Coupling AFM, DSC and FT-IR towards Elucidation of Film-Forming Systems Transformation to Dermal Films: A Betamethasone Dipropionate Case Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Evaluation and Stability Screening of FFS
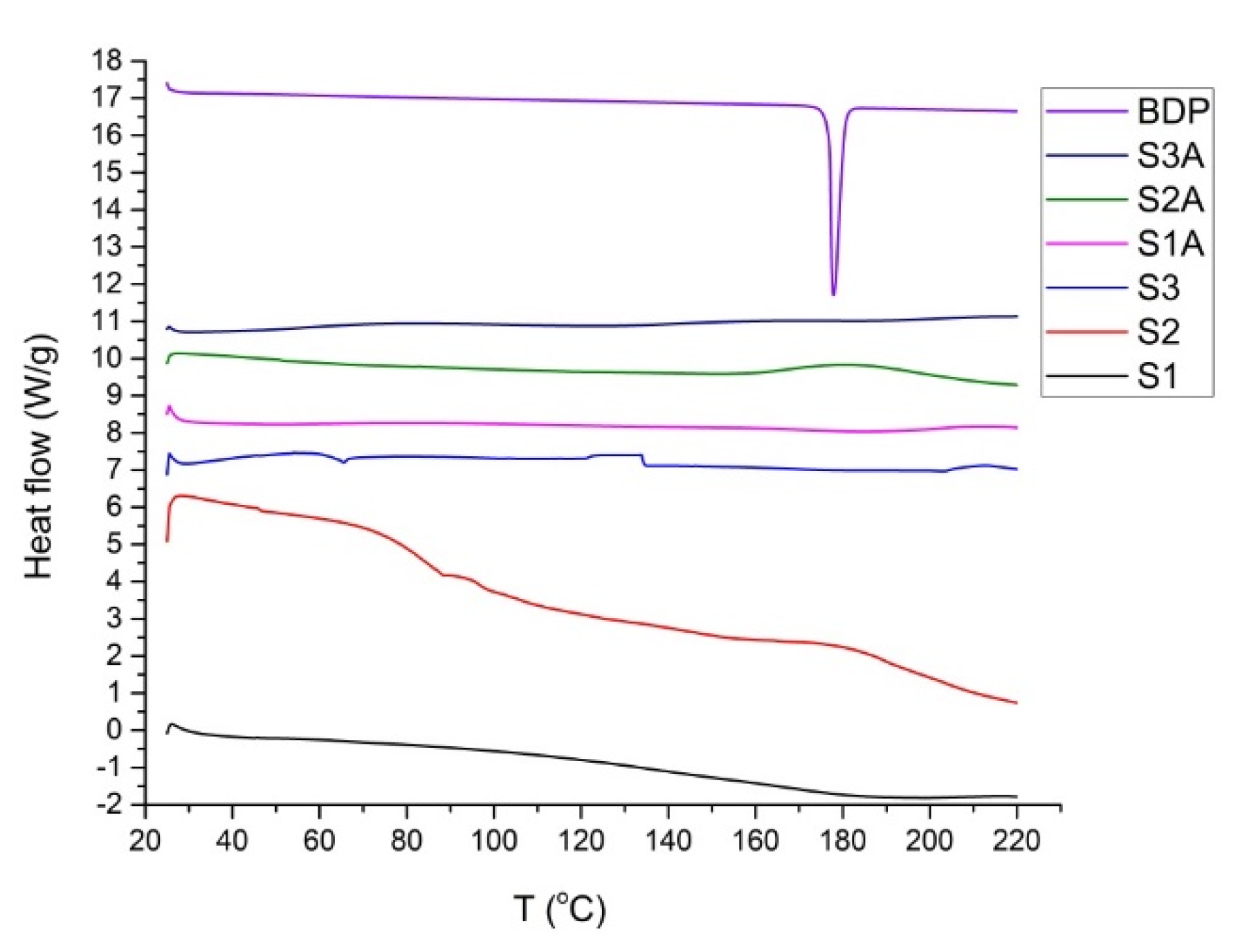
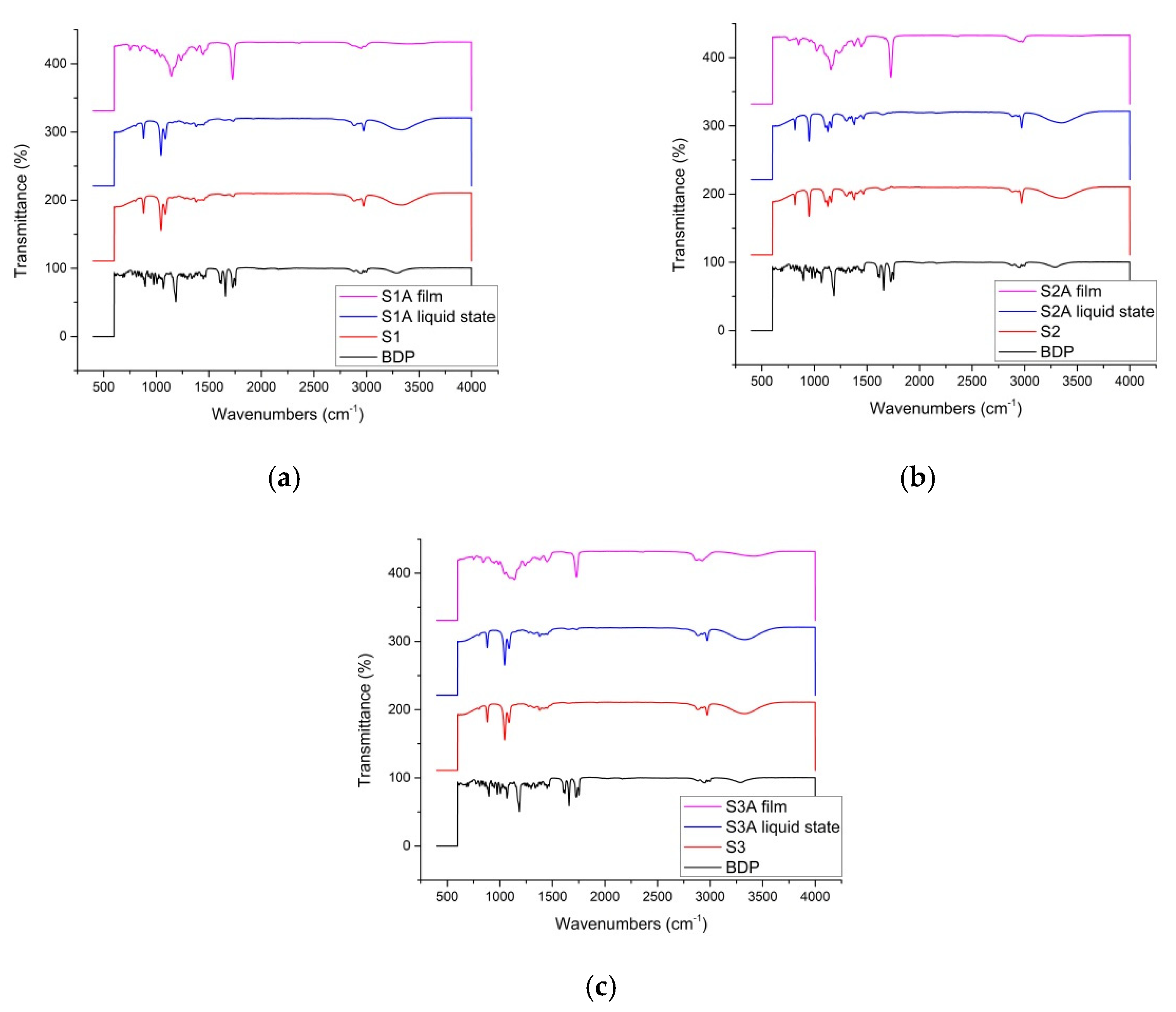
2.2. Thermal and Spectroscopic Analysis
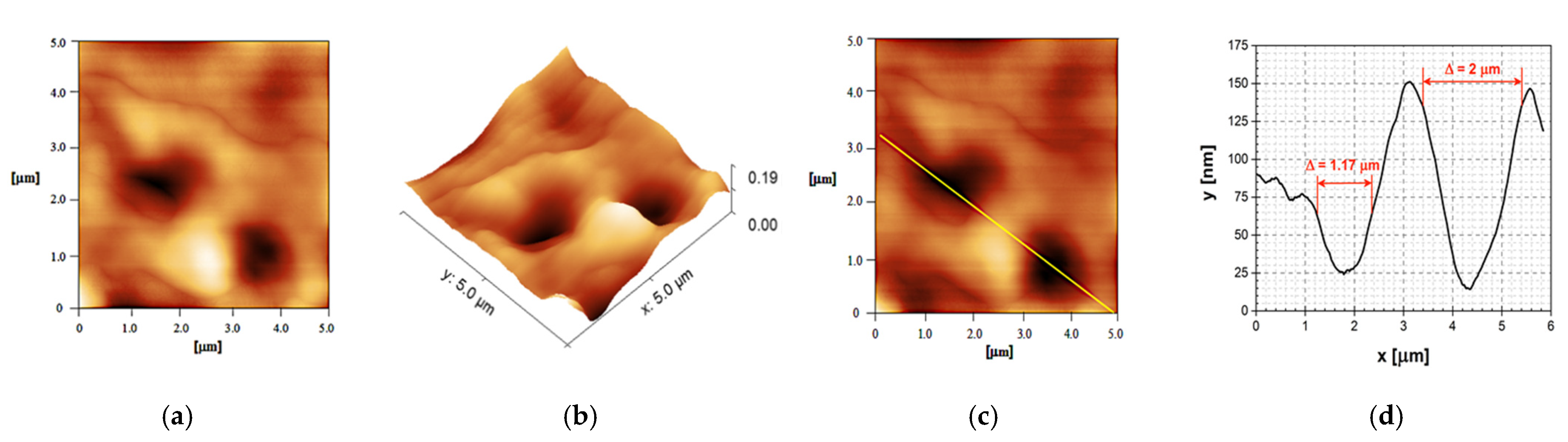
2.3. AFM Analysis of the Film Surface Morphology
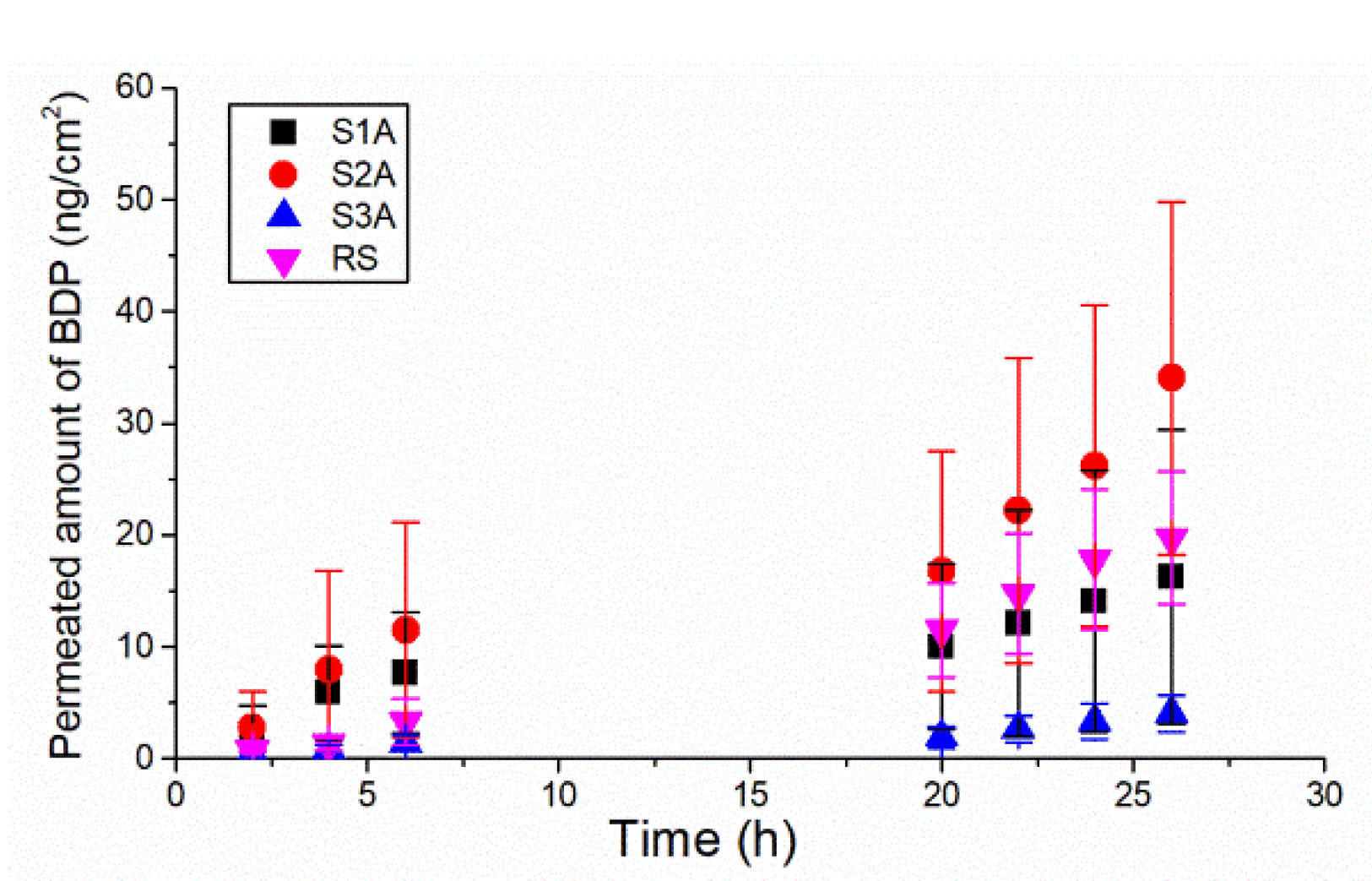
2.4. In Vitro Permeation of BDP through the Porcine Ear Epidermis
