5. [Google Scholar] [CrossRef]
- Frenkel-Mullerad, H.; Ben-Knaz, R.; Avnir, D. Preserving the activity of enzymes under harsh oxidizing conditions: Sol–gel entrapped alkaline phosphatase exposed to bromine. J. Sol-Gel Sci. Technol. 2014, 69, 453–456. [Google Scholar] [CrossRef]
- Pierre, A. The sol-gel encapsulation of enzymes. Biocatal. Biotransform. 2004, 22, 145–170. [Google Scholar] [CrossRef]
- Wang, J.; Monton, M.R.N.; Zhang, X.; Filipe, C.D.; Pelton, R.; Brennan, J.D. Hydrophobic sol–gel channel patterning strategies for paper-based microfluidics. Lab Chip 2014, 14, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Seidel, K.; Türkkan, S.; Warwas, D.P.; Dubich, T.; Rohde, M.; Hauser, H.; Behrens, P.; Kirschning, A.; Köster, M.; et al. Macrophage entrapped silica coated superparamagnetic iron oxide particles for controlled drug release in a 3D cancer model. J. Control. Release 2019, 294, 327–336. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Spain, J.C.; Naik, R.R.; Stone, M.O. Enzyme immobilization in a biomimetic silica support. Nat. Biotechnol. 2004, 22, 211–213. [Google Scholar] [CrossRef]
- Brennan, J.D.; Benjamin, D.; DiBattista, E.; Gulcev, M.D. Using sugar and amino acid additives to stabilize enzymes within sol-gel derived silica. Chem. Mater. 2003, 15, 737–745. [Google Scholar] [CrossRef]
- Vinogradov, V.V.; Avnir, D. Exceptional thermal stability of therapeutical enzymes entrapped in alumina sol–gel matrices. J. Mater. Chem. B 2014, 2, 2868–2873. [Google Scholar] [CrossRef]
