The Short-Day Cycle Induces Intestinal Epithelial Purine Metabolism Imbalance and Hepatic Disfunctions in Antibiotic-Mediated Gut Microbiota Perturbation Mice
Abstract
1. Introduction
2. Results
2.1. Validation of the Model of Antibiotic-Induced Gut Dysbiosis in Mice
2.2. Body Mass, Body Temperature, Organ Indexes, and Serum Biochemical Indicators in ABX Mice under NLD and SD
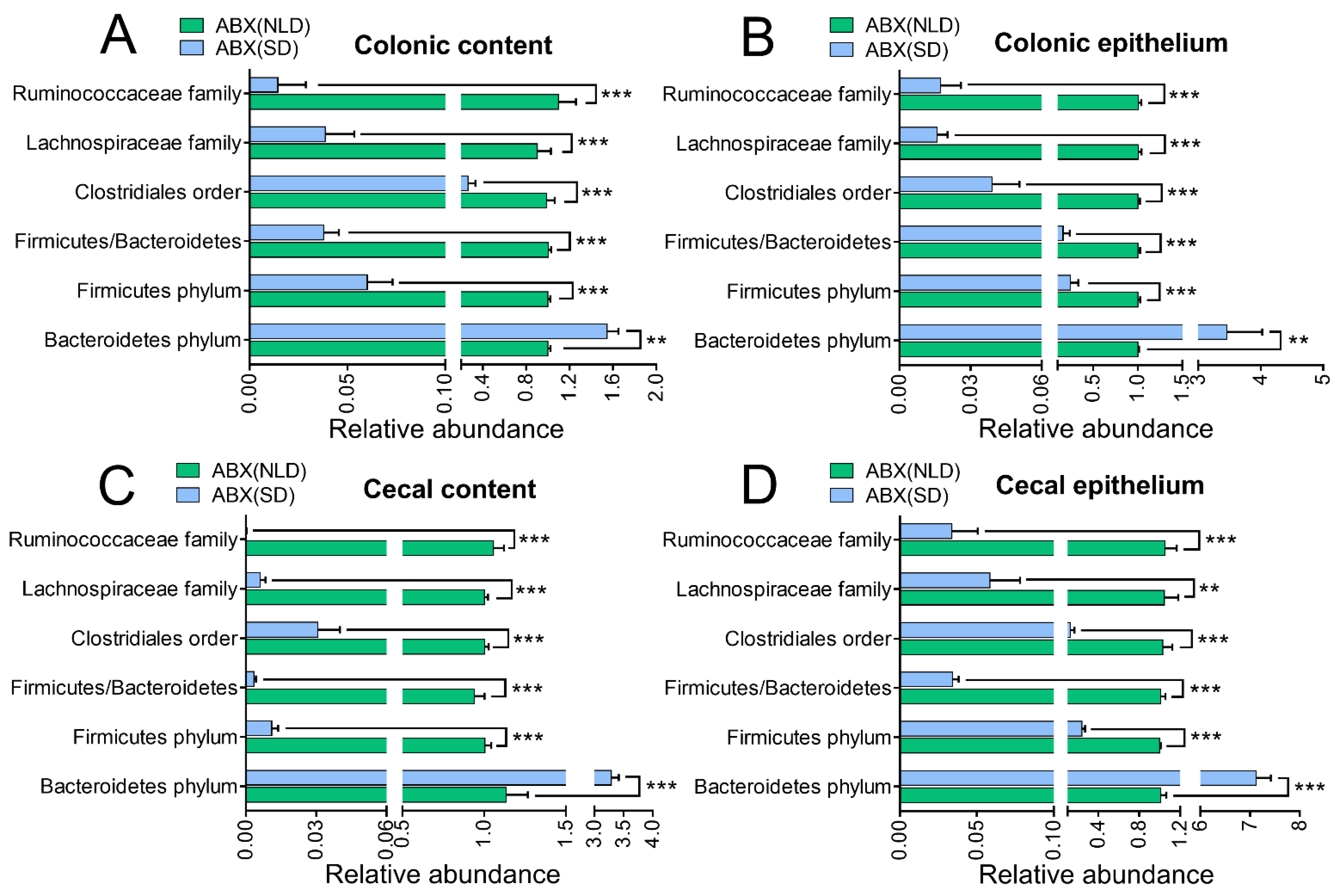
2.3. Gut Microbiota Profiles in ABX Mice under NLD and SD
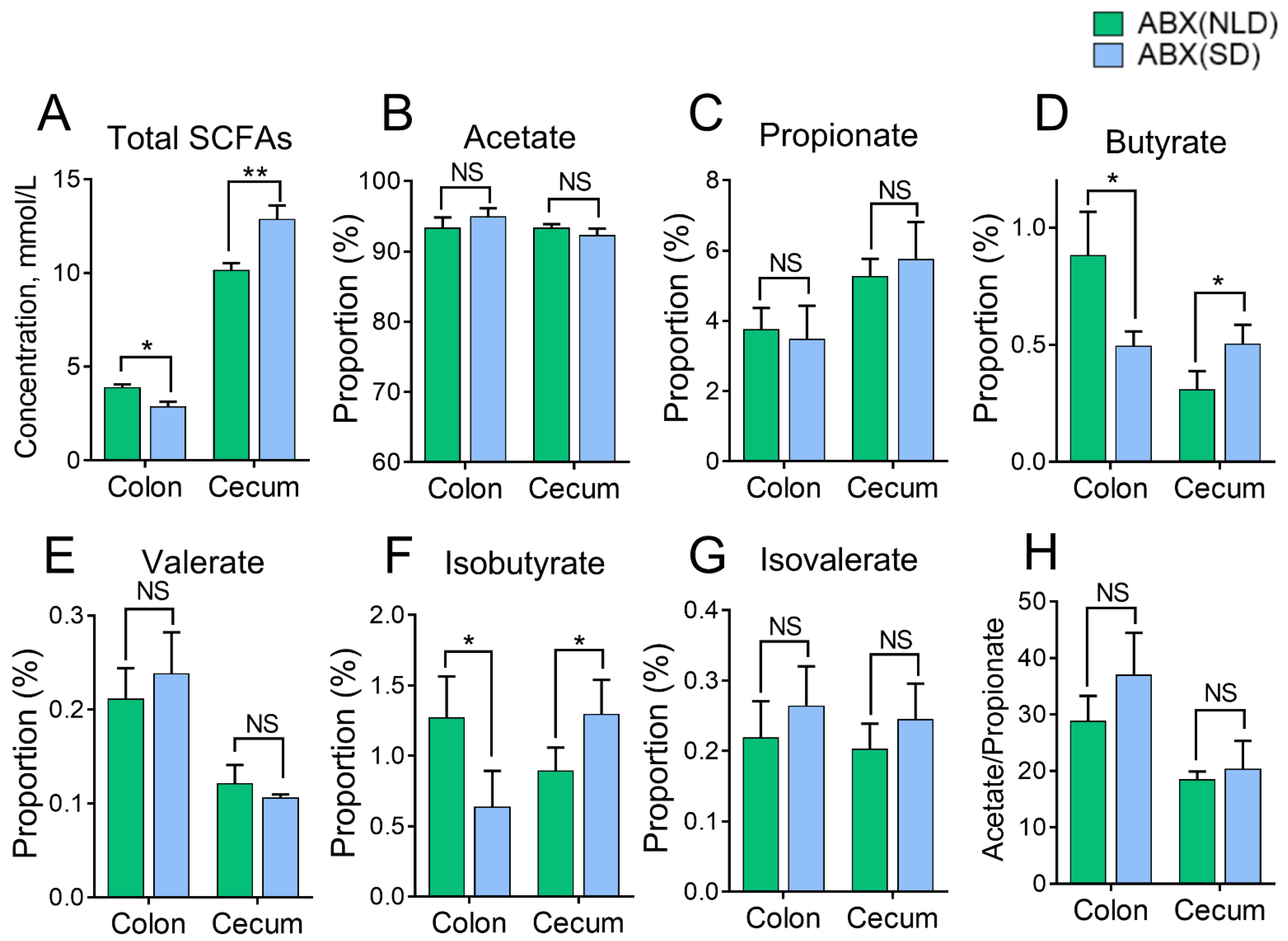
2.4. The Proportions of SCFAs in Colonic and Cecal Contents of ABX Mice under NLD and SD
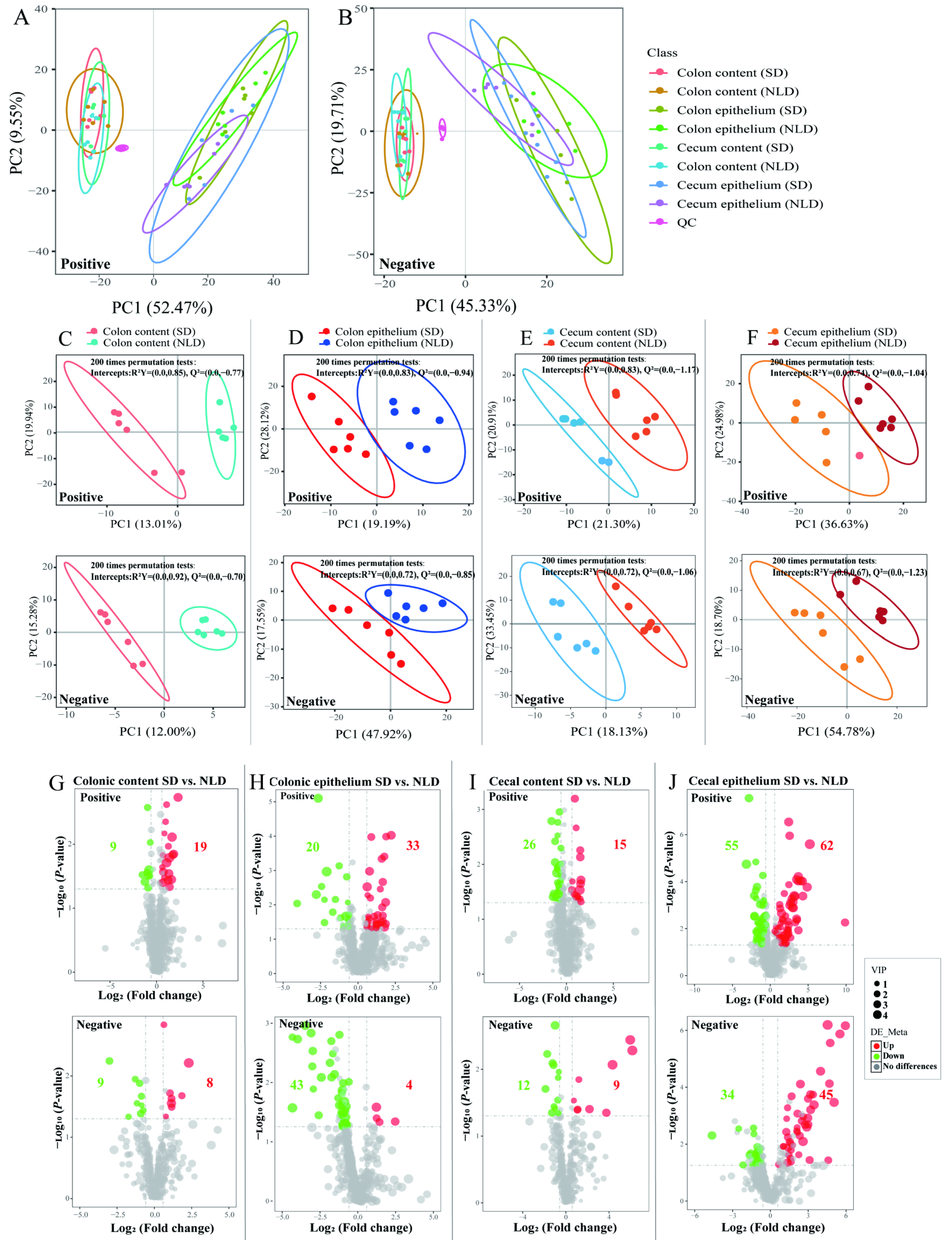
2.5. Overview of Metabolomics Profiles and the Volcano Plots of Differentially Altered Metabolites of Intestinal Contents and Epithelium in ABX Mice under NLD and SD
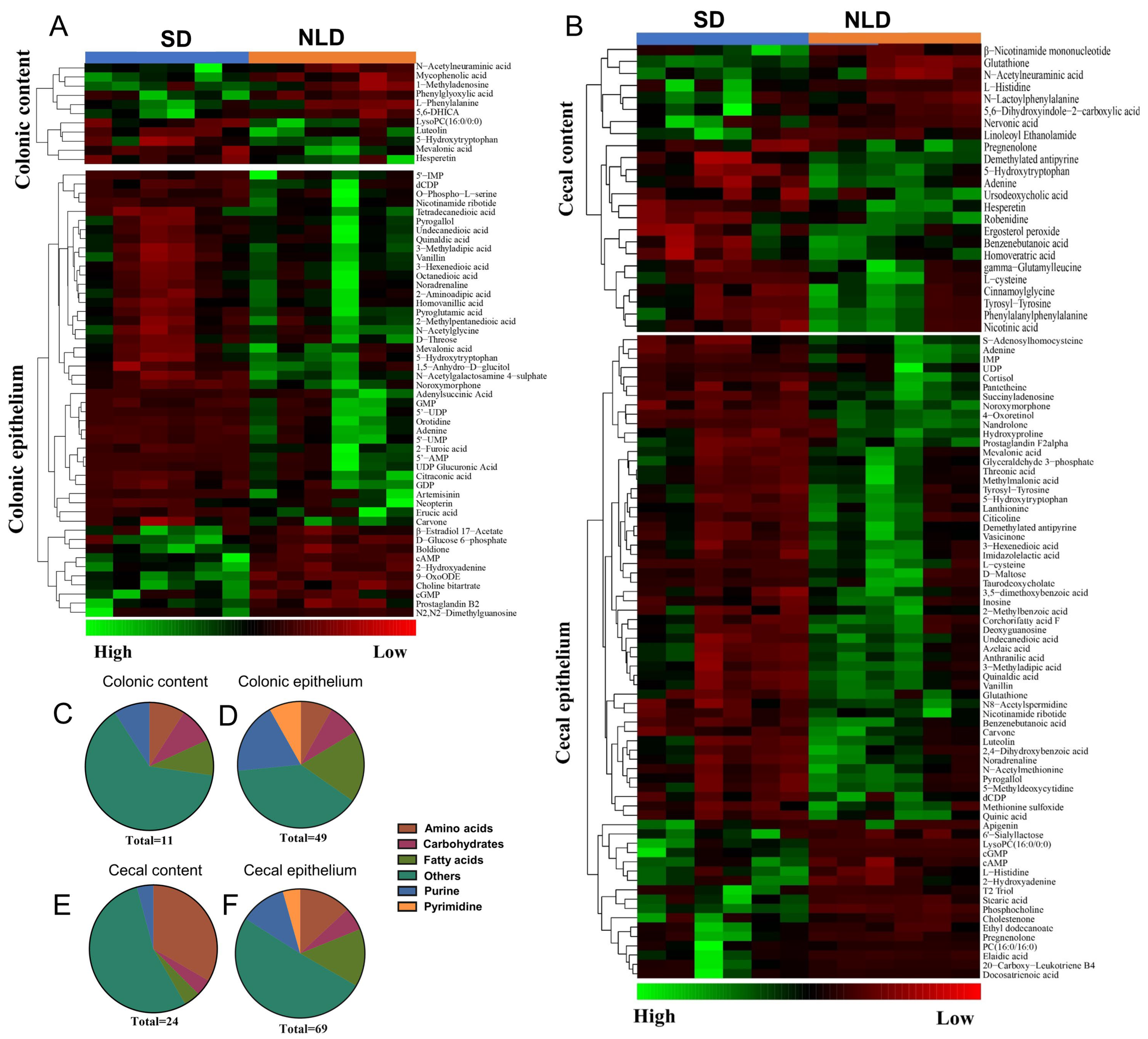
2.6. Identification and Classification of Differentially Altered Metabolites of Metabolomics Data in ABX Mice under NLD and SD
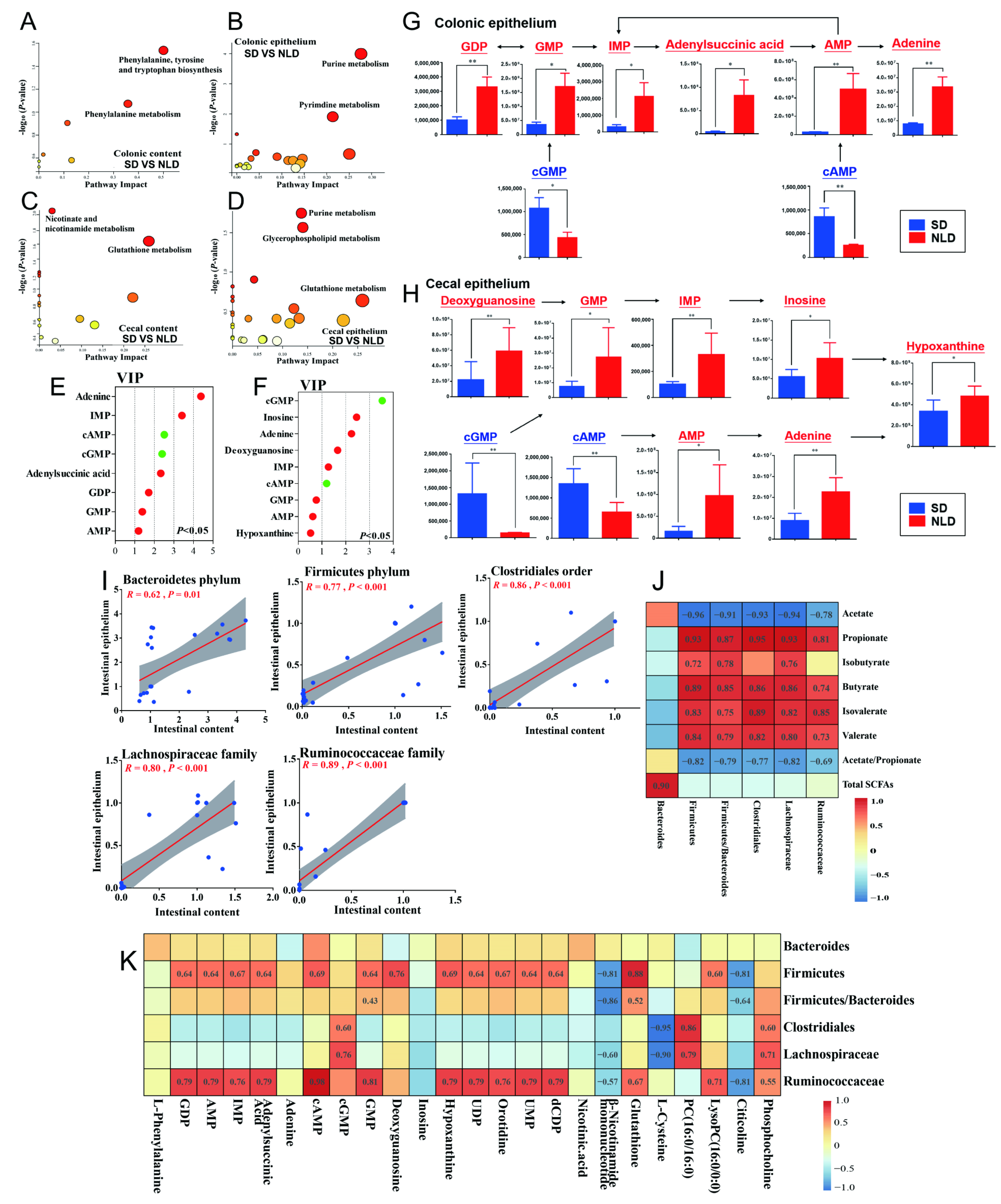
2.7. Characterization and Functional Analysis of Key Metabolic Pathways of Metabolomics Data in ABX Mice under NLD and SD
2.8. Correlations between Gut Microbes, SCFA Proportions, and Metabolites
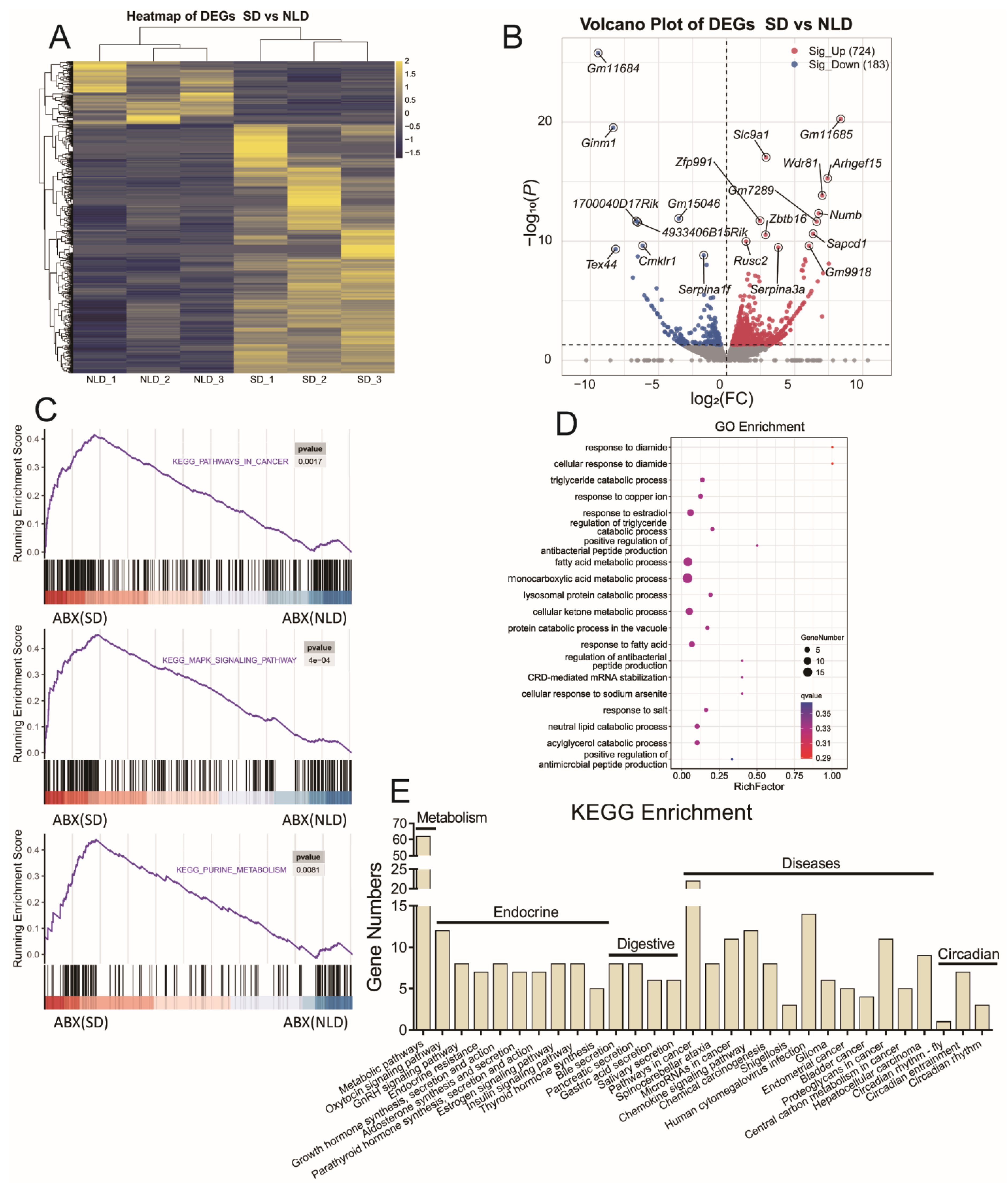
2.9. Hepatic Transcriptome Sequencing Profiles and Pathways Enrichment in ABX Mice under NLD and SD
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Mice Management and Experimental Design
4.3. Sample Collection
4.4. Detection of Serum Biochemical Parameters
4.5. Determination of SCFAs
4.6. Colonic and Cecal Contents and Epithelial DNA Extraction
4.7. Quantitative Real-Time PCR of Gut Microbes
4.8. Metabolite Extraction in Intestinal Contents and Epithelium
4.9. Non-Targeted Metabolomics Analysis
4.10. Hepatic Transcriptome Sequencing and Data Processing
