Benzoic Acid, Chlorine Dioxide, and 1-Methylcyclopropene Induce Flavonoid Metabolic Shifts in Postharvest Flowering Chinese Cabbage Revealed by High-Dimensional Analytical Data
Abstract
:1. Introduction
2. Results and Discussion
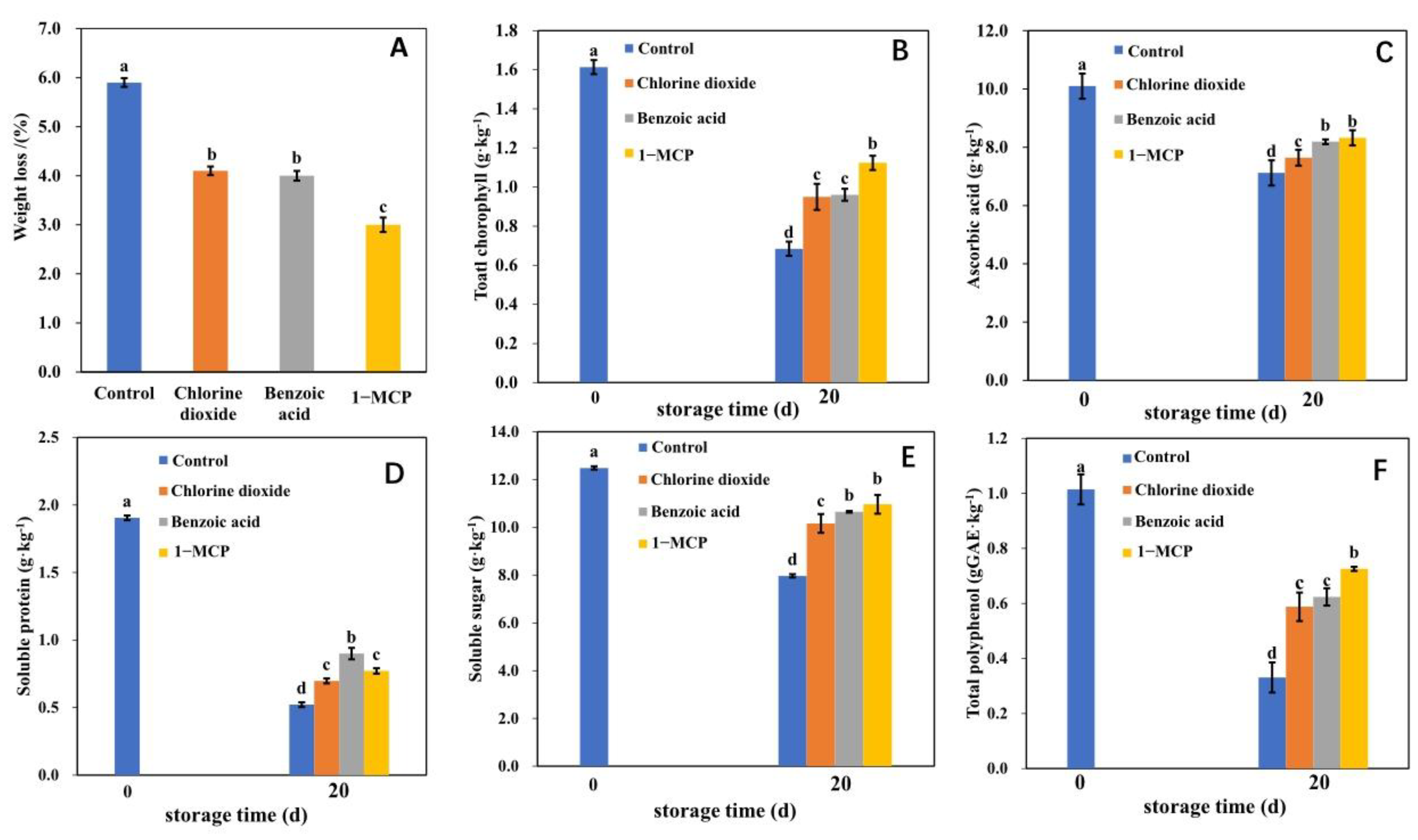
2.1. The Phenotype and Quality of Postharvest Flowering Chinese Cabbage Treated with Chlorine Dioxide, Benzoic Acid, and 1-MCP
2.2. Transcript-Metabolite Profiling of Postharvest Flowering Chinese Cabbage Treated with Chlorine Dioxide, Benzoic Acid, and 1-MCP
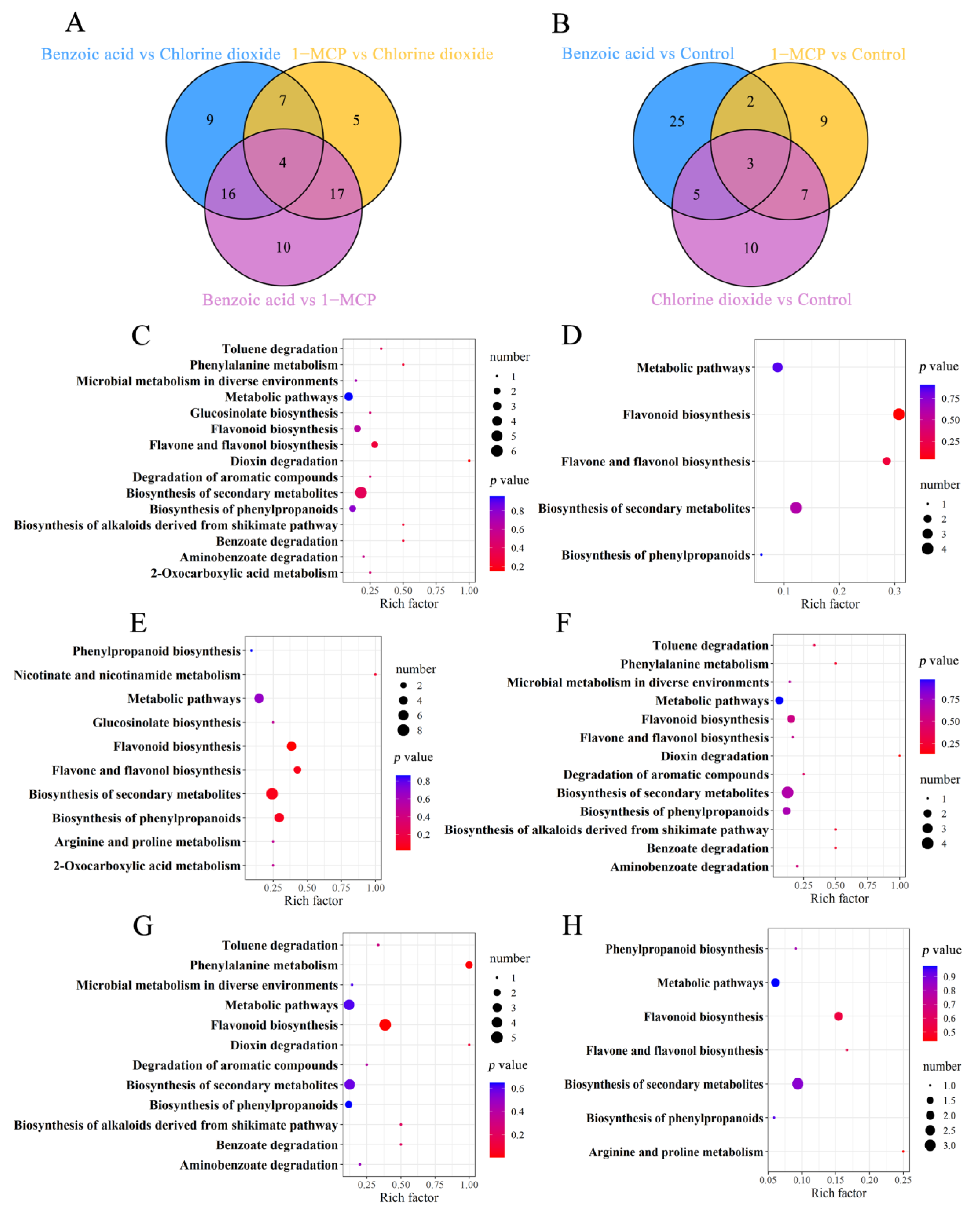
2.2.1. Transcriptome Analysis
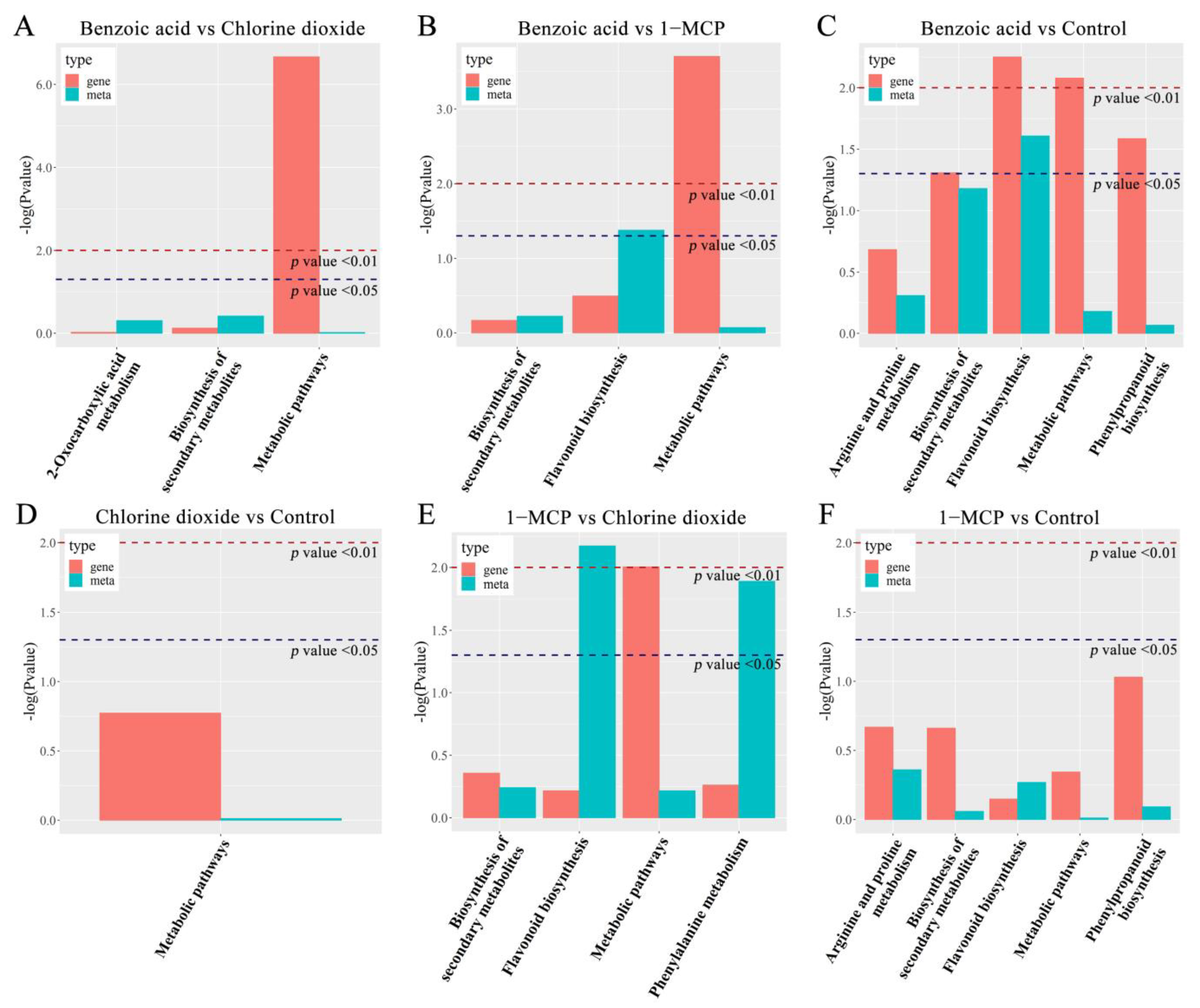
2.2.2. Metabolite Analysis
2.2.3. Transcript-Metabolite Correlation Network
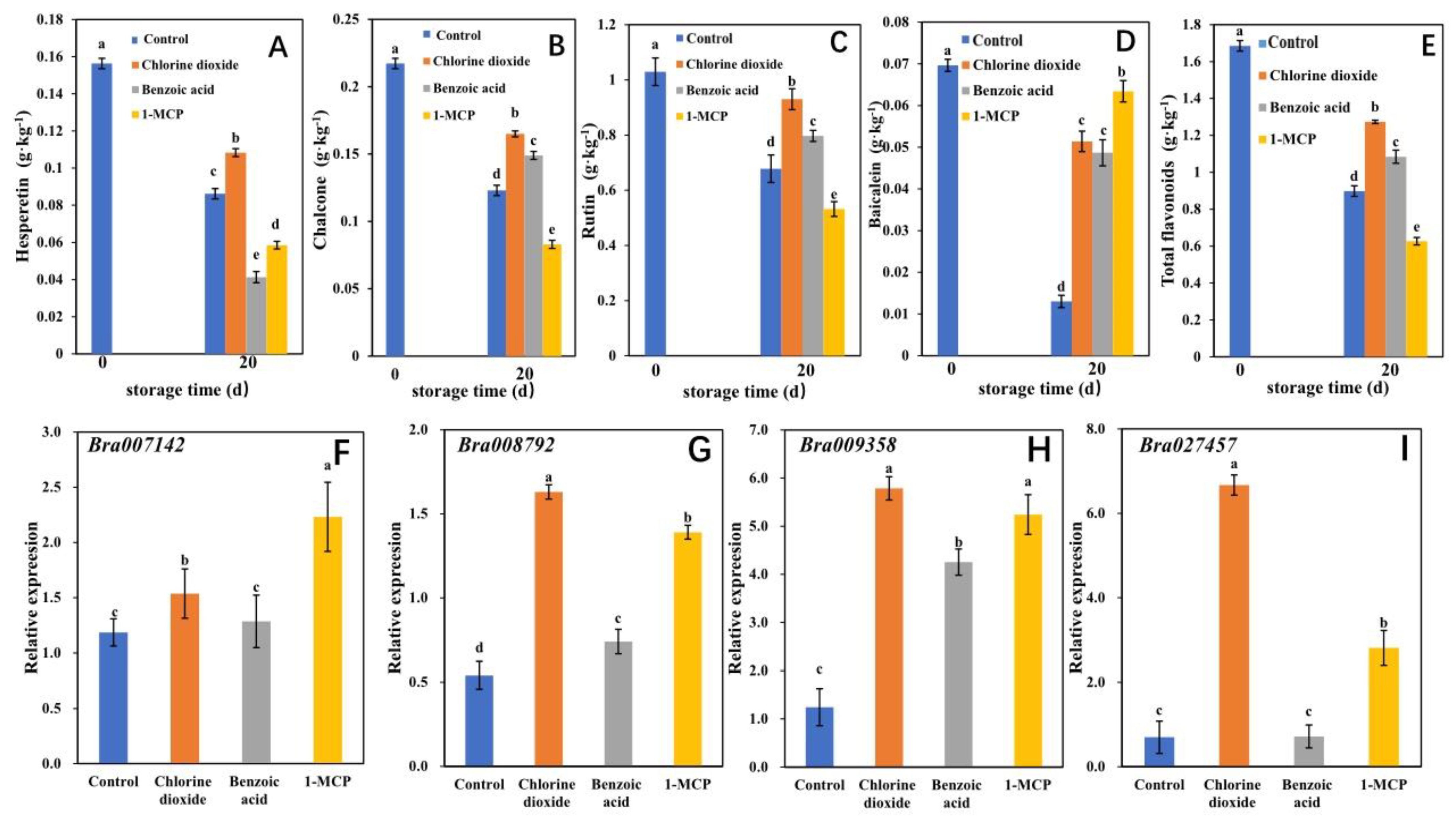
2.3. The Confirmation of Functional Differential Genes and Their Regulating Flavonoids Metabolites
3. Materials and Methods
3.1. Materials and Reagents
3.2. Quality Analysis
3.2.1. Determination of Chlorophyll
3.2.2. Determination of Ascorbic Acid
3.2.3. Determination of Soluble Protein
3.2.4. Determination of Soluble Sugar
3.2.5. Determination of Total Polyphenolic
3.2.6. Determination of Total Flavonoids
3.3. Transcriptome-Metabolite Profiling
3.3.1. Transcriptome Sequencing and Analysis
3.3.2. Metabolite Detection and Analysis
3.4. qRT-PCR Analysis of Functional Genes
3.5. Confirmation of Functional Metabolites with HPLC
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biol. Technol. 2018, 139, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.F.; Yang, C.; Fei, Y.; Ma, L.Z. Physiological and proteomic analyses of 1-MCP treatment on lignification in fresh common bean (Phaseolus vulgaris L.) during storage. Postharvest Biol. Technol. 2020, 160, 111041. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellan-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Jing, P. Anthocyanins in Brassicaceae: Composition, stability, bioavailability, and potential health benefits. Crit. Rev. Food Sci. Nutr. 2020, 62, 2205–2220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Kang, Y.Y.; Zhong, M.; Zhang, L.; Chai, X.R.; Jiang, X.X.; Yang, X. Effects of iron deficiency stress on plant growth and quality in flowering Chinese cabbage and its adaptive response. Agronomy 2022, 12, 875. [Google Scholar] [CrossRef]
