Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction †
Abstract
:1. Introduction
2. Porphyrin-Based Systems as Electrochemical OER Catalysts for Water Oxidation
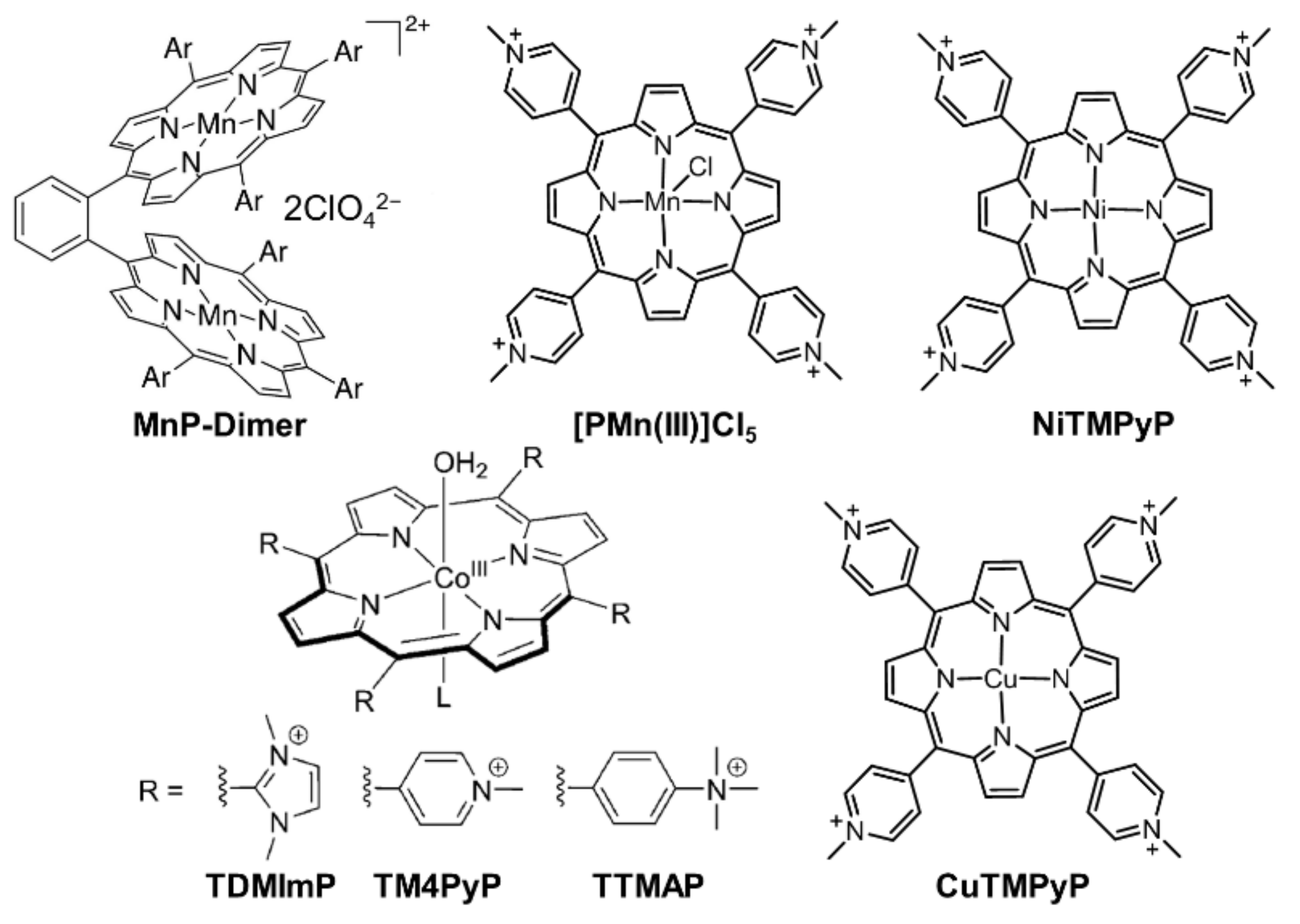
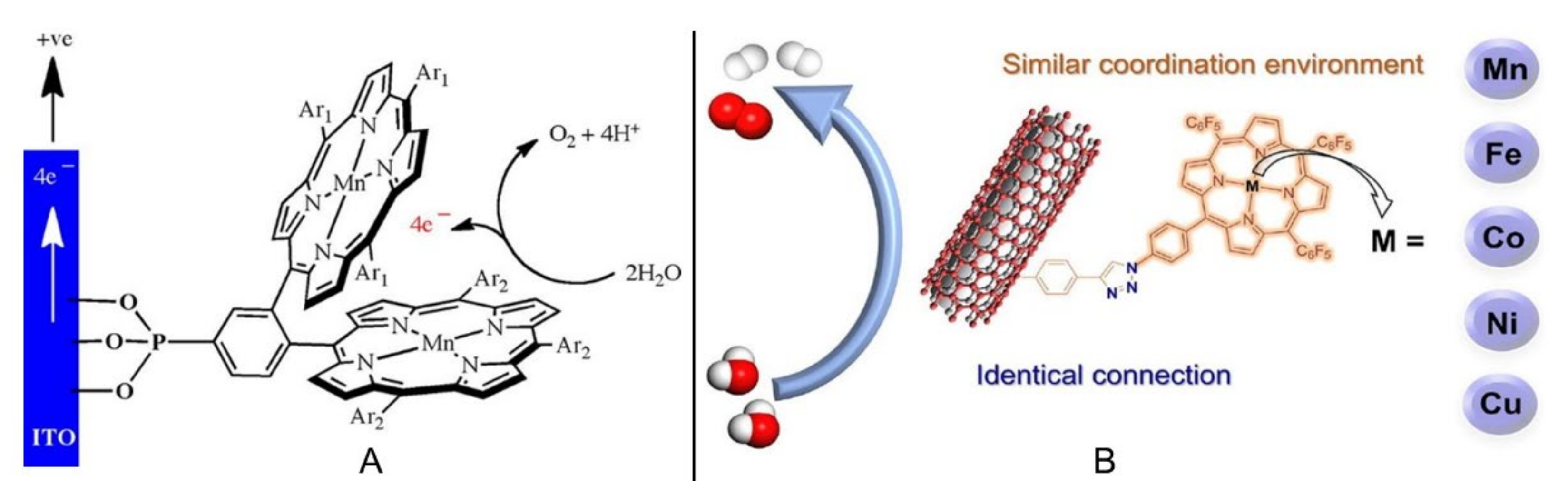
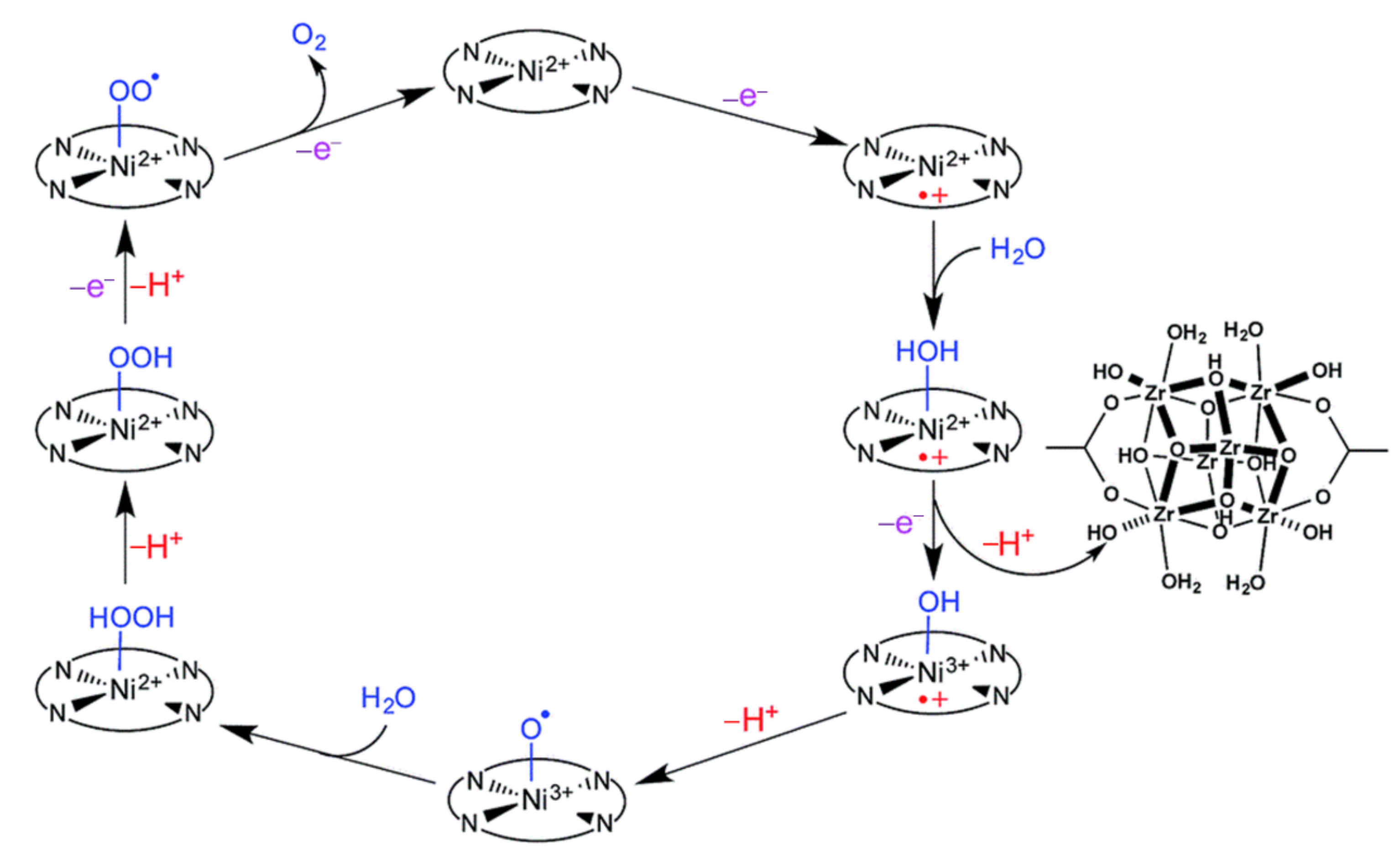
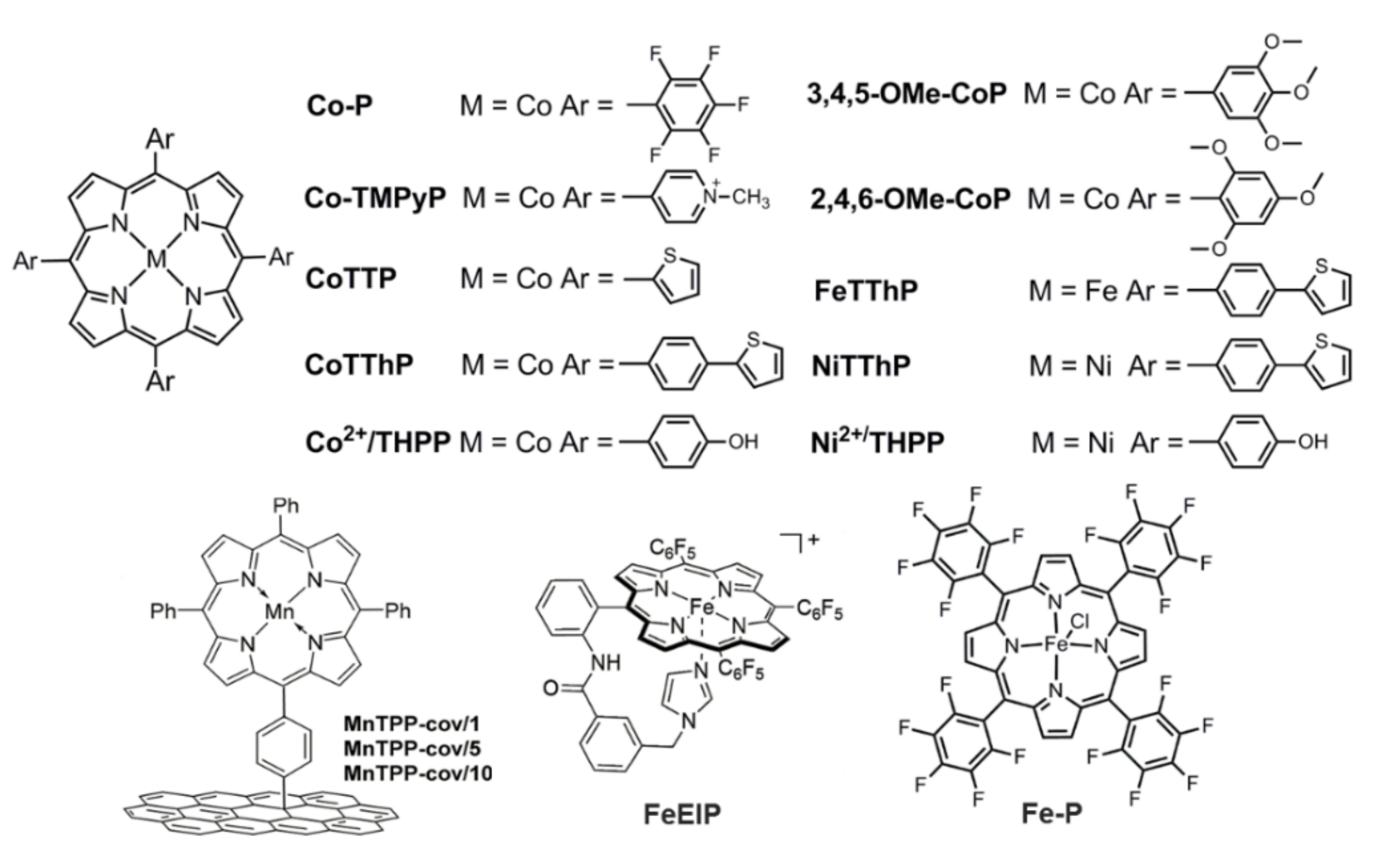
2.1. Porphyrin-Based Small Molecules for Electrochemical OER