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Valekunja, U.K.; Stangherlin, A.; Howell, S.A.; Snijders, A.P.; Damodaran, G.; Reddy, A.B. Circadian rhythms in the absence of the clock gene Bmal1. Science 2020, 367, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.K.; Yamada, R.G.; Ukai, H.; Baggs, J.E.; Miraglia, L.J.; Kobayashi, T.J.; Welsh, D.K.; Kay, S.A.; Ueda, H.R.; Hogenesch, J.B. Feedback repression is required for mammalian circadian clock function. Nat. Genet. 2006, 38, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Puram, R.V.; Kowalczyk, M.S.; de Boer, C.G.; Schneider, R.K.; Miller, P.G.; McConkey, M.; Tothova, Z.; Tejero, H.; Heckl, D.; Järås, M.; et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell 2016, 165, 303–316. [Google Scholar] [CrossRef]
- Mure, L.S.; Vinberg, F.; Hanneken, A.; Panda, S. Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science 2019, 366, 1251–1255. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef]
- Stokes, K.; Cooke, A.; Chang, H.; Weaver, D.R.; Breault, D.T.; Karpowicz, P. The Circadian Clock Gene BMAL1 Coordinates Intestinal Regeneration. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 95–114. [Google Scholar] [CrossRef]
- Yoshida, D.; Aoki, N.; Tanaka, M.; Aoyama, S.; Shibata, S. The circadian clock controls fluctuations of colonic cell proliferation during the light/dark cycle via feeding behavior in mice. Chronobiol. Int. 2015, 32, 1145–1155. [Google Scholar] [CrossRef]
- Bishehsari, F.; Levi, F.; Turek, F.W.; Keshavarzian, A. Circadian Rhythms in Gastrointestinal Health and Diseases. Gastroenterology 2016, 151, e1–e5. [Google Scholar] [CrossRef]
- Voigt, R.M.; Summa, K.C.; Forsyth, C.B.; Green, S.J.; Engen, P.; Naqib, A.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcohol. Clin. Exp. Res. 2016, 40, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Liu, F.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef] [PubMed]
- Alhopuro, P.; Bjorklund, M.; Sammalkorpi, H.; Turunen, M.; Tuupanen, S.; Bistrom, M.; Niittymaki, I.; Lehtonen, H.J.; Kivioja, T.; Launonen, V.; et al. Mutations in the circadian gene CLOCK in colorectal cancer. Mol. Cancer Res. 2010, 8, 952–960. [Google Scholar] [CrossRef]