3. Materials and Methods
3.1. Materials
3.2. Preparation Method of the Polymeric FFSs with a Lipophilic Model Drug
3.3. Characterization of Physicochemical, Mechanical and Sensory Properties
3.3.1. Assessment of Drying Time, Thickness, Spreadability, Flexibility and Stickiness of the Casted Films
3.3.2. pH Values of FFS
3.3.3. Viscosity Measurements
3.4. Morphology and Methamorphosis Characterization
3.4.1. Differential Scanning Calorimetry (DSC)
3.4.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
3.4.3. Atomic Force Microscopy
3.5. In Vitro Skin Permeation Study
3.6. HPLC-MS/MS
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, X.; Feldman, S.R.; Chang, J.; Balkrishnan, R. Topical drug delivery systems in dermatology: A review of patient adherence issues. Expert Opin. Drug Deliv. 2012, 9, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Rottke, M.; Lunter, D.J.; Daniels, R. In vitro studies on release and skin permeation of nonivamide from novel oil-in-oil-emulsions. Eur. J. Pharm. Biopharm. 2014, 86, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Qi, S.; Liu, F.; Brown, M.B.; McAuley, W.J. Rationalising polymer selection for supersaturated film forming systems produced by an aerosol spray for the transdermal delivery of methylphenidate. Eur. J. Pharm. Biopharm. 2017, 114, 164–174. [Google Scholar] [CrossRef]
- Hifumi, H.; Ewing, A.V.; Kazarian, S.G. ATR-FTIR spectroscopic imaging to study the drying and dissolution of pharmaceutical polymer-based films. Int. J. Pharm. 2016, 515, 57–68. [Google Scholar] [CrossRef]
- Liqui-Patch®. Available online: https://epinamics.com/the-liqui-patch (accessed on 3 May 2022).