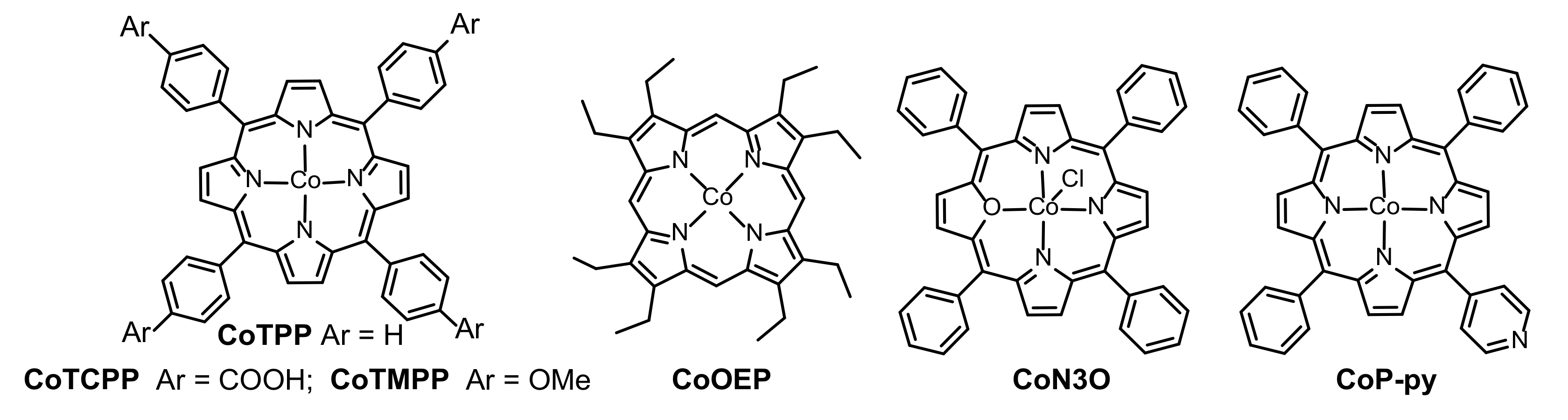
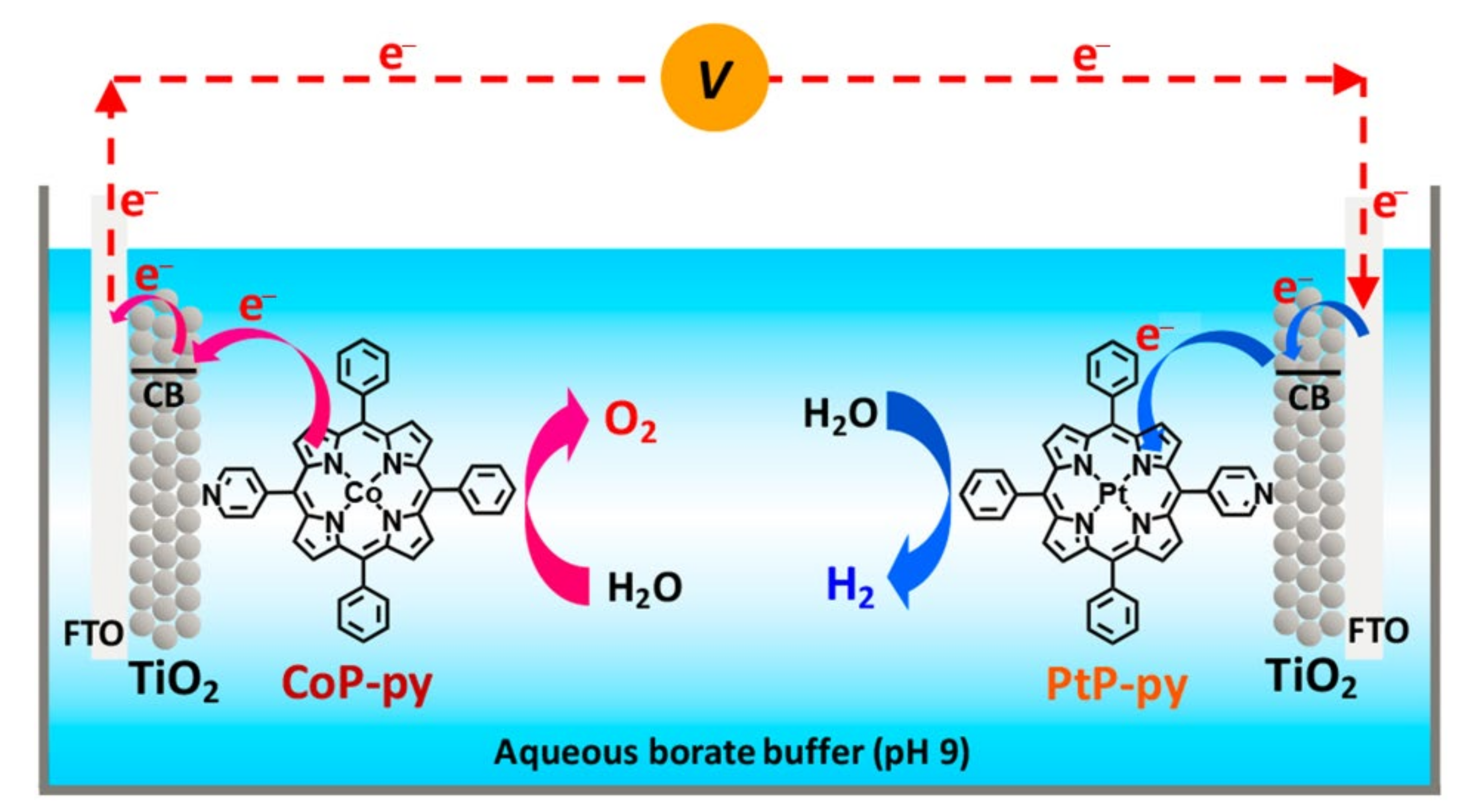
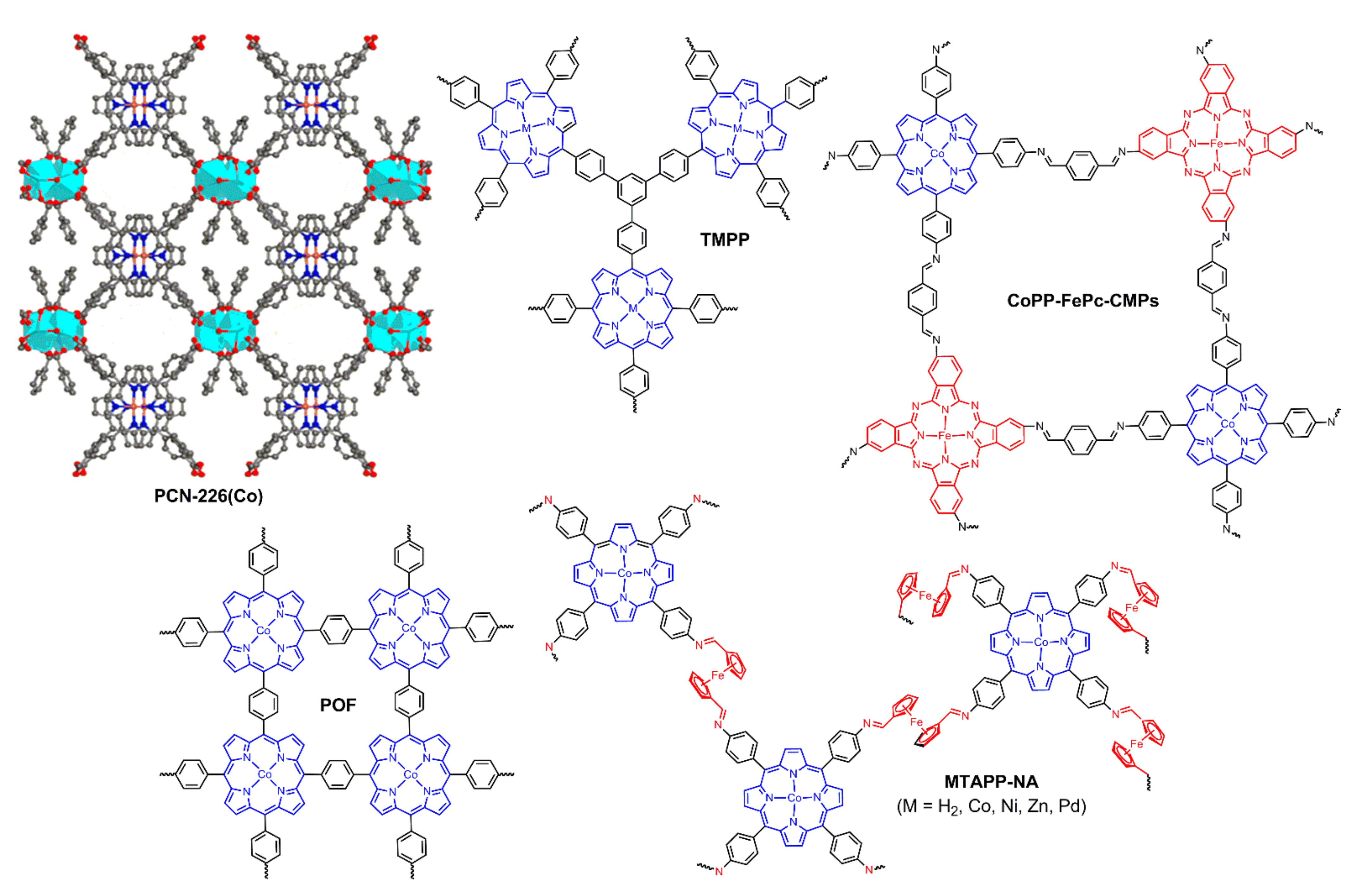
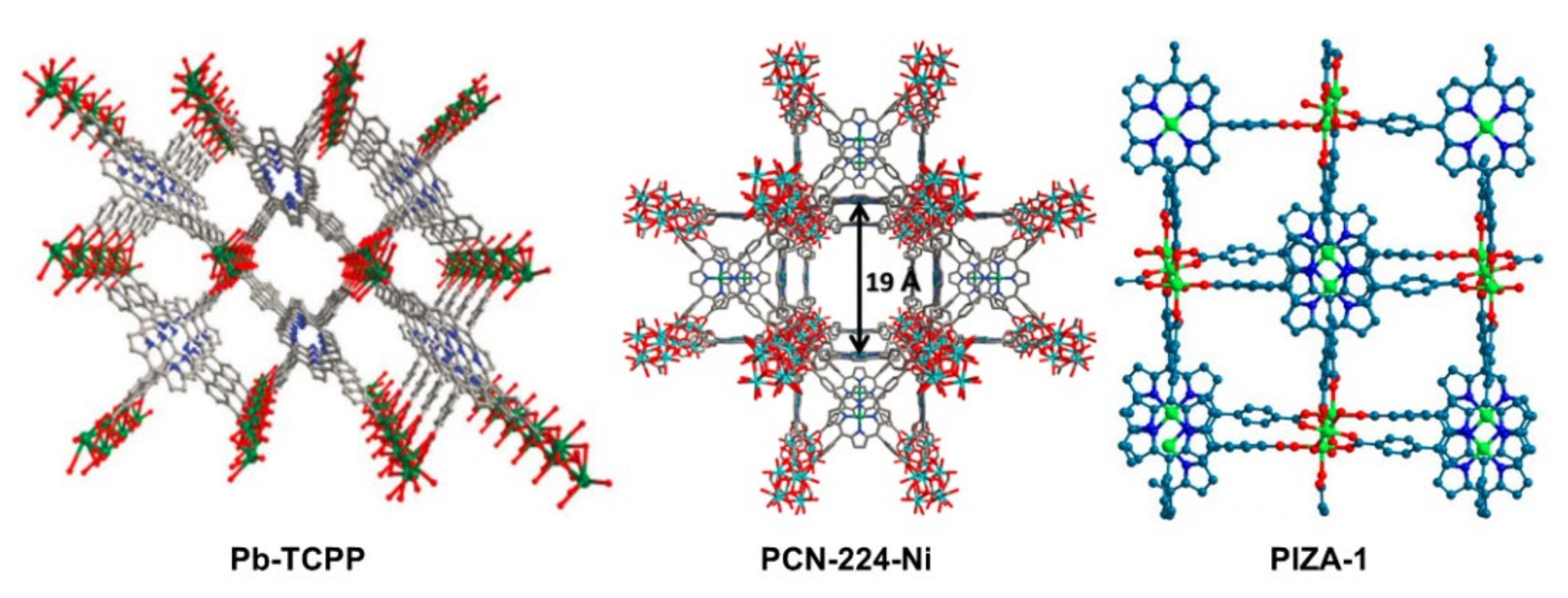
2.2. Porphyrin Coordination Polymers for Electrochemical OER
2.3. Porphyrin-Based Porous Organic Polymers for Electrochemical OER
| Catalysts | Working Electrode | Electrolyte | Loading | η10 (mV) | Tafel Slope (mV·dec−1) | Ref |
|---|---|---|---|---|---|---|