- Skene, D.J.; Skornyakov, E.; Chowdhury, N.R.; Gajula, R.P.; Middleton, B.; Satterfield, B.C.; Porter, K.I.; Van Dongen, H.P.A.; Gaddameedhi, S. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 7825–7830. [Google Scholar] [CrossRef]
- van Mark, A.; Spallek, M.; Groneberg, D.A.; Kessel, R.; Weiler, S.W. Correlates shift work with increased risk of gastrointestinal complaints or frequency of gastritis or peptic ulcer in H. pylori-infected shift workers? Int. Arch. Occup. Environ. Health 2010, 83, 423–431. [Google Scholar] [CrossRef]
- Janney, A.; Powrie, F.; Mann, E.H. Host-microbiota maladaptation in colorectal cancer. Nature 2020, 585, 509–517. [Google Scholar] [CrossRef]
- Gomez, A.; Luckey, D.; Taneja, V. The gut microbiome in autoimmunity: Sex matters. Clin. Immunol. 2015, 159, 154–162. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Chevalier, G.; Laveissière, A.; Desachy, G.; Barnich, N.; Sivignon, A.; Maresca, M.; Nicoletti, C.; Di Pasquale, E.; Martinez-Medina, M.; Simpson, K.W.; et al. Blockage of bacterial FimH prevents mucosal inflammation associated with Crohn’s disease. Microbiome 2021, 9, 176. [Google Scholar] [CrossRef]
- Jin, L.; Shi, X.; Yang, J.; Zhao, Y.; Xue, L.; Xu, L.; Cai, J. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell 2021, 12, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.F., 2nd; Behrendt, C.L.; Ruhn, K.A.; Lee, S.; Raj, P.; Takahashi, J.S.; Hooper, L.V. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell 2021, 184, 4154–4167.e4112. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Callejon-Leblic, B.; Selma-Royo, M.; Collado, M.C.; Abril, N.; Garcia-Barrera, T. Impact of Antibiotic-Induced Depletion of Gut Microbiota and Selenium Supplementation on Plasma Selenoproteome and Metal Homeostasis in a Mice Model. J. Agric. Food Chem. 2021, 69, 7652–7662. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Fu, R.; Duan, Z.; Wang, P.; Zhu, C.; Fan, D. Ginsenoside Rk3 alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Res. Int. 2021, 146, 110465. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef]
