Plasminogen Binding and Activation at the Mesothelial Cell Surface Promotes Invasion through a Collagen Matrix
Abstract
1. Introduction
2. Results
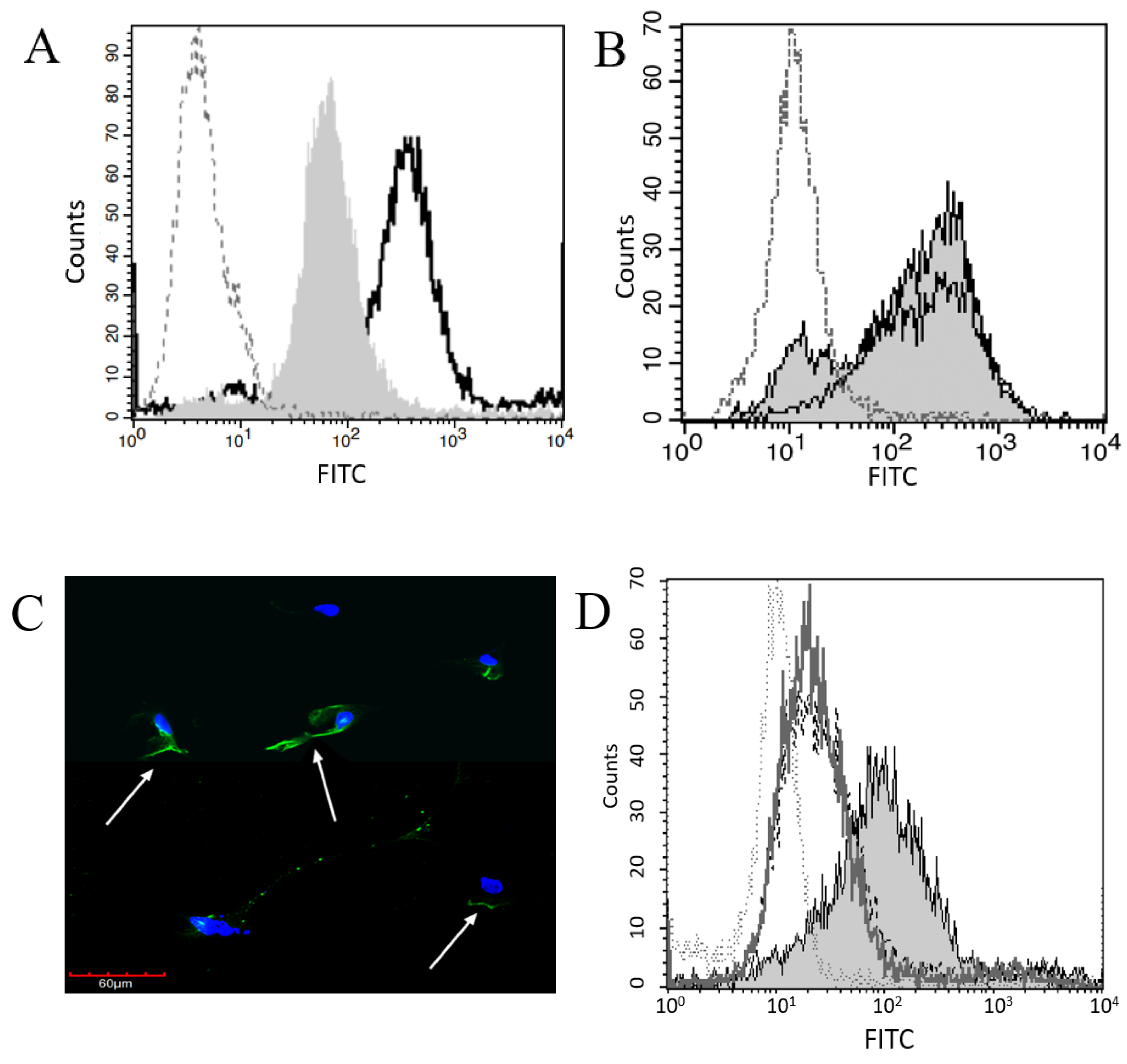
2.1. Plg Binds to Pleural and Peritoneal Mesothelial Cells
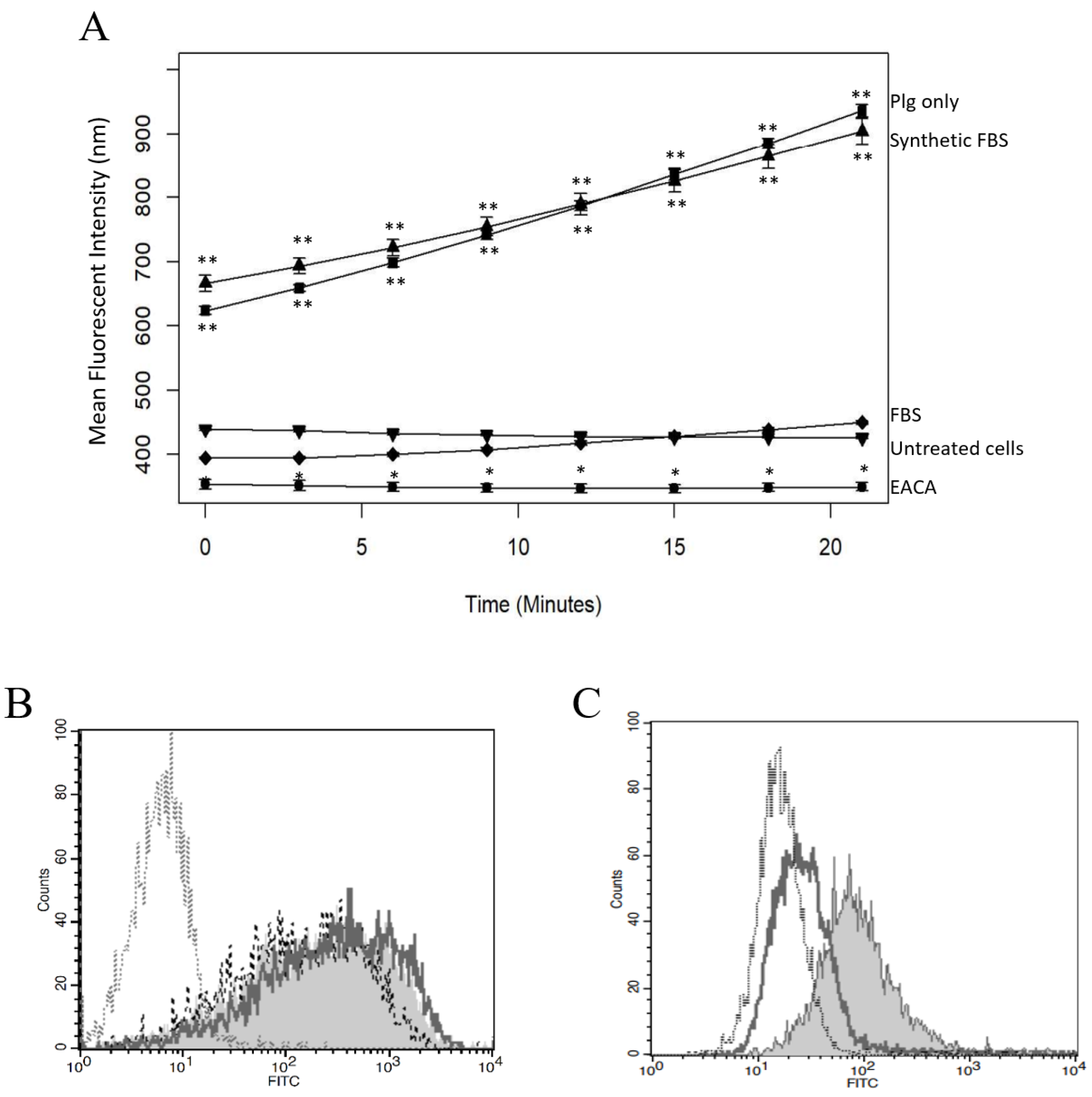
2.2. Plg Activation Is Blocked by uPA Inhibitor
2.3. Plg Binding and Activity Are Reduced by EACA or FBS
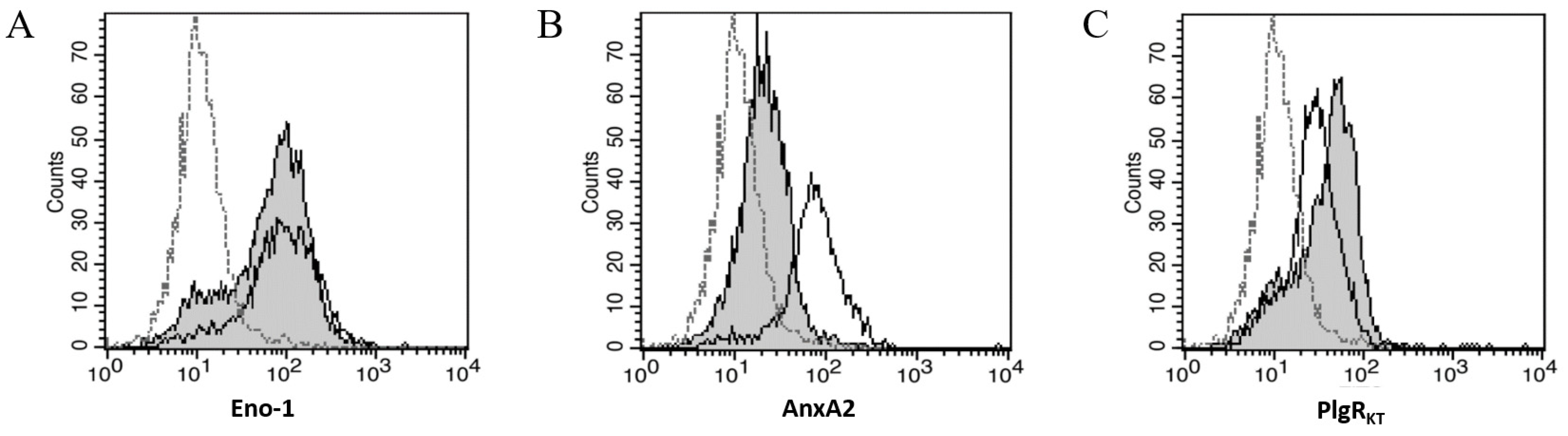
2.4. Three PlgRs Were Detected on the MeT5A Cell Surface
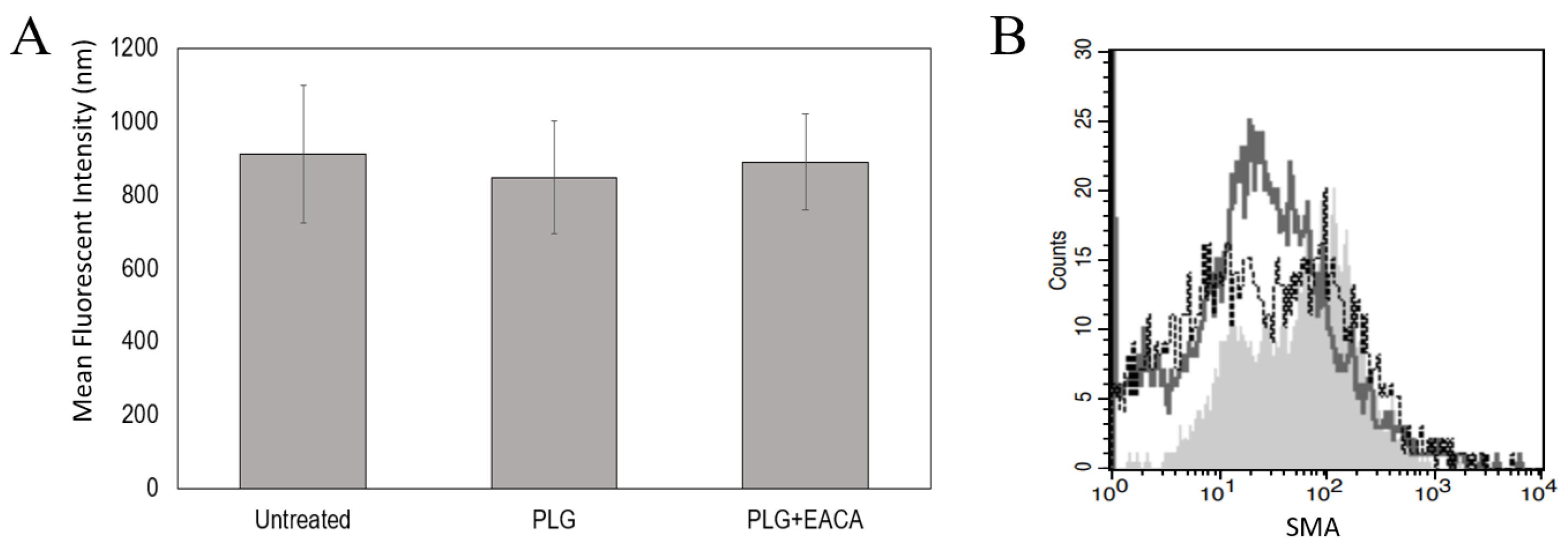
2.5. Plg Does Not Affect MeT5A Proliferation or Differentiation
2.6. Plg Increases MeT5A Chemotaxis through a Collagen Matrix
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Cultures
4.3. Fluorescent Microscopy
4.4. Flow Cytometry
4.5. Plasmin Activity Assays
4.6. Plg Blocking Assays
4.7. Proliferation Assays
4.8. Migration Assays
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugimoto, M.A.; Ribeiro, A.L.C.; Costa, B.R.C.; Vago, J.P.; Lima, K.M.; Carneiro, F.S.; Ortiz, M.M.O.; Lima, G.L.N.; Carmo, A.A.F.; Rocha, R.M.; et al. Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1. Blood 2017, 129, 2896–2907. [Google Scholar] [CrossRef] [PubMed]
- Vago, J.P.; Sugimoto, M.A.; Lima, K.M.; Negreiros-Lima, G.L.; Baik, N.; Teixeira, M.M.; Perretti, M.; Parmer, R.J.; Miles, L.A.; Sousa, L.P. Plasminogen and the Plasminogen Receptor, Plg-RKT, Regulate Macrophage Phenotypic, and Functional Changes. Front. Immunol. 2019, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E.; Birnie, K.; Lansley, S.; Herrick, S.E.; Lim, C.B.; Prele, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. [Google Scholar] [CrossRef]