- MedSpray®. Available online: https://www.medpharm.com/us/expertise/routes-of-delivery/ (accessed on 3 May 2022).
- Durapeel®. Available online: https://www.crescitatherapeutics.com/durapeel (accessed on 3 May 2022).
- Estradiol MTDS. Available online: https://www.acrux.com.au/product/estradiol-mdts/ (accessed on 4 May 2022).
- Axiron®. Available online: https://www.acrux.com.au/product/testosterone-solution/ (accessed on 4 May 2022).
- Lamisil® Once 1% Cutaneous Solution. Available online: https://www.medicines.org.uk/emc/product/179/smpc#gref (accessed on 6 May 2022).
- Kis, N.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Csányi, E.; Csóka, I.; Berkó, S. Investigation of silicone-containing semisolid in situ film-forming systems using QbD tools. Pharmaceutics 2019, 11, 660. [Google Scholar] [CrossRef] [Green Version]
- Kovács, A.; Kis, N.; Budai-Szűcs, M.; Gácsi, A.; Csányi, E.; Csóka, I.; Berkó, S. QbD-Based Investigation of Dermal Semisolid in situ Film-Forming Systems for Local Anaesthesia. Drug Des. Devel. Ther. 2020, 14, 5059–5076. [Google Scholar] [CrossRef]
- [EMA] European Medicines Agency. CHMP/QWP/708282/2018 Draft Guideline on Quality and Equivalence of Topical Products; Committee for Medicinal Products for Human Use (CHMP): Amsterdam, The Netherlands, 2018. [Google Scholar]
- Timotijević, M.D.; Ilić, T.; Savić, S.; Pantelić, I. Simultaneous physico-mechanical and in vivo assessment towards factual skin performance profile of topical polymeric film-forming systems. Pharmaceutics 2022, 14, 223. [Google Scholar] [CrossRef]
- Buechler, C.R.; Veenstra, J.; Gold, L.S. New topical therapies for psoriasis. Dermatol. Rev. 2021, 2, 262–268. [Google Scholar] [CrossRef]
- Horn, E.J.; Domm, S.; Katz, H.I.; Lebwohl, M.; Mrowietz, U.; Kragballe, K. Topical corticosteroids in psoriasis: Strategies for improving safety. JEADV 2010, 24, 119–124. [Google Scholar] [CrossRef]
- Psomadakis, C.E.; Han, G. New and emerging topical therapies for psoriasis and atopic dermatitis. J. Clin. Aesthet. Dermatol. 2019, 12, 28–34. [Google Scholar]
- Hadgraft, J.; Lane, M.E. Drug crystallization-implications for topical and transdermal delivery. Expert Opin. Drug Deliv. 2016, 6, 817–830. [Google Scholar] [CrossRef]
- Parhi, R.; Goli, V.V.N. Design and optimization of film-forming gel of etoricoxib using research surface methodology. Drug Deliv. Transl. Res. 2020, 10, 498–514. [Google Scholar] [CrossRef]
- Van Bocxlaer, K.; McArthur, K.-N.; Harris, A.; Alavijeh, M.; Braillard, S.; Mowbray, C.E.; Croft, S.L. Film-Forming Systems for the Delivery of DNDI-0690 to Treat Cutaneous Leishmaniasis. Pharmaceutics 2021, 13, 516. [Google Scholar] [CrossRef]
- Woertz, C.; Kleinebudde, P. Development of orodispersible polymer films containing poorly water soluble active pharmaceutical ingredients with focus on different drug loadings and storage stability. Int. J. Pharm. 2015, 493, 134–145. [Google Scholar] [CrossRef]