- He, B.; Liu, Y.; Hoang, T.K.; Tian, X.; Taylor, C.M.; Luo, M.; Tran, D.Q.; Tatevian, N.; Rhoads, J.M. Antibiotic-modulated microbiome suppresses lethal inflammation and prolongs lifespan in Treg-deficient mice. Microbiome 2019, 7, 145. [Google Scholar] [CrossRef]
- Ojima, M.N.; Gotoh, A.; Takada, H.; Odamaki, T.; Xiao, J.-Z.; Katoh, T.; Katayama, T. Bifidobacterium bifidum Suppresses Gut Inflammation Caused by Repeated Antibiotic Disturbance Without Recovering Gut Microbiome Diversity in Mice. Front. Microbiol. 2020, 11, 1349. [Google Scholar] [CrossRef]
- Pan, J.; Gong, G.; Wang, Q.; Shang, J.; He, Y.; Catania, C.; Birnbaum, D.; Li, Y.; Jia, Z.; Zhang, Y.; et al. A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea. Nat. Commun. 2022, 13, 2117. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N.; Rakicioglu, N. Do Rotating Night Shifts Change Nurses’ Nutritional Status? A Cross-Sectional Study. J. Am. Coll. Nutr. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X.; et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yue, F.; Xing, L.; Wu, S.; Shi, Y.; Li, J.; Xiang, X.; Lam, S.M.; Shui, G.; Russell, R.; et al. Constant Light Exposure Alters Gut Microbiota and Promotes the Progression of Steatohepatitis in High Fat Diet Rats. Front. Microbiol. 2020, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Huang, F.; Zhao, A.; Lei, S.; Zhang, Y.; Xie, G.; Chen, T.; Qu, C.; Rajani, C.; Dong, B.; et al. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017, 15, 120. [Google Scholar] [CrossRef]
- Tsukuda, N.; Yahagi, K.; Hara, T.; Watanabe, Y.; Matsumoto, H.; Mori, H.; Higashi, K.; Tsuji, H.; Matsumoto, S.; Kurokawa, K.; et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 2021, 15, 2574–2590. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut. Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Marko, L.; Hoges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Gobel, A.; Rauner, M.; Hofbauer, L.C.; Rachner, T.D. Cholesterol and beyond—The role of the mevalonate pathway in cancer biology. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188351. [Google Scholar] [CrossRef]