- Yi, Y.; Chen, Y.; Brook, M.A.; Brennan, J.D. Development of macroporous titania monoliths by a biocompatible method. Part 2: Enzyme entrapment studies. Chem. Mater. 2006, 18, 5336–5342. [Google Scholar] [CrossRef]
- Sinha, R.; Ganesana, M.; Andreescu, S.; Stanciu, L. AChE biosensor based on zinc oxide sol–gel for the detection of pesticides. Anal. Chim. Acta 2010, 661, 195–199. [Google Scholar] [CrossRef]
- Shi, X.; Gu, W.; Li, B.; Chen, N.; Zhao, K.; Xian, Y. Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim. Acta 2014, 181, 1–22. [Google Scholar] [CrossRef]
- Shabanova, E.M.; Drozdov, A.S.; Ivanovski, V.; Suvorova, I.I.; Vinogradov, V.V. Collagenase@ magnetite: Proteolytic composite for magnetically targeted minimally invasive surgery. RSC Adv. 2016, 6, 84354–84362. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Shapovalova, O.E.; Ivanovski, V.; Avnir, D.; Vinogradov, V.V. Entrapment of enzymes within sol–gel-derived magnetite. Chem. Mater. 2016, 28, 2248–2253. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Vinogradov, V.V. Leach-proof magnetic thrombolytic nanoparticles and coatings of enhanced activity. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreeva, Y.I.; Drozdov, A.S.; Avnir, D.; Vinogradov, V.V. Enzymatic nanocomposites with radio frequency field-modulated activity. ACS Biomater. Sci. Eng. 2018, 4, 3962–3967. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.C.; Soares, C.M.; dos Santos, O.A.; de Castro, H.F.; de Moraes, F.F.; Zanin, G.M. Influence of gelation time on the morphological and physico-chemical properties of the sol–gel entrapped lipase. J. Mol. Catal. B Enzym. 2008, 52, 27–33. [Google Scholar] [CrossRef]