- Pfau, J.C.; McNew, T.; Hanley, K.; Swan, L.; Black, B. Autoimmune markers for progression of Libby amphibole lamellar pleural thickening. Inhal. Toxicol. 2019, 31, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Black, B.; Szeinuk, J.; Whitehouse, A.C.; Levin, S.M.; Henschke, C.I.; Yankelevitz, D.F.; Flores, R.M. Rapid progression of pleural disease due to exposure to Libby amphibole: “Not your grandfather’s asbestos related disease”. Am. J. Ind. Med. 2014, 57, 1197–1206. [Google Scholar] [CrossRef]
- Hanson, R.; Evilia, C.; Gilmer, J.; Woods, L.; Black, B.; Flores, R.; Pfau, J.C. Libby amphibole-induced mesothelial cell autoantibodies bind to surface plasminogen and alter collagen matrix remodeling. Physiol. Rep. 2016, 4, e12881. [Google Scholar] [CrossRef]
- Marchand, L.S.; St-Hilaire, S.; Putnam, E.A.; Serve, K.M.; Pfau, J.C. Mesothelial cell and anti-nuclear autoantibodies associated with pleural abnormalities in an asbestos exposed population of Libby MT. Toxicol. Lett. 2012, 208, 168–173. [Google Scholar] [CrossRef][Green Version]
- Owens, S.; Jeffers, A.; Boren, J.; Tsukasaki, Y.; Koenig, K.; Ikebe, M.; Idell, S.; Tucker, T.A. Mesomesenchymal transition of pleural mesothelial cells is PI3K and NF-kappaB dependent. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L1265–L1273. [Google Scholar] [CrossRef]
- Nicholl, S.M.; Roztocil, E.; Galaria, I.I.; Davies, M.G. Plasmin induces smooth muscle cell proliferation. J. Surg. Res. 2005, 127, 39–45. [Google Scholar] [CrossRef]
- Menshikov, M.; Plekhanova, O.; Cai, H.; Chalupsky, K.; Parfyonova, Y.; Bashtrikov, P.; Tkachuk, V.; Berk, B.C. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 801–807. [Google Scholar] [CrossRef]