- Zhu, J.J.; Djukovic, D.; Deng, L.L.; Gu, H.W.; Himmati, F.; Abu Zaid, M.; Chiorean, E.G.; Raftery, D. Targeted serum metabolite profiling and sequential metabolite ratio analysis for colorectal cancer progression monitoring. Anal. Bioanal. Chem. 2015, 407, 7857–7863. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.C.; Philip, N.H.; Hui, L.; Zhou, X.; Franklin, R.A.; Kong, Y.; Medzhitov, R. Desynchronization of the molecular clock contributes to the heterogeneity of the inflammatory response. Sci. Signal 2019, 12, 1851. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, J.; Navas-Carrillo, D.; Orenes-Pinero, E. Alterations of circadian rhythms and their impact on obesity, metabolic syndrome and cardiovascular diseases. Crit. Rev. Food Sci. 2020, 60, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Guerrero-Vargas, N.N. Synchrony between suprachiasmatic nucleus-driven signals and the light/dark cycle is essential for liver homeostasis. Hepatology 2017, 65, 2110–2112. [Google Scholar] [CrossRef]
- Kettner, N.M.; Voicu, H.; Finegold, M.J.; Coarfa, C.; Sreekumar, A.; Putluri, N.; Katchy, C.A.; Lee, C.; Moore, D.D.; Fu, L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 2016, 30, 909–924. [Google Scholar] [CrossRef]
- Chiaro, T.R.; Soto, R.; Stephens, W.Z.; Kubinak, J.L.; Petersen, C.; Gogokhia, L.; Bell, R.; Delgado, J.C.; Cox, J.; Voth, W.; et al. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci. Transl. Med. 2017, 9, eaaf9044. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Cheng, P.; Qian, C.; Xu, F.; Yang, Y.; Wang, A.; Chen, W.; Sun, Z.; Lu, Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 2020, 10, 10665–10679. [Google Scholar] [CrossRef]
- Mishima, E.; Ichijo, M.; Kawabe, T.; Kikuchi, K.; Akiyama, Y.; Toyohara, T.; Suzuki, T.; Suzuki, C.; Asao, A.; Ishii, N.; et al. Germ-Free Conditions Modulate Host Purine Metabolism, Exacerbating Adenine-Induced Kidney Damage. Toxins 2020, 12, 547. [Google Scholar] [CrossRef]