- Pirozzi, D.; Fanelli, E.; Aronne, A.; Pernice, P.; Mingione, A. Lipase entrapment in a zirconia matrix: Sol–gel synthesis and catalytic properties. J. Mol. Catal. B Enzym. 2009, 59, 116–120. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Bin Mat, H. Catalytic activity and stability of laccase entrapped in sol–gel silica with additives. J. Sol-Gel Sci. Technol. 2012, 61, 96–103. [Google Scholar] [CrossRef]
- Anastasova, E.I.; Ivanovski, V.; Fakhardo, A.F.; Lepeshkin, A.I.; Omar, S.; Drozdov, A.S.; Vinogradov, V.V. A pure magnetite hydrogel: Synthesis, properties and possible applications. Soft Matter 2017, 13, 8651–8660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilton, R.F., Jr.; Dewan, J.C.; Petsko, G.A. Effects of temperature on protein structure and dynamics: X-ray crystallographic studies of the protein ribonuclease-A at nine different temperatures from 98 to 320 K. Biochemistry 1992, 31, 2469–2481. [Google Scholar] [CrossRef]
- Qi, X.L.; Holt, C.; Mcnulty, D.; Clarke, D.T.; Brownlow, S.; Jones, G.R. Effect of temperature on the secondary structure of β-lactoglobulin at pH 6.7, as determined by CD and IR spectroscopy: A test of the molten globule hypothesis. Biochem. J. 1997, 324, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Brazhnikov, E.; Chirgadze, Y.N.; Dolgikh, D.; Ptitsyn, O. Noncooperative temperature melting of a globular protein without specific tertiary structure: Acid form of bovine carbonic anhydrase B. Biopolym. Orig. Res. Biomol. 