- Menshikov, M.; Torosyan, N.; Elizarova, E.; Plakida, K.; Vorotnikov, A.; Parfyonova, Y.; Stepanova, V.; Bobik, A.; Berk, B.; Tkachuk, V. Urokinase induces matrix metalloproteinase-9/gelatinase B expression in THP-1 monocytes via ERK1/2 and cytosolic phospholipase A2 activation and eicosanoid production. J. Vasc. Res. 2006, 43, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Ganapathy, S.; Settle, M.; Plow, E.F. Plasminogen promotes macrophage phagocytosis in mice. Blood 2014, 124, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Ny, L.; Parmer, R.J.; Shen, Y.; Holmberg, S.; Baik, N.; Bäckman, A.; Broden, J.; Wilczynska, M.; Ny, T.; Miles, L.A. The plasminogen receptor, Plg-RKT, plays a role in inflammation and fibrinolysis during cutaneous wound healing in mice. Cell Death Dis. 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Thaler, B.; Baik, N.; Hohensinner, P.J.; Baumgartner, J.; Panzenbock, A.; Stojkovic, S.; Demyanets, S.; Huk, I.; Rega-Kaun, G.; Kaun, C.; et al. Differential expression of Plg-RKT and its effects on migration of proinflammatory monocyte and macrophage subsets. Blood 2019, 134, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.A.; Parmer, R.J. Plasminogen receptors: The first quarter century. Semin. Thromb. Hemost. 2013, 39, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.F.; Miles, L.A. Plasminogen receptors in the mediation of pericellular proteolysis. Cell Differ. Dev. 1990, 32, 293–298. [Google Scholar] [CrossRef]
- Plow, E.F.; Doeuvre, L.; Das, R. So many plasminogen receptors: Why? J. Biomed. Biotechnol. 2012, 2012, 141806. [Google Scholar] [CrossRef]
- Miles, L.; Hawley, S.; Baik, N.; Andronicos, N.; Castellino, F.; Parmer, R. Plasminogen receptors: The sine qua non of cell surface plasminogen activation. Front. Biosci. A J. Virtual Libr. 2005, 10, 1754–1762. [Google Scholar]