- Okugawa, Y.; Toiyama, Y.; Shigeyasu, K.; Yamamoto, A.; Shigemori, T.; Yin, C.Z.; Ichikawa, T.; Yasuda, H.; Fujikawa, H.; Yoshiyama, S.; et al. Enhanced AZIN1 RNA editing and overexpression of its regulatory enzyme ADAR1 are important prognostic biomarkers in gastric cancer. J. Transl. Med. 2018, 16, 366. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Camici, M.; Allegrini, S.; Pesi, R.; Petrotto, E.; Tozzi, M.G. Emerging Role of Purine Metabolizing Enzymes in Brain Function and Tumors. Int. J. Mol. Sci. 2018, 19, 3598. [Google Scholar] [CrossRef]
- Ojani, R.; Alinezhad, A.; Abedi, Z. A highly sensitive electrochemical sensor for simultaneous detection of uric acid, xanthine and hypoxanthine based on poly(L-methionine) modified glassy carbon electrode. Sens. Actuat. B-Chem. 2013, 188, 621–630. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bortolotti, M.; Polito, L.; Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. BBA-Mol. Basis Dis. 2018, 1864, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S.S.; Kulkarni, S.; Saha, M.; Sylvia, K.E.; Sears, C.L.; Pasricha, P.J. Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology 2020, 159, 200–213.e208. [Google Scholar] [CrossRef] [PubMed]
- Steinman, M.Q.; Knight, J.A.; Trainor, B.C. Effects of photoperiod and food restriction on the reproductive physiology of female California mice. Gen. Comp. Endocrinol. 2012, 176, 391–399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhat, G.K.; Hamm, M.L.; Igietseme, J.U.; Mann, D.R. Does leptin mediate the effect of photoperiod on immune function in mice? Biol. Reprod. 2003, 69, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Gronseth, T.; Vestby, L.K.; Nesse, L.L.; Thoen, E.; Habimana, O.; von Unge, M.; Silvola, J.T. Lugol’s solution eradicates Staphylococcus aureus biofilm in vitro. Int. J. Pediatr. Otorh. 2017, 103, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Tan, F.; Zhou, X.; Zhao, X. Lactobacillus fermentum CQPC06 in naturally fermented pickles prevents non-alcoholic fatty liver disease by stabilizing the gut-liver axis in mice. Food Funct. 