- Hanson, P.; Yang, R.Y.; Chang, L.C.; Ledesma, L.; Ledesma, D. Carotenoids, ascorbic acid, minerals, and total glucosinolates in choysum (Brassica rapa cvg. parachinensis) and kailaan (B. oleraceae Alboglabra group) as affected by variety and wet and dry season production. J. Food. Compos. Anal. 2011, 24, 950–962. [Google Scholar]
- Kovalikova, Z.; Kubes, J.; Skalicky, M.; Kuchtickova, N.; Maskova, L.; Tuma, J.; Vachova, P.; Hejnak, V. Changes in content of polyphenols and ascorbic acid in leaves of white cabbage after pest infestation. Molecules 2019, 24, 2622. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.D.; Liu, Z.H.; Liu, B.; Wang, Y.C.; Wang, J.J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Song, S.W.; Lei, Y.L.; Huang, X.M.; Su, W.; Chen, R.Y.; Hao, Y.W. Crosstalk of cold and gibberellin effects on bolting and flowering in flowering Chinese cabbage. J. Integr. Agric. 2019, 18, 992–1000. [Google Scholar] [CrossRef]
- Tan, X.L.; Zhao, Y.T.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.G.; Tao, N.G. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Fang, Y.; Ren, Z.X.; Chen, C.; Jin, C.; Raman, S.T.; Ganeshan, A.K.P.G.; Jia, J.Q.; Gui, Z.Z. Effects of treatment with 1-methylcyclopropene and ClO2 on the postharvest shelf-life and physiological quality of mulberry fruit (Morus Alba L.). J. Appl. Bot. Food Qual. 2016, 39, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Mischek, D.; Krapfenbauer-Cermak, C. Exposure assessment of food preservatives (sulphites, benzoic and sorbic acid) in Austria. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Kok, D.; Bal, E. Changes on bioactive compounds and electrochemical characteristics of cv. Horoz Karası table grape (V. vinifera L.) induced by various doses of preharvest applications of benzoic acid, citric acid and oxalic acid at berry setting and verasion periods. Erwerbs-Obstbau 2019, 61, 17–24. [Google Scholar] [CrossRef]
- Plesoianu, A.M.; Tutulescu, F.; Nour, V. Postharvest antimicrobial treatments with organic acids to improve the shelf life of fresh blueberries. Not. Bot. Horti Agrobot. 2020, 48, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Perez, J.J.; Vero, S.; Diaz-Rivera, E.; Lara-Capistran, L.; Noa-Carrazana, J.C.; Hernandez-Montiel, L.G. Application of chlorine dioxide (ClO2) and marine yeasts to control postharvest anthracnose disease in mango (Mangifera indica L.). Cienc. Investig. Agrar. 2019, 46, 266–275. [Google Scholar] [CrossRef]