| CuTMPyP | FTO | 0.10 M phosphate buffer (pH 7.0) | 1.0 mM | 310 a | — | [41] |
| CoTCPP/ZrP | GC | 0.1 M KOH | 3.38 wt% | 476 | 76.4 | [50] |
| CoP-py/TiO2 | FTO | 0.1 M borate buffer (pH 9.0) | 0.10 μmol/cm2 | 540 | — | [51] |
| CoTMPP@ZIF-8 | GC | 0.1 M KCl (pH 7.0) | one CoTMPP per 15 cages | 387.4 b | 210.3 | [52] |
| Mn2DP-a|ITO | ITO | 25 mM Na2B4O7/0.1 M NaClO4 (pH 7.0) | 6.8 × 10−12 mol/cm2 | 470 | 90 | [55] |
| CoP@CNT | GC | 0.1 M KOH | 5.25 μg/mg c | 422 | — | [56] |
| FeTPyP-Co | Au(111) | 0.1 M NaOH | — | 310 | — | [59] |
| TiCP-PCP | CFP | 1.0 M KOH | 0.33 g/cm2 | 310 | 117 | [60] |
| 0.2TiCP-PCP@Co1−xS | CFP | 1.0 M KOH | 0.33 g/cm2 | 157 | 65 | [61] |
| Pb-TCPP | GC | 1.0 M KOH | — | 470 | 106.2 | [64] |
| PCN-224-Ni | FTO | 0.1 M NaClO4 (pH 7.0) | 8 × 1013 sites/cm2 | 450 | 150 | [65] |