- Andronicos, N.M.; Chen, E.I.; Baik, N.; Bai, H.; Parmer, C.M.; Kiosses, W.B.; Kamps, M.P.; Yates, J.R., 3rd; Parmer, R.J.; Miles, L.A. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood 2010, 115, 1319–1330. [Google Scholar] [CrossRef]
- Tucker, T.A.; Idell, S. The Contribution of the Urokinase Plasminogen Activator and the Urokinase Receptor to Pleural and Parenchymal Lung Injury and Repair: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 1437. [Google Scholar] [CrossRef]
- Miles, L.A.; Dahlberg, C.M.; Plescia, J.; Felez, J.; Kato, K.; Plow, E.F. Role of cell-surface lysines in plasminogen binding to cells: Identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry 1991, 30, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Nagai, H.; Toyokuni, S. Receptor role of the annexin A2 in the mesothelial endocytosis of crocidolite fibers. Lab. Investig. 2015, 95, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Kassam, G.; Le, B.H.; Choi, K.S.; Kang, H.M.; Fitzpatrick, S.L.; Louie, P.; Waisman, D.M. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry 1998, 37, 16958–16966. [Google Scholar] [CrossRef]
- Violand, B.N.; Byrne, R.; Castellino, F.J. The effect of alpha-omega-amino acids on human plasminogen structure and activation. J. Biol. Chem. 1978, 253, 5395–5401. [Google Scholar] [CrossRef]
- Miles, L.A.; Castellino, F.J.; Gong, Y. Critical role for conversion of glu-plasminogen to Lys-plasminogen for optimal stimulation of plasminogen activation on cell surfaces. Trends Cardiovasc. Med. 2003, 13, 21–30. [Google Scholar] [CrossRef]