- Wei, F.; Fu, M.R.; Li, J.P.; Yang, X.Y.; Chen, Q.M.; Tian, S.P. Chlorine dioxide delays the reddening of postharvest green peppers by affecting the chlorophyll degradation and carotenoid synthesis pathways. Postharvest Biol. Technol. 2019, 156, 110939. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, B.; Peng, X.Y.; Wang, J.D.; Li, Q.P.; Jin, J.; Ha, Y.M. Effects of chlorine dioxide treatment on respiration rate and ethylene synthesis of postharvest tomato fruit. Postharvest Biol. Technol. 2014, 93, 9–14. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B. Comparison of hydrogen sulphide with 1-methylcyclopropene (1-MCP) to inhibit senescence of the leafy vegetable, pak choy. Postharvest Biol. Technol. 2018, 137, 129–133. [Google Scholar] [CrossRef]
- Cao, S.F.; Yang, Z.F.; Zheng, Y.H. Effect of 1-methylcyclopene on senescence and quality maintenance of green bell pepper fruit during storage at 20 °C. Postharvest Biol. Technol. 2012, 70, 1–6. [Google Scholar] [CrossRef]
- Gómez-Lobato, M.E.; Hasperué, J.H.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. Effect of 1-MCP on the expression of chlorophyll degrading genes during senescence of broccoli (Brassica oleracea L.). Sci. Hortic. 2012, 144, 208–211. [Google Scholar] [CrossRef]
- Gong, Y.P.; Mattheis, J.P. Effect of ethylene and 1-methylcyclopropene on chlorophyll catabolism of broccoli florets. Plant Growth Regul. 2003, 40, 33–38. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Mahfouz, S.A. Effect of 1-methylcyclopropene (1-MCP) on the postharvest senescence of coriander leaves during storage and its relation to antioxidant enzyme activity. Sci. Hortic. 2012, 141, 69–75. [Google Scholar] [CrossRef]
- Ili’c, Z.; Aharon, Z.; Perzelan, Y.; Alkalai-Tuvia, S.; Fallik, E. The influence of 1-MCP on chlorophyll, antioxidants activity and quality changes in “Ever-Green” and Red Pepper fruits after harvest. Acta. Hortic. 2008, 830, 643–650. [Google Scholar]
- Zhang, X.M.; Li, J.P.; Fu, M.R.; Jin, T.; Yang, X.Y.; Han, C.; Chen, Q.M.; Yue, F.L. Effects of the combination of 1-MCP and ClO2 on shelf life quality of green pepper. Sci. Technol. Food Ind. 2018, 39, 275–280. [Google Scholar]