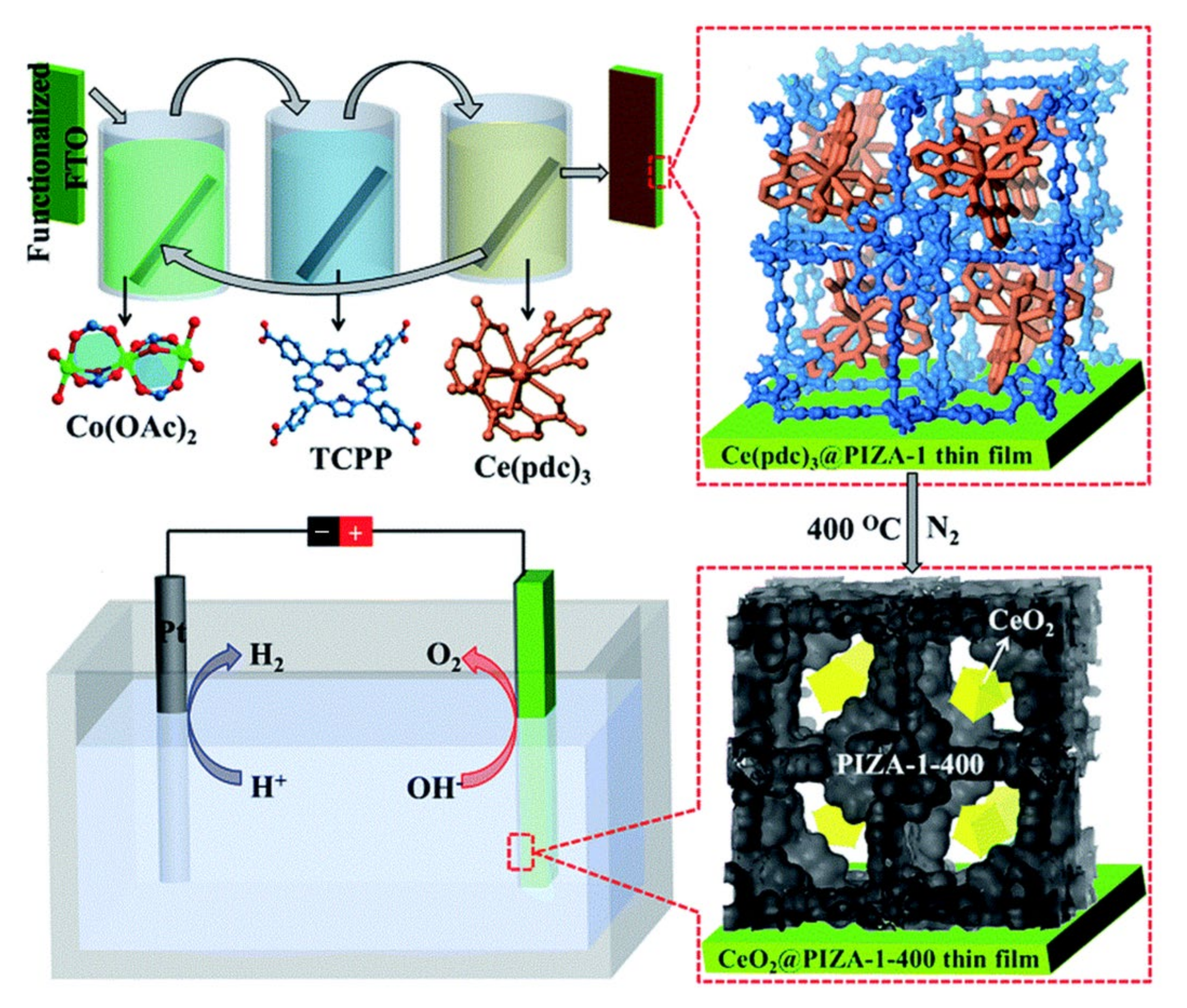
| CeO2@PIZA-1-400 | FTO | 1.0 M KOH | — | 370 | 47.6 | [66] |
| Fe(Salen)@PIZA-1-400 | FTO | 1.0 M KOH | — | 340 | 56 | [67] |
| CoP-2ph-CMP-800 | GC | 1.0 M KOH | — | 370 | 86 | [73] |
| Cu-CMP850 | GC | 1.0 M KOH | 0.28 mg/cm2 | 350 | 135 | [74] |
| Co-MPPy-1 | GC | 1.0 M NaOH | 4.23 × 10−9 mol/cm2 | 420 | 58 | [75] |