1985, 24, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolym. Orig. Res. Biomol. 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Montich, G.G. Partly folded states of bovine carbonic anhydrase interact with zwitterionic and anionic lipid membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1468, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Grdadolnik, J.; Maréchal, Y. Bovine serum albumin observed by infrared spectrometry. I. Methodology, structural investigation, and water uptake. Biopolym. Orig. Res. Biomol. 2001, 62, 40–53. [Google Scholar] [CrossRef]
- Grobelnik, B.; Grdadolnik, J. Calculation of the Absorption Spectrum from an ATR Infrared Experiment. Acta Chim. Slov. 2008, 55, 978–984. [Google Scholar]
- Li, Y.S.; Church, J.S.; Woodhead, A.L. Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent bio-applications of sol–gel materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Jafari, H.; Amiri, M.; Abdi, E.; Navid, S.L.; Bouckaert, J.; Jijie, R.; Boukherroub, R.; Szunerits, S. Entrapment of uropathogenic E. coli cells into ultra-thin sol-gel matrices on gold thin films: A low cost alternative for impedimetric bacteria sensing. Biosens. Bioelectron. 2019, 124, 161–166. [Google Scholar] [CrossRef]
- Ganonyan, N.; Benmelech, N.; Bar, G.; Gvishi, R.; Avnir, D. Entrapment of enzymes in silica aerogels. Mater. Today 2020, 33, 24–35. [Google Scholar] [CrossRef]
- Fernandez Caresani, J.R.; Dallegrave, A.; dos Santos, J.H. Amylases immobilization by sol–gel entrapment: Application for starch hydrolysis. J. Sol-Gel Sci. Technol. 2020, 94, 229–240. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A. Removal of diclofenac, paracetamol, and carbamazepine from model aqueous solutions by magnetic sol–gel encapsulated horseradish peroxidase and lignin peroxidase composites. Nanomaterials 2020, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Drozdov, A.S.; Fakhardo, A.F.; Vinogradov, V.V. Sol-gel composites based on alumina and ferria for cardiovascular diseases treatment. In Biocompatible Hybrid Oxide Nanoparticles for Human Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–179. [Google Scholar]
- Shapovalova, O.E.; Levy, D.; Avnir, D.; Vinogradov, V.V. Protection of enzymes from photodegradation by entrapment within alumina. Colloids Surf. B Biointerfaces 2016, 146, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ju, H. Preparation of porous titania sol- gel matrix for immobilization of horseradish peroxidase by a vapor deposition method. Anal. Chem. 2002, 74, 3579–3583. [Google Scholar] [CrossRef] [PubMed]
- Kochana, J.; Gala, A.; Parczewski, A.; Adamski, J. Titania sol–gel-derived tyrosinase-based amperometric biosensor for determination of phenolic compounds in water samples. Examination of interference effects. Anal. Bioanal. Chem. 2008, 391, 1275–1281. [Google Scholar] [CrossRef]