- Kan, J.; Huo, T.; Xie, W.; Jin, C. Effect of 1-MCP and UV-C on antioxidant capacity in apple (Malus pumila Mil.) fruit during storage. LWT 2019, 40, 281–285. [Google Scholar]
- Wei, J. The Effects of Nitric Oxide and Chlorine Dioxide on Postharvest Disease and Pesticide Residues of Hami Melon and Table Grape; Xinjiang University: Urumqi, China, 2018. [Google Scholar]
- Du, Y.M.; An, T.; Zhao, H.D.; Han, C.; Sun, F.; Chen, Q.M.; Yue, F.L.; Luo, Z.S.; Fu, M.R. Synergistic inhibitory effect of 1-methylcyclopropene (1-MCP) and chlorine dioxide (ClO2) treatment on chlorophyll degradation of green pepper fruit during storage. Postharvest Biol. Technol. 2021, 171, 111363. [Google Scholar] [CrossRef]
- Picchi, V.; Migliori, C.; Lo Scalzo, R.; Campanelli, G.; Ferrari, V.; Di Cesare, L.F. Phytochemical content in organic and conventionally grown Italian cauliflower. Food Chem. 2012, 130, 501–509. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, F.M. Phytochemistry and biological activity of mustard (Brassica juncea): A review. Cyta. J. Food. 2020, 18, 704–718. [Google Scholar] [CrossRef]
- Mazzucotelli, C.A.; Gonzalez-Aguilar, G.A.; Villegas-Ochoa, M.A.; Dominguez-Avila, A.J.; Ansorena, M.R.; Di Scala, K.C. Chemical characterization and functional properties of selected leafy vegetables for innovative mixed salads. J. Food Biochem. 2018, 42, e12461. [Google Scholar] [CrossRef] [Green Version]
- Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive compounds in Brassicaceae vegetables with a role in the prevention of chronic diseases. Molecules 2018, 23, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trejo-Tellez, L.I.; Estrada-Ortiz, E.; Gomez-Merino, F.C.; Becker, C.; Krumbein, A.; Schwarz, D. Flavonoid, nitrate and glucosinolate concentrations in Brassica species are differentially affected by photosynthetically active radiation, phosphate and phosphite. Front. Plant Sci. 2019, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Zaghdoud, C.; Carvajal, M.; Moreno, D.A.; Ferchichi, A.; Martinez-Ballesta, M.D. Health-promoting compounds of broccoli (Brassica oleracea L. var. italica) plants as affected by nitrogen fertilisation in projected future climatic change environments. J. Sci. Food Agric. 