- Van Hinsbergh, V.W.; Kooistra, T.; Scheffer, M.A.; van Bockel, J.H.; van Muijen, G.N. Characterization and fibrinolytic properties of human omental tissue mesothelial cells: Comparison with endothelial cells. Blood 1990, 75, 1490–1497. [Google Scholar] [CrossRef]
- Idell, S.; Zwieb, C.; Kumar, A.; Koenig, K.B.; Johnson, A.R. Pahtways of fibrin turnover of human pleural mesothelial cells in vitro. Am. J. Respir. Cell Mol. Biol. 1992, 7, 414–426. [Google Scholar] [CrossRef]
- Imamura, S.; Namangala, B.; Tajima, T.; Tembo, M.E.; Yasuda, J.; Ohashi, K.; Onuma, M. Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine 2006, 24, 2230–2237. [Google Scholar] [CrossRef]
- Singh, S.; Saleem, S.; Reed, G.L. Alpha2-Antiplasmin: The Devil You Don’t Know in Cerebrovascular and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 608899. [Google Scholar] [CrossRef]
- Wygrecka, M.; Marsh, L.M.; Morty, R.E.; Henneke, I.; Guenther, A.; Lohmeyer, J.; Markart, P.; Preissner, K.T. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood 2009, 113, 5588–5598. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, N.; Won, C.; Kim, H.R.; Hwang, Y.I.; Song, Y.W.; Kang, J.S.; Lee, W.J. α-Enolase expressed on the surfaces of monocytes and macrophages induces robust synovial inflammation in rheumatoid arthritis. J. Immunol. 2012, 189, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.A.; Lighvani, S.; Baik, N.; Andronicos, N.M.; Chen, E.I.; Parmer, C.M.; Khaldoyanidi, S.; Diggs, J.E.; Kiosses, W.B.; Kamps, M.P.; et al. The plasminogen receptor, Plg-R(KT), and macrophage function. J. Biomed. Biotechnol. 2012, 2012, 250464. [Google Scholar] [CrossRef]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Nanninga, L.B. Molar concentrations of fibrinolytic components, especially free fibrinolysin, in vivo. Thromb. Diath. Haemorrh. 1975, 33, 244–255. [Google Scholar] [CrossRef]