2020, 11, 8707–8723. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, A.; Liu, X.Y.; Ma, Y.; Zhao, F.F.; Wang, M.Z.; Loor, J.J.; Wang, H.R. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Liu, A.; Zhang, T.S.; Li, Y.; Zhao, H. Gas Chromatography Detection Protocol of Short-chain Fatty Acids in Mice Feces. Bio-Protocol 2020, 10, 3672. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Zhang, G.; Zhang, T.; Lou, J.; Liu, J. L-Arabinose Elicits Gut-Derived Hydrogen Production and Ameliorates Metabolic Syndrome in C57BL/6J Mice on High-Fat-Diet. Nutrients 2019, 11, 3054. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi-Abyane, M.; Alipour, D.; Moghimi, H.R. Effects of different sources of nitrogen on performance, relative population of rumen microorganisms, ruminal fermentation and blood parameters in male feedlotting lambs. Animal 2020, 14, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, X.; Tang, R.; Wang, K. Real-time PCR quantification of the predominant bacterial divisions in the distal gut of Meishan and Landrace pigs. Anaerobe 2008, 14, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Gacias, M.; Gaspari, S.; Santos, P.M.G.; Tamburini, S.; Andrade, M.; Zhang, F.; Shen, N.; Tolstikov, V.; Kiebish, M.A.; Dupree, J.L.; et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife 2016, 5, 13442. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Zhang, H.; Yu, L.H.; Dong, L.; Gong, D.Q.; Yao, J.H.; Wang, H.R. Illumina Sequencing and Metabolomics Analysis Reveal Thiamine Modulation of Ruminal Microbiota and Metabolome Characteristics in Goats Fed a High-Concentrate Diet. Front. Microbiol. 2021, 12, 653283. [Google Scholar] [CrossRef]
- Li, K.P.; Yuan, M.; Wu, Y.L.; Pineda, M.; Zhang, C.M.; Chen, Y.F.; Chen, Z.Q.; Rong, X.L.; Turnbull, J.E.; Guo, J. A High-Fat High-Fructose Diet Dysregulates the Homeostatic Crosstalk Between Gut Microbiome, Metabolome, and Immunity in an Experimental Model of Obesity. Mol. Nutr. Food Res. 2022, 66, e2100950. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]