2016, 96, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Cefola, M.; Amodio, M.L.; Rinaldi, R.; Vanadia, S.; Colelli, G. Exposure to 1-methylcyclopropene (1-MCP) delays the effects of ethylene on fresh-cut broccoli raab (Brassica rapa L.). Postharvest Biol. Technol. 2010, 58, 29–35. [Google Scholar] [CrossRef]
- Yao, X.T.; Zhang, L.; Li, T.T.; Li, L.; Li, S.Y.; Zhang, Z.Z.; Wu, J.H. Effect of benzoic acid and gibberellin on preservation of cut Chinese narcissus flowers. Chin. J. Trop. Crops 2020, 41, 184–191. [Google Scholar]
- Jiang, L.Q.; Feng, W.Y.; Li, F.; Xu, J.Y.; Ma, Y.P.; Ma, H.L. Effect of One-methylcyclopropene (1-MCP) and chlorine dioxide (ClO2) on preservation of green walnut fruit and kernel traits. J. Food Sci. Technol. 2015, 52, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Del, O.A.; Calzada, J.; Nunez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar]
- Zhao, Y.; Zheng, Y.Z.; Wang, J.; Ma, S.Y.; Yu, Y.M.; White, W.L.; Yang, S.P.; Yang, F.; Lu, J. Fucoidan extracted from Undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.C.; Wu, J.C.; Shahid, M.Q.; He, Y.H.; Lin, S.Q.; Liu, Z.H.; Yang, X.H. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef]
- Park, B.B.; Choi, J.W.; Park, D.; Choi, D.; Paek, J.; Kim, H.J.; Son, S.Y.; Ul Mushtaq, A.; Shin, H.; Kim, S.H. Structure-activity relationships of baicalein and its analogs as novel TSLP inhibitors. Sci. Rep. 2019, 9, 8762. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Cedillo-Rivera, R.; Mata, R. Antiprotozoal activity of the constituents of Conyxa filaginoides. J. Nat. Prod. 2001, 64, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Kakizaki, T.; Morimitsu, Y.; Ohara, T.; Hatakeyama, K.; Yoshiaki, H.; Kohori, J.; Nishio, T. Novel glucosinolate composition lacking 4-methylthio-3-butenyl glucosinolate in Japanese white radish (Raphanus sativus L.). Theor. Appl. Genet. 2015, 128, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Eum, H.L.; Yi, T.G.; Hong, S.J.; Park, N.I. Variations of bioactive compound contents and antioxidant capacity of Asparagus seedlings in 23 varieties. Hortic. Sci. 2020, 38, 291–302. [Google Scholar]