- Hanna, P.A.; Ghorab, M.M.; Gad, S. Development of betamethasone dipropionate-loaded nanostructured lipid carriers for topical and transdermal delivery. Antiinflamm. Antiallergy Agents Med. Chem. 2019, 18, 26–44. [Google Scholar] [CrossRef]
- Tran, T.T.D.; Tran, P.H.L. Molecular interactions in solid dispersions of poorly water-soluble drugs. Pharmaceutics 2020, 12, 745. [Google Scholar] [CrossRef]
- García-Couce, J.; Vernhes, M.; Bada, N.; Agüero, L.; Valdés, O.; Alvarez-Barreto, J.; Fuentes, G.; Almirall, A.; Cruz, L.J. Synthesis and Evaluation of AlgNa-g-Poly(QCL-co-HEMA) Hydrogels as Platform for Chondrocyte Proliferation and Controlled Release of Betamethasone. Int. J. Mol. Sci. 2021, 22, 5730. [Google Scholar] [CrossRef]
- Zayed, G.M.; Rasoul, S.A.; Ibrahim, M.A.; Saddik, M.S.; Alshora, D.H. In vitro and in vivo characterization of domperidone-loaded fast dissolving buccal films. Saudi Pharm. J. 2020, 28, 266–273. [Google Scholar] [CrossRef]
- Garvie-Cook, H.; Frederiksen, K.; Petersson, K.; Guy, R.H.; Gordeev, S. Characterization of topical film-forming systems using atomic force microscopy and Raman microspectroscopy. Mol. Pharm. 2015, 12, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Pünnel, L.C.; Lunter, D.J. Film-forming systems for dermal drug delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef] [PubMed]
- Garvie-Cook, H.; Frederiksen, K.; Petersson, K.; Guy, R.H.; Gordeev, S.N. Biophysical elucidation of the mechanism of enhanced drug release and topical delivery from polymeric film-forming systems. J. Control. Release 2015, 212, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Gennari, C.G.M.; Semin, F.; Franzè, S.; Musazzi, U.M.; Quaroni, G.M.G.; Casiraghi, A.; Cilurzo, F. A glimpse in critical attributes to design cutaneous film forming systems based on ammonium methacrylate. J. Drug Deliv. Sci. Technol. 2017, 41, 157–163. [Google Scholar] [CrossRef]
- Zurdo Schroeder, I.; Franke, P.; Schaefer, U.F.; Lehr, C.M. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur. J. Pharm. Biopharm. 2007, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Ali, A.; Sultana, Y.; Najmi, A.K. Fabrication and evaluation of polymeric films for transdermal delivery of pinacidil. Pharmazie 2004, 59, 631–635. [Google Scholar] [PubMed]
- Ilić, T.; Pantelić, I.; Lunter, D.; Đorđević, S.; Marković, B.; Ranković, D.; Daniels, R.; Savić, S. Critical quality attributes, in vitro release and correlated in vitro skin permeation—in vivo tape stripping collective data for demonstrating therapeutic (non)equivalence of topical semisolids: A case study of “ready-to-use” vehicles. Int. J. Pharm. 2017, 528, 253–267. [Google Scholar] [CrossRef] [PubMed]

| Sample Composition (%, m/m) | S1A | S2A | S3A |
|---|---|---|---|
| Betamethasone dipropionate | 0.064 | 0.064 | 0.064 |
| Eudragit® RS PO | 8.5 | - | 4.0 |
| Eudragit® NE 30D | - | 6.0 | - |
| Klucel® GF | - | - | 1.0 |
| Propylene glycol | 1.0 | - | 0.5 |
| Ethanol, 96% (v/v) | 86.7 | - | 92.9 |
| Isopropyl alcohol | - | 85.0 | - |
| Polysorbate 80 | 1.0 | - | 0.3 |
| Water, purified | up to 100 | up to 100 | up to 100 |