- Eggers, D.K.; Valentine, J.S. Crowding and hydration effects on protein conformation: A study with sol-gel encapsulated proteins. J. Mol. Biol. 2001, 314, 911–922. [Google Scholar] [CrossRef]
- Lin, S.; Van den Bergh, W.; Baker, S.; Jones, J.R. Protein interactions with nanoporous sol–gel derived bioactive glasses. Acta Biomater. 2011, 7, 3606–3615. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Smit, M.; Dunn, B.; Zink, J.I. Stabilization of creatine kinase encapsulated in silicate sol- gel materials and unusual temperature effects on its activity. Chem. Mater. 2002, 14, 4300–4306. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Ivanovski, V.; Avnir, D.; Vinogradov, V.V. A universal magnetic ferrofluid: Nanomagnetite stable hydrosol with no added dispersants and at neutral pH. J. Colloid Interface Sci. 2016, 468, 307–312. [Google Scholar] [CrossRef]
- Portaccio, M.; Della Ventura, B.; Mita, D.; Manolova, N.; Stoilova, O.; Rashkov, I.; Lepore, M. FT-IR microscopy characterization of sol–gel layers prior and after glucose oxidase immobilization for biosensing applications. J. Sol-Gel Sci. Technol. 2011, 57, 204–211. [Google Scholar] [CrossRef]
- Delfino, I.; Portaccio, M.; Della Ventura, B.; Mita, D.; Lepore, M. Enzyme distribution and secondary structure of sol–gel immobilized glucose oxidase by micro-attenuated total reflection FT-IR spectroscopy. Mater. Sci. Eng. C 2013, 33, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Rumyantceva, V.; Rumyantceva, V.; Andreeva, Y.; Tsvetikova, S.; Radaev, A.; Vishnevskaya, M.; Vinogradov, V.; Drozdov, A.S.; Koshel, E. Magnetically controlled carbonate nanocomposite with ciprofloxacin for biofilm eradication. Int. J. Mol. Sci. 2021, 22, 6187. [Google Scholar] [CrossRef] [PubMed]
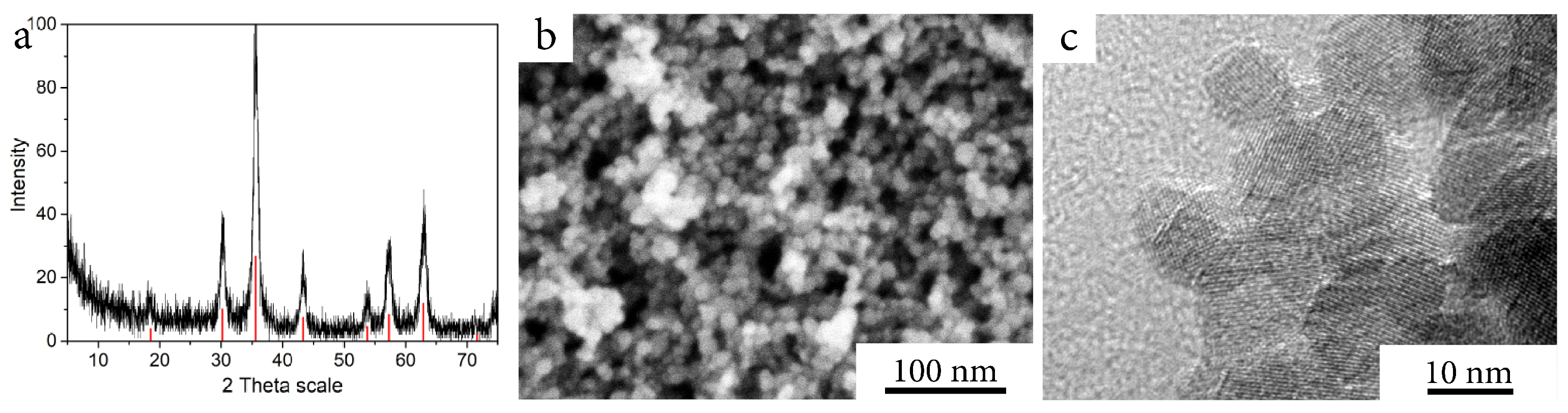
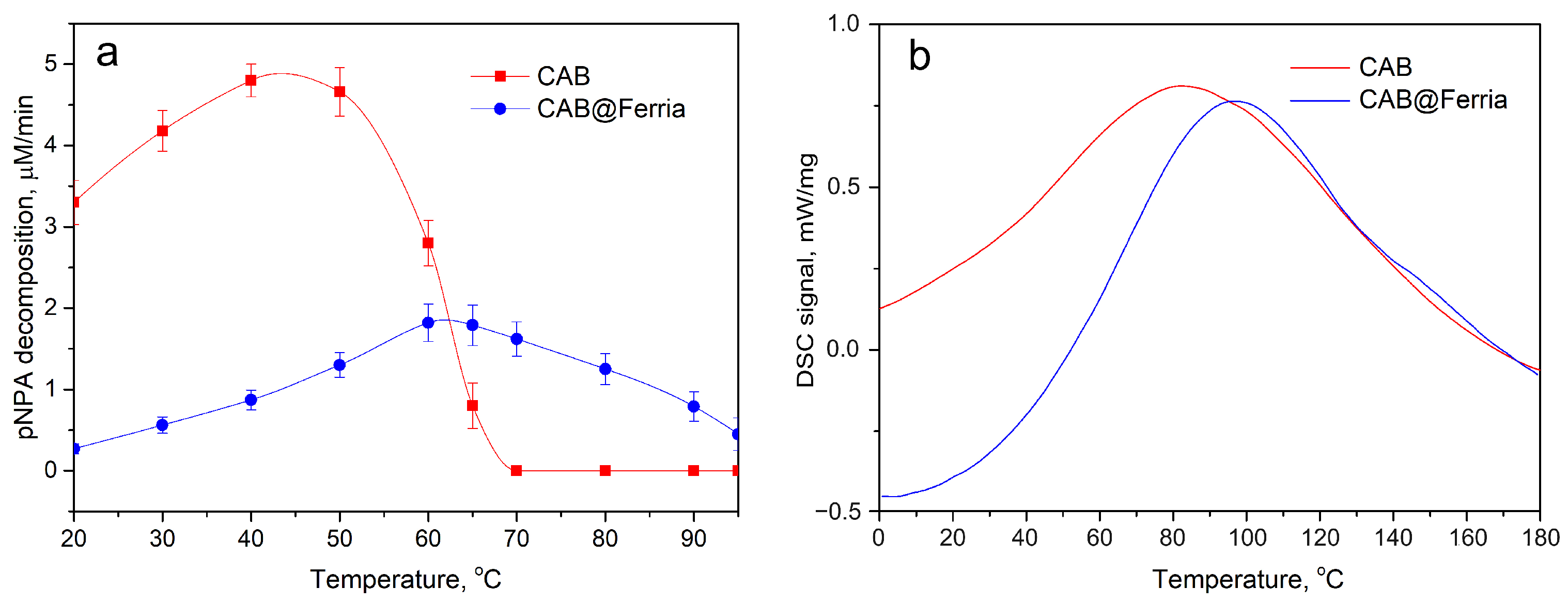
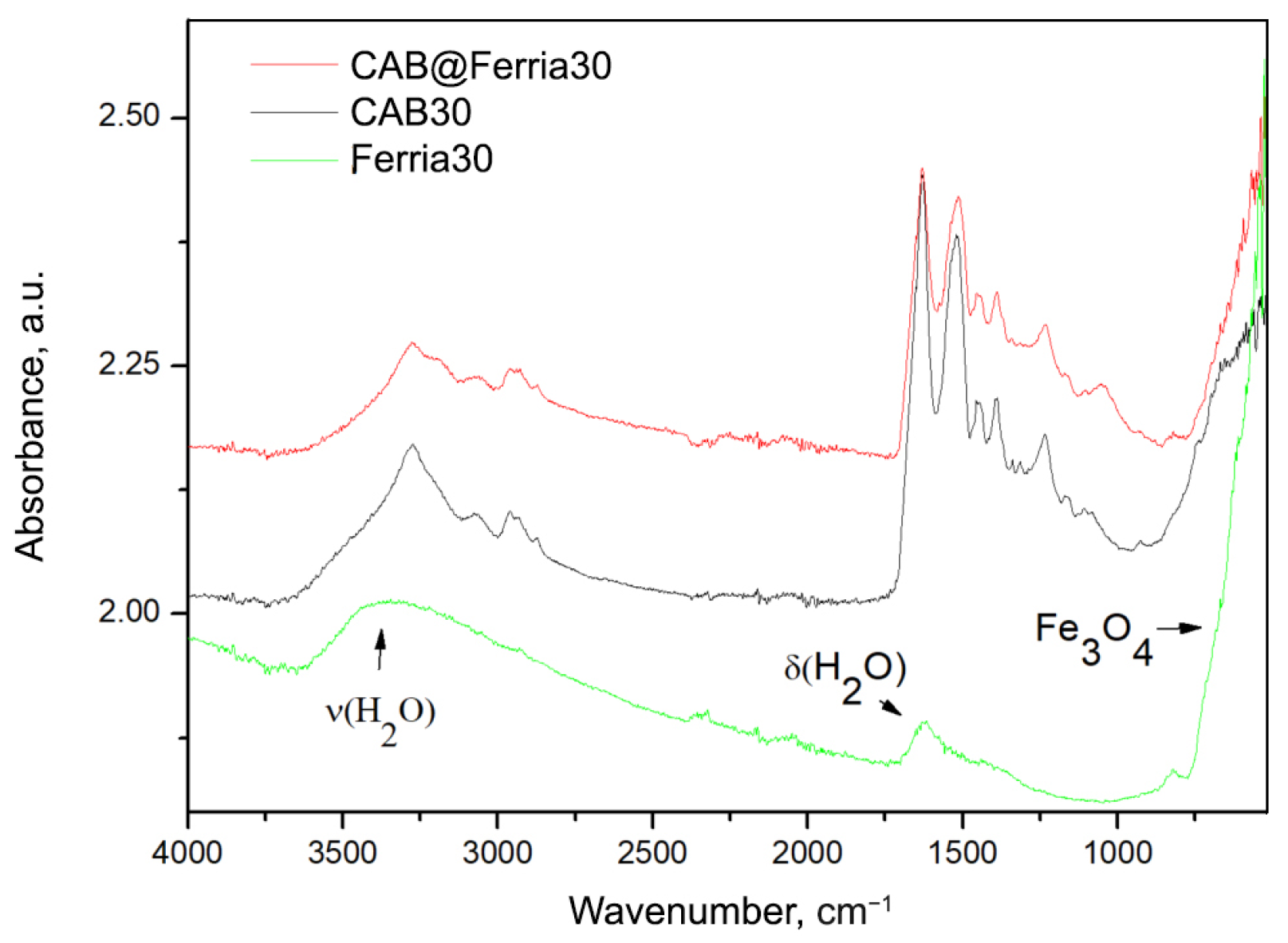
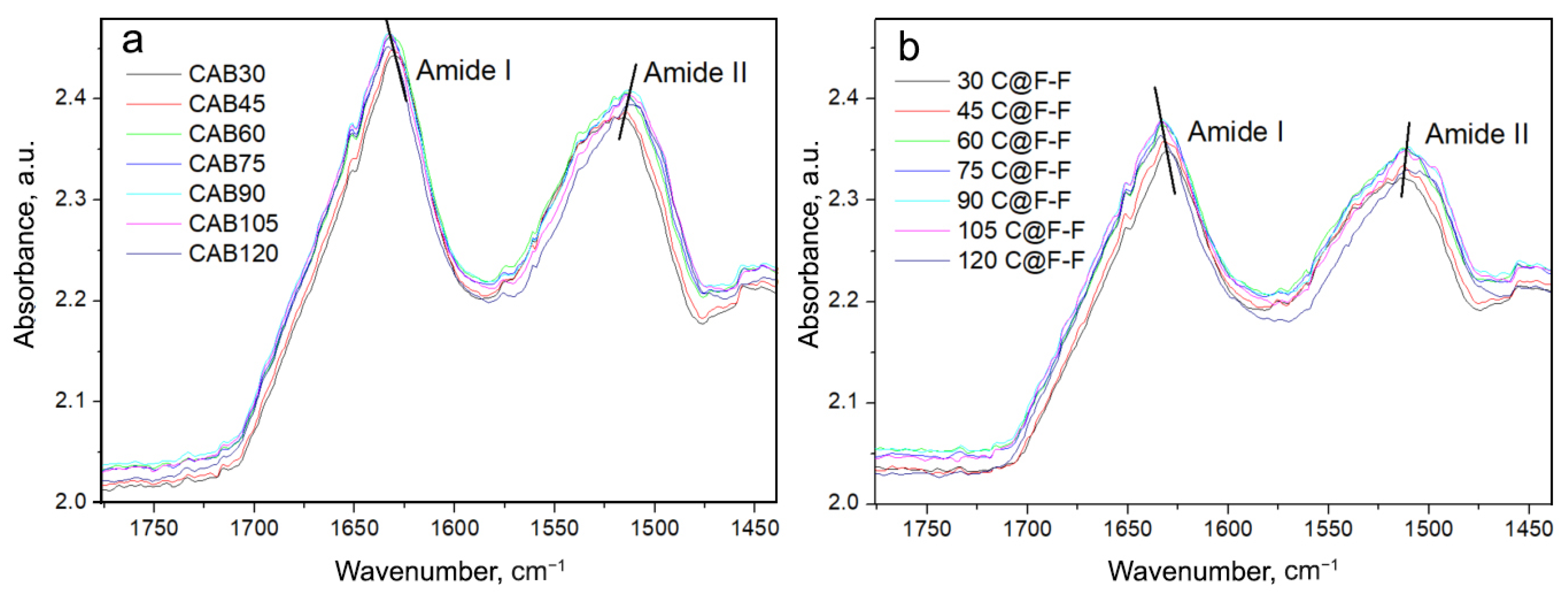

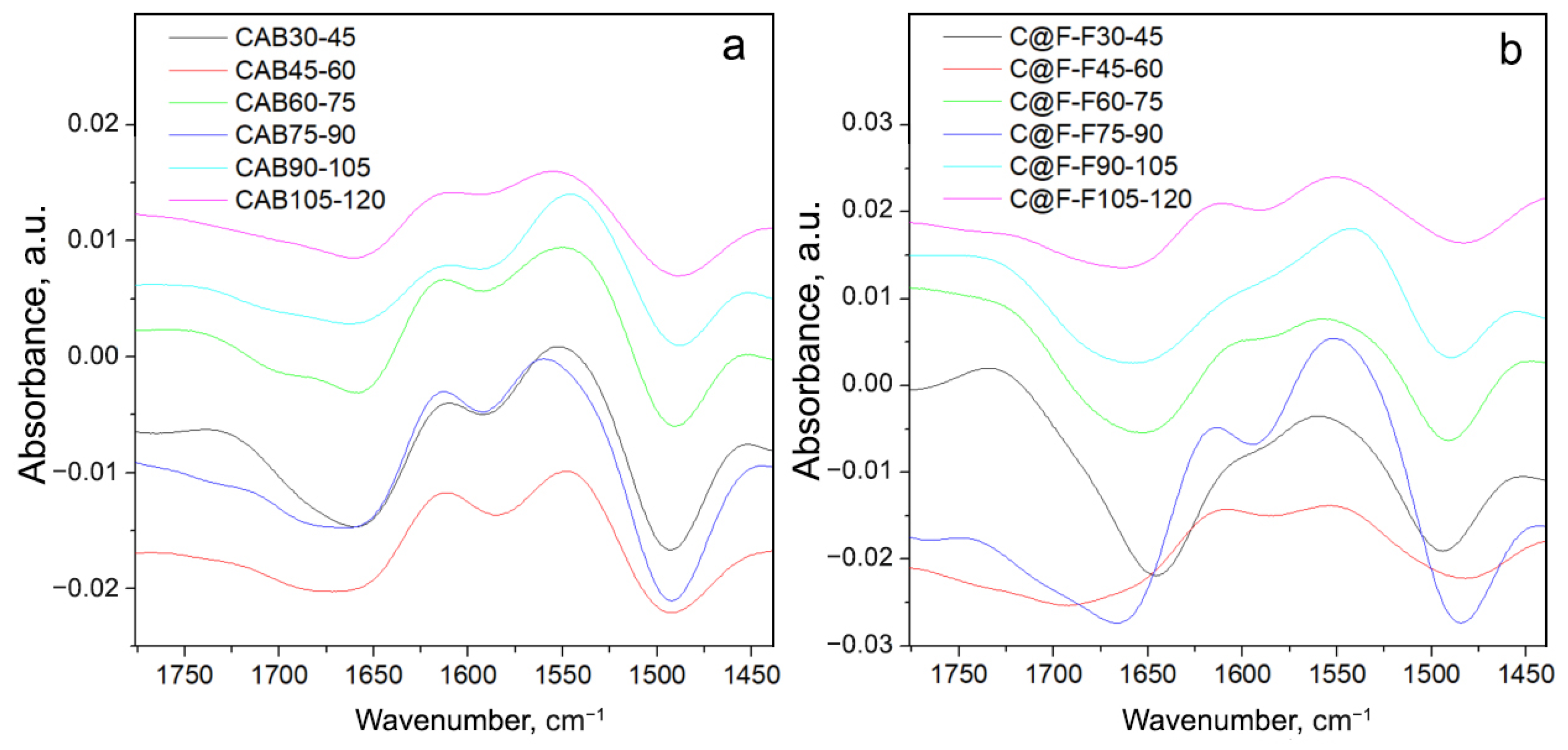
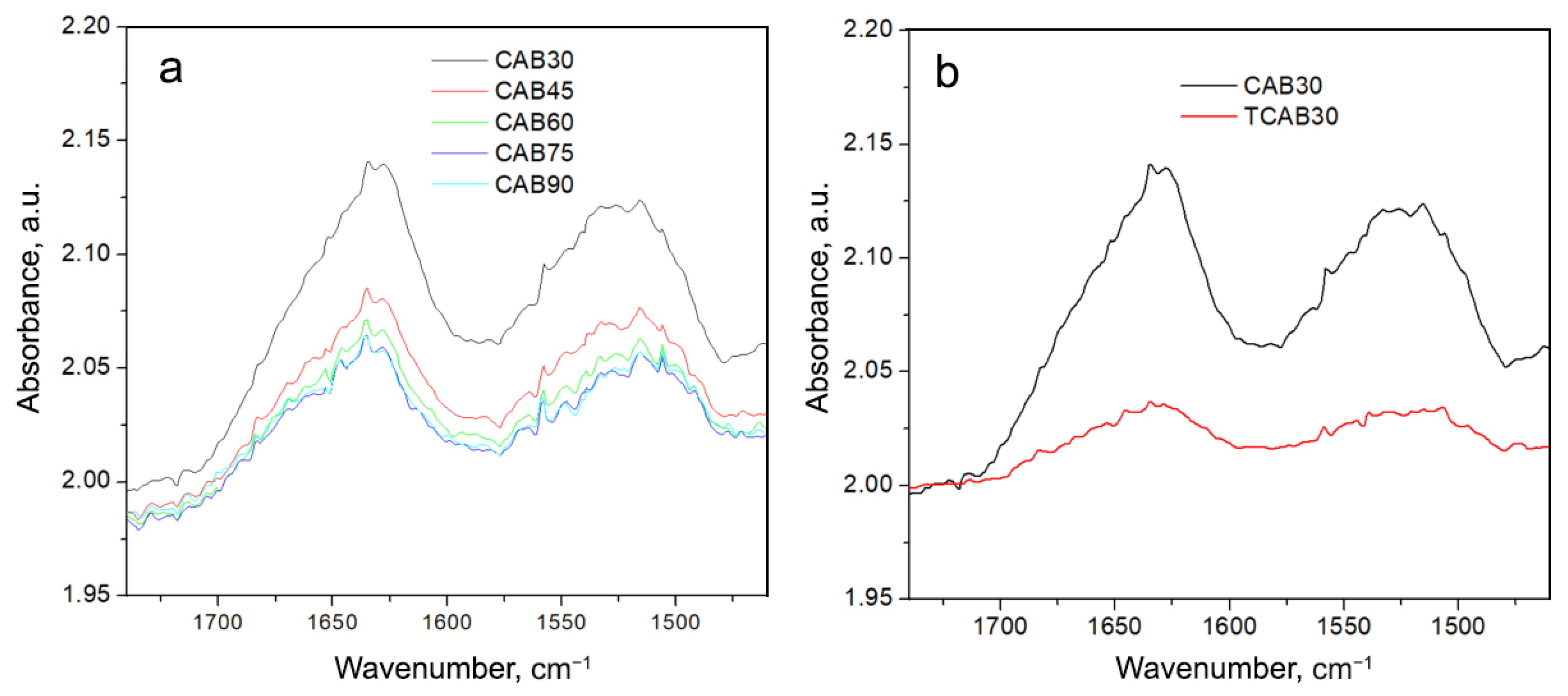

| Temperature, °C | CAB, M/min | CAB@Ferria, M/min |
|---|---|---|
| 20 | 3.30 | 0.27 |
| 30 | 4.18 | 0.56 |
| 40 | 4.80 | 0.87 |
| 50 | 4.66 | 1.30 |
| 60 | 2.80 | 1.82 |
| 65 | 0.78 | 1.79 |
| 70 | 0 | 1.62 |
| 80 | 0 | 1.30 |
| 90 | 0 | 0.79 |
| 95 | 0 | 0.45 |
| Sample | Turns, Bends | -Structure | Turns, Bends | Turns, Bends | -Helix | -Structure | HO, (Side Groups) | Amide II | ||
|---|---|---|---|---|---|---|---|---|---|---|
| /cm | ||||||||||
| 30CAB | 1695 | 1681 | 1671 | 1664 | 1656 | 1628 | 1593 | 1536 | 1511 | 1496 |
| 120CAB | 1696 | 1682 | 1679 | 1666 | 1655 | 1632 | 1601 | 1533 | 1510 | 1495 |
| 30C@F-F | 1685 | 1675 | 1666 | 1655 | 1631 | 1592 | 1539 | 1510 | 1495 | |
| 120C@F-F | 1707, 1695 | 1682 | 1666 | 1651 | 1632 | 1598 | 1537 | 1513 | 1496 | |
| Band integrated area/% | ||||||||||
| 30CAB | 0.93 | 3.15 | 3.08 | 1.18 | 10.28 | 26.88 | 19.15 | 22.91 | 10.85 | 1.59 |
| 120CAB | 1.06 | 1.36 | 5.38 | 2.97 | 8.15 | 26.59 | 17.25 | 20.18 | 11.77 | 5.29 |