- Yuan, G.F.; Wang, X.P.; Guo, R.F.; Wang, Q.M. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Harbaum, B.; Hubbermann, E.M.; Zhu, Z.J.; Schwarz, K. Free and bound phenolic compounds in leaves of pak choi (Brassica campestris L. ssp chinensis var. communis) and Chinese leaf mustard (Brassica juncea Coss). Food Chem. 2008, 110, 838–846. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.F.; Zhang, L.G. Anthocyanin accumulation, antioxidant ability and stability, and a transcriptional analysis of anthocyanin biosynthesis in purple heading chinese cabbage (Brassica rapa L. ssp pekinensis). J. Agric. Food Chem. 2016, 64, 132–145. [Google Scholar] [CrossRef]
- Francisco, M.; Cartea, M.E.; Butron, A.M.; Sotelo, T.; Velasco, P. Environmental and genetic effects on yield and secondary metabolite production in Brassica rapa crops. J. Agric. Food Chem. 2012, 60, 5507–5514. [Google Scholar] [CrossRef]
- Jeong, H.R.; Cho, H.S.; Cho, Y.S.; Kim, D.O. Changes in phenolics, soluble solids, vitamin C, and antioxidant capacity of various cultivars of hardy kiwifruits during cold storage. Food Sci. Biotechnol. 2020, 29, 1763–1770. [Google Scholar] [CrossRef]
- MacLean, D.D.; Murr, D.P.; DeEll, J.R.; Mackay, A.B.; Kupferman, E.M. Inhibition of PAL, CHS, and ERS1 in ‘Redd Anjou’ pear (Pyrus communis L.) by 1-MCP. Postharvest Biol. Technol. 2007, 45, 46–55. [Google Scholar] [CrossRef]
- Guo, S.; Li, T.; Wu, C.; Fan, G.; Wang, H.; Shen, D. Melatonin and 1-methylcyclopropene treatments on delay senescence of apricots during postharvest cold storage by enhancing antioxidant system activity. J. Food Process. Preserv. 2021, 45, e15863. [Google Scholar] [CrossRef]
- Xie, G.F.; Feng, Y.C.; Chen, Y.; Zhang, M.S. Effects of 1-methylcyclopropene (1-MCP) and ethylene on postharvest lignification of common beans (Phaseolus vulgaris L.). ACS Omega 2020, 5, 8659–8666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kereamy, A.; Chervin, C.; Roustan, J.P.; Cheynier, V.; Souquet, J.M.; Moutounet, M.; Raynal, J.; Ford, C.; Latché, A.; Pech, J.C. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant. 2003, 119, 175–182. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Li, Y.Y.; Song, L.Q.; Zhao, L.L.; You, C.X.; Hao, Y.J. EIN3-LIKE1, MYB1, and ETHYLENE RESPONSE FACTOR3 act in a regulatory loop that synergistically modulates ethylene biosynthesis and anthocyanin accumulation. Plant Physiol. 2018, 178, 808–823. [Google Scholar] [CrossRef]