- Serve, K.M.; Black, B.; Szeinuk, J.; Pfau, J.C. Asbestos-associated mesothelial cell autoantibodies promote collagen deposition in vitro. Inhal. Toxicol. 2013, 25, 774–784. [Google Scholar] [CrossRef][Green Version]
- Yurka, H.G.; Wissler, R.N.; Zanghi, C.N.; Liu, X.; Tu, X.; Eaton, M.P. The effective concentration of epsilon-aminocapric acid for inhibition of fibrinolysis in neonatal plasma in vitro. Anesth. Analg. 2010, 111, 180–184. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditzig, Z.; Wilson, C.M.; Salas, J.; Serve, K.M. Plasminogen Binding and Activation at the Mesothelial Cell Surface Promotes Invasion through a Collagen Matrix. Int. J. Mol. Sci. 2022, 23, 5984. https://doi.org/10.3390/ijms23115984
Ditzig Z, Wilson CM, Salas J, Serve KM. Plasminogen Binding and Activation at the Mesothelial Cell Surface Promotes Invasion through a Collagen Matrix. International Journal of Molecular Sciences. 2022; 23(11):5984. https://doi.org/10.3390/ijms23115984
Chicago/Turabian StyleDitzig, Zachary, Caleb M. Wilson, Jesse Salas, and Kinta M. Serve. 2022. "Plasminogen Binding and Activation at the Mesothelial Cell Surface Promotes Invasion through a Collagen Matrix" International Journal of Molecular Sciences 23, no. 11: 5984. https://doi.org/10.3390/ijms23115984
APA StyleDitzig, Z., Wilson, C. M., Salas, J., & Serve, K. M. (2022). Plasminogen Binding and Activation at the Mesothelial Cell Surface Promotes Invasion through a Collagen Matrix. International Journal of Molecular Sciences, 23(11), 5984. https://doi.org/10.3390/ijms23115984