| Gut Microbes | Forward Primer Sequence | Reverse Primer Sequence | References |
|---|---|---|---|
| Firmicutes phylum | GGAGYATGTGGTTTAATTCGAAGCA | AGCTGACGACAACCATGCAC | [63] |
| Bacteroidetes phylum | GGARCATGTGGTTTAATTCGATGAT | AGCTGACGACAACCATGCAG | [63] |
| Clostridiales order | GCGTTATCCGGATTTAC | ACACCTAGTATTCATCG | [64] |
| Lachnospiraceae family | CGGTACCTGACTAAGAAGC | AGTTTTATTCTTGCGAACG | [64] |
| Ruminococcaceae family | TTAACACAATAAGTWATCCACCTGG | ACCTTCCTCCGTTTTGTCAAC | [64] |
| Total bacteria | CGGCAACGAGCGCAACCC | CCATTGTAGCACGTGTGTAGCC | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, Y.; Chen, Y.; Ge, L.; Wei, W.; Wang, Y.; Hu, L.; Loor, J.J.; Wang, M.; Yin, J. The Short-Day Cycle Induces Intestinal Epithelial Purine Metabolism Imbalance and Hepatic Disfunctions in Antibiotic-Mediated Gut Microbiota Perturbation Mice. Int. J. Mol. Sci. 2022, 23, 6008. https://doi.org/10.3390/ijms23116008
Zhen Y, Chen Y, Ge L, Wei W, Wang Y, Hu L, Loor JJ, Wang M, Yin J. The Short-Day Cycle Induces Intestinal Epithelial Purine Metabolism Imbalance and Hepatic Disfunctions in Antibiotic-Mediated Gut Microbiota Perturbation Mice. International Journal of Molecular Sciences. 2022; 23(11):6008. https://doi.org/10.3390/ijms23116008
Chicago/Turabian StyleZhen, Yongkang, Yifei Chen, Ling Ge, Wenjun Wei, Yusu Wang, Liangyu Hu, Juan J. Loor, Mengzhi Wang, and Junliang Yin. 2022. "The Short-Day Cycle Induces Intestinal Epithelial Purine Metabolism Imbalance and Hepatic Disfunctions in Antibiotic-Mediated Gut Microbiota Perturbation Mice" International Journal of Molecular Sciences 23, no. 11: 6008. https://doi.org/10.3390/ijms23116008
APA StyleZhen, Y., Chen, Y., Ge, L., Wei, W., Wang, Y., Hu, L., Loor, J. J., Wang, M., & Yin, J. (2022). The Short-Day Cycle Induces Intestinal Epithelial Purine Metabolism Imbalance and Hepatic Disfunctions in Antibiotic-Mediated Gut Microbiota Perturbation Mice. International Journal of Molecular Sciences, 23(11), 6008. https://doi.org/10.3390/ijms23116008