- Chaudhary, P.R.; Bang, H.; Jayaprakasha, G.K.; Patil, B.S. Effect of ethylene degreening on flavonoid pathway gene expression and phytochemicals in Rio Red grapefruit (Citrus paradisi Macf). Phytochem. Lett. 2017, 22, 270–279. [Google Scholar] [CrossRef]
- Andrea, M.; Reyes, J.; María, E.; Gómez, L.; Pedro, M.; Civello, G.A. Phenylalanine ammonia lyase is more relevant than chalcone synthase and chalcone isomerase in the biosynthesis of flavonoids during postharvest senescence of broccoli. J. Food Biochem. 2022, 46, e14054. [Google Scholar]
- Wu, X.; An, X.; Yu, M.; Ma, R.; Yu, Z. 1-Methylcyclopropene treatment on phenolics and the antioxidant system in postharvest peach combined with the liquid chromatography/mass spectrometry technique. J. Agric. Food Chem. 2018, 66, 6364–6372. [Google Scholar] [CrossRef]
- MacLean, D.D.; Murr, D.P.; DeEll, J.R.; Horvath, C.R. Postharvest variation in apple (Malus domestica Borkh.) flavonoids following harvest, storage, and 1-MCP treatment. J. Agric. Food Chem. 2006, 54, 870–878. [Google Scholar] [CrossRef]
- Caesar, J.; Tamm, A.; Ruckteschler, N.; Leifke, A.L.; Weber, B. Revisiting chlorophyll extraction methods in biological soil crusts -methodology for determination of chlorophyll a and chlorophyll a plus b as compared to previous methods. Biogeosciences 2018, 15, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, A.; Wilodarczyk-Stasiak, M.; Pankiewicz, U.; Kowalski, R.; Jamroz, J. Development and validation of a differential pulse polarography method for determination of total vitamin C and dehydroascorbic acid contents in foods. LWT 2020, 118, 108828. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, H.H.; Cui, C.H.; Wang, M.; Guo, J.J.; Wen, Z.P. Improvement of the assay of soluble sugar content (anthrone method). Lab. Sci. 2013, 16, 19–20. [Google Scholar]
- Attard, E. A Rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Cent. Eur. J. Chem. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Qin, M.J.; Qi, J.; Yu, B.Y.; Tang, L.I.; Xu, P.H. Influence of harvest times and processing methods on contents of total flavonoids and total saponins in roots of Ophiopogon japonicus. China J. Chin. Mater. Med. 2010, 35, 821. [Google Scholar]