| FeTAPP-NiTCPP-POP | GC | 1.0 M KOH | — | 338 | 52 | [76] |
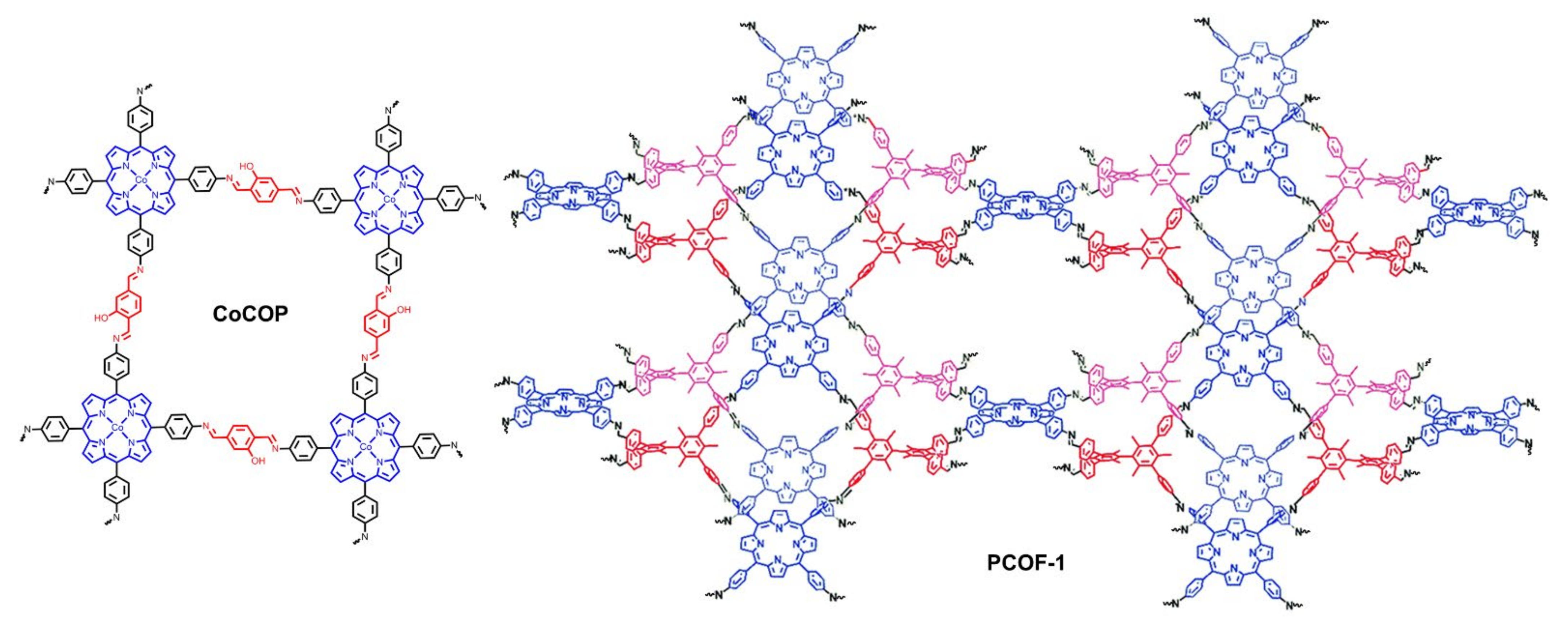
| CoCOP | CFP | 1.0 M KOH | — | 350 | 151 | [81] |
| PCOF-1-Co | GC | 1.0 M KOH | — | 386 | 89 | [82] |
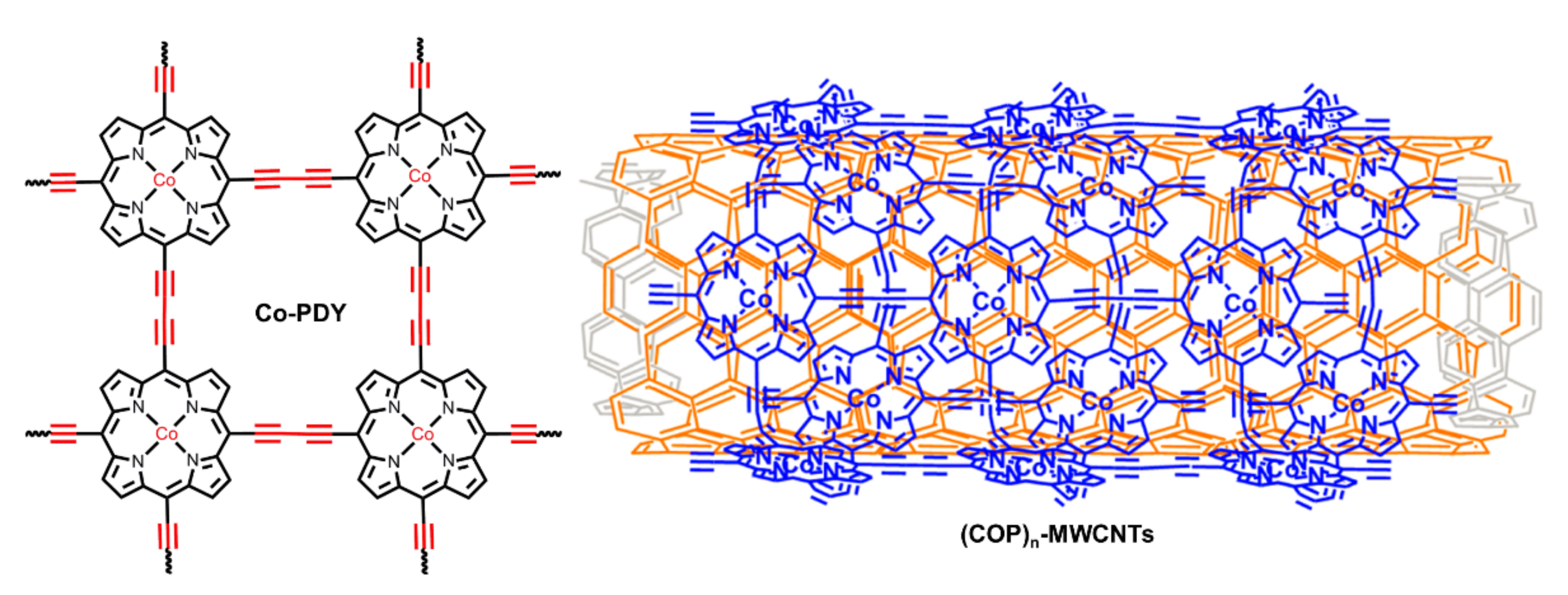
| Co-PDY | CF | 1.0 M KOH | — | 270 | 99 | [86] |
| (CoP)n-MWCNTs | GC | 1.0 M KOH | 0.14 mg/cm2 | 430 | 60.8 | [88] |