| Sample | Time Point | Viscosity (mPa·s) | pH Value |
|---|---|---|---|
| S1A | Initially | 1.67 ± 0.03 | 7.1 ± 0.1 |
| After 3 months | 1.77 ± 0.20 | 6.8 ± 0.2 | |
| After 6 months | 1.74 ± 0.16 | 6.6 ± 0.1 | |
| S2A | Initially | 26.10 ± 0.62 | 7.7 ± 0.1 |
| After 3 months | 25.16 ± 0.63 | 7.5 ± 0.1 | |
| After 6 months | 24.54 ± 0.62 | 7.3 ± 0.1 | |
| S3A | Initially | 47.17 ± 3.06 | 7.0 ± 0.2 |
| After 3 months | 49.07 ± 3.49 | 6.8 ± 0.1 | |
| After 6 months | 47.32 ± 3.16 | 6.7 ± 0.1 |
| Sample | Film’s Organoleptic Appearance | Film-Drying Time at 32.0 ± 0.1 °C (min) | Film-Drying Time at 25 ± 2 °C (min) | Film Surface (mm2) | Folding Endurance Value | Film Thickness (mm) |
|---|---|---|---|---|---|---|
| S1A | Colorless, transparent, structured, homogenous, glossy, low stickiness | 6.6 ± 0.1 a | 24.0 ± 0.6 c | 210.0 ± 14.2 c | 111.0 ± 2.0 | 0.007 ± 0.001 c |
| S2A | Colorless, mildly turbid, fine-structured, homogenous, low stickiness | 6.3 ± 0.1 | 47.0 ± 0.6 c | 77.0 ± 1.0 c | 54.0 ± 2.0 d | 0.015 ± 0.002 c |
| S3A | Whitish, mildly turbid, honeycomb-like structure, homogenous, matt, low stickiness | 5.0 ± 0.1 b | 31.0 ± 1.2 c | 84.0 ± 3.0 c | 107.0 ± 1.0 | 0.032 ± 0.006 c |
| Sample | Permeation Rate (ng/cm2 h) | Q26 h * (ng/cm2) | Permeation Coefficient (mg/cm2 h) |
|---|---|---|---|
| S1A | 1.039 ± 0.783 | 16.277 ± 13.130 | 0.0016 ± 0.0012 |
| S2A | 2.964 ± 0.851 a | 34.049 ± 15.795 a | 0.0046 ± 0.0013 a |
| S3A | 0.348 ± 0.157 b | 4.026 ± 1.615 b | 0.0005 ± 0.0002 b |
| RS | 1.247 ± 0.209 | 19.726 ± 5.953 | 0.0019 ± 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timotijević, M.D.; Ilić, T.; Marković, B.; Randjelović, D.; Cekić, N.; Nikolić, I.; Savić, S.; Pantelić, I. Coupling AFM, DSC and FT-IR towards Elucidation of Film-Forming Systems Transformation to Dermal Films: A Betamethasone Dipropionate Case Study. Int. J. Mol. Sci. 2022, 23, 6013. https://doi.org/10.3390/ijms23116013
Timotijević MD, Ilić T, Marković B, Randjelović D, Cekić N, Nikolić I, Savić S, Pantelić I. Coupling AFM, DSC and FT-IR towards Elucidation of Film-Forming Systems Transformation to Dermal Films: A Betamethasone Dipropionate Case Study. International Journal of Molecular Sciences. 2022; 23(11):6013. https://doi.org/10.3390/ijms23116013
Chicago/Turabian StyleTimotijević, Mirjana D., Tanja Ilić, Bojan Marković, Danijela Randjelović, Nebojša Cekić, Ines Nikolić, Snežana Savić, and Ivana Pantelić. 2022. "Coupling AFM, DSC and FT-IR towards Elucidation of Film-Forming Systems Transformation to Dermal Films: A Betamethasone Dipropionate Case Study" International Journal of Molecular Sciences 23, no. 11: 6013. https://doi.org/10.3390/ijms23116013
APA StyleTimotijević, M. D., Ilić, T., Marković, B., Randjelović, D., Cekić, N., Nikolić, I., Savić, S., & Pantelić, I. (2022). Coupling AFM, DSC and FT-IR towards Elucidation of Film-Forming Systems Transformation to Dermal Films: A Betamethasone Dipropionate Case Study. International Journal of Molecular Sciences, 23(11), 6013. https://doi.org/10.3390/ijms23116013