- Rothenberg, D.O.; Yang, H.J.; Chen, M.B.; Zhang, W.T.; Zhang, L.Y. Metabolome and transcriptome sequencing analysis reveals anthocyanin metabolism in pink flowers of anthocyanin-rich tea (Camellia sinensis). Molecules 2019, 24, 1064. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zheng, P.C.; Liu, P.P.; Song, X.W.; Guo, F.; Li, Y.Y.; Ni, D.J.; Jiang, C.J. Novel insight into the role of withering process in characteristic flavor formation of teas using transcriptome analysis and metabolite profiling. Food Chem. 2019, 272, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chawla, V.; Sharma, E.; Mahajan, P.; Shankar, R.; Yadav, S.K. Comparative transcriptome analysis of chinary, assamica and cambod tea (Camellia sinensis) types during development and seasonal variation using RNA-seq technology. Sci. Rep. 2016, 6, 37244. [Google Scholar] [CrossRef]
- Guo, H.H.; Guo, H.X.; Zhang, L.; Tang, Z.M.; Yu, X.M. Metabolome and transcriptome association analysis reveals dynamic regulation of purine metabolism and flavonoid synthesis in transdifferentiation during somatic embryogenesis in cotton. Int. J. Mol. Sci. 2019, 20, 2070. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, L.; Li, Y.; Zhong, M.; Chai, X.; Zhao, P.; Huang, R.; Kang, Y.; Yang, X. Benzoic Acid, Chlorine Dioxide, and 1-Methylcyclopropene Induce Flavonoid Metabolic Shifts in Postharvest Flowering Chinese Cabbage Revealed by High-Dimensional Analytical Data. Int. J. Mol. Sci. 2022, 23, 6011. https://doi.org/10.3390/ijms23116011
Yue L, Li Y, Zhong M, Chai X, Zhao P, Huang R, Kang Y, Yang X. Benzoic Acid, Chlorine Dioxide, and 1-Methylcyclopropene Induce Flavonoid Metabolic Shifts in Postharvest Flowering Chinese Cabbage Revealed by High-Dimensional Analytical Data. International Journal of Molecular Sciences. 2022; 23(11):6011. https://doi.org/10.3390/ijms23116011
Chicago/Turabian StyleYue, Lingqi, Yongshen Li, Min Zhong, Xirong Chai, Puyan Zhao, Riming Huang, Yunyan Kang, and Xian Yang. 2022. "Benzoic Acid, Chlorine Dioxide, and 1-Methylcyclopropene Induce Flavonoid Metabolic Shifts in Postharvest Flowering Chinese Cabbage Revealed by High-Dimensional Analytical Data" International Journal of Molecular Sciences 23, no. 11: 6011. https://doi.org/10.3390/ijms23116011