| 30C@F-F | 3.03 | 2.71 | 3.48 | 3.63 | 33.48 | 17.71 | 20.84 | 12.10 | 3.01 | |
| 120C@F-F | 2.00 | 4.44 | 8.06 | 5.18 | 28.97 | 15.05 | 14.20 | 11.44 | 10.68 | |
| Position | Min | Min | Max | Min | Max | Min |
|---|---|---|---|---|---|---|
| (CAB)/cm | 1700 | 1661 | 1611 | 1591 | 1550 | 1490 |
| (C@F-F)/cm | 1663 | 1604 | 1594 | 1551 | 1487 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanovski, V.; Shapovalova, O.E.; Drozdov, A.S. Structural Rearrangements of Carbonic Anhydrase Entrapped in Sol-Gel Magnetite Determined by ATR–FTIR Spectroscopy. Int. J. Mol. Sci. 2022, 23, 5975. https://doi.org/10.3390/ijms23115975
Ivanovski V, Shapovalova OE, Drozdov AS. Structural Rearrangements of Carbonic Anhydrase Entrapped in Sol-Gel Magnetite Determined by ATR–FTIR Spectroscopy. International Journal of Molecular Sciences. 2022; 23(11):5975. https://doi.org/10.3390/ijms23115975
Chicago/Turabian StyleIvanovski, Vladimir, Olga E. Shapovalova, and Andrey S. Drozdov. 2022. "Structural Rearrangements of Carbonic Anhydrase Entrapped in Sol-Gel Magnetite Determined by ATR–FTIR Spectroscopy" International Journal of Molecular Sciences 23, no. 11: 5975. https://doi.org/10.3390/ijms23115975
APA StyleIvanovski, V., Shapovalova, O. E., & Drozdov, A. S. (2022). Structural Rearrangements of Carbonic Anhydrase Entrapped in Sol-Gel Magnetite Determined by ATR–FTIR Spectroscopy. International Journal of Molecular Sciences, 23(11), 5975. https://doi.org/10.3390/ijms23115975