3. Porphyrin-Based Systems as Bifunctional Oxygen Electrocatalysts for Zn–Air Batteries
3.1. Porphyrin-Based Small Molecules as Bifunctional Oxygen Electrocatalysts
3.2. Porphyrin-Based Porous Polymers as Bifunctional Oxygen Electrocatalysts

| Catalysts | OER a | ORR a | Zn–air Battery d | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Electrolyte | η10 (mV) | Tafel Slope (mV·dec−1) | Electrolyte | E1/2 (V) | Tafel Slope (mV·dec−1) | Voltage Gap ∆E (V) | Peak Power Density (mW/cm−2) | ||
| CoTPP/MWCNT/Py–Py | 1.0 M KOH | 440 | — | 0.5 M H2SO4 | 0.375 b | — | — | — | [90] |
| CoTPP/CNTs | 1.0 M KOH | 407 | 60.3 | 0.1 M KOH | 0.81 | 36.9 | — | 155.7 | [91] |
| Co-P/CNTs | 1.0 M KOH | 480 | 71.6 | 0.1 M KOH | 0.76 | 43.5 | — | 84.5 | [91] |
| 3,4,5-OMe-CoP/CNT | 1.0 M KOH | 482 | 81 | 0.1 M KOH | 0.80 | 49 | 0.8 | 144.8 | [92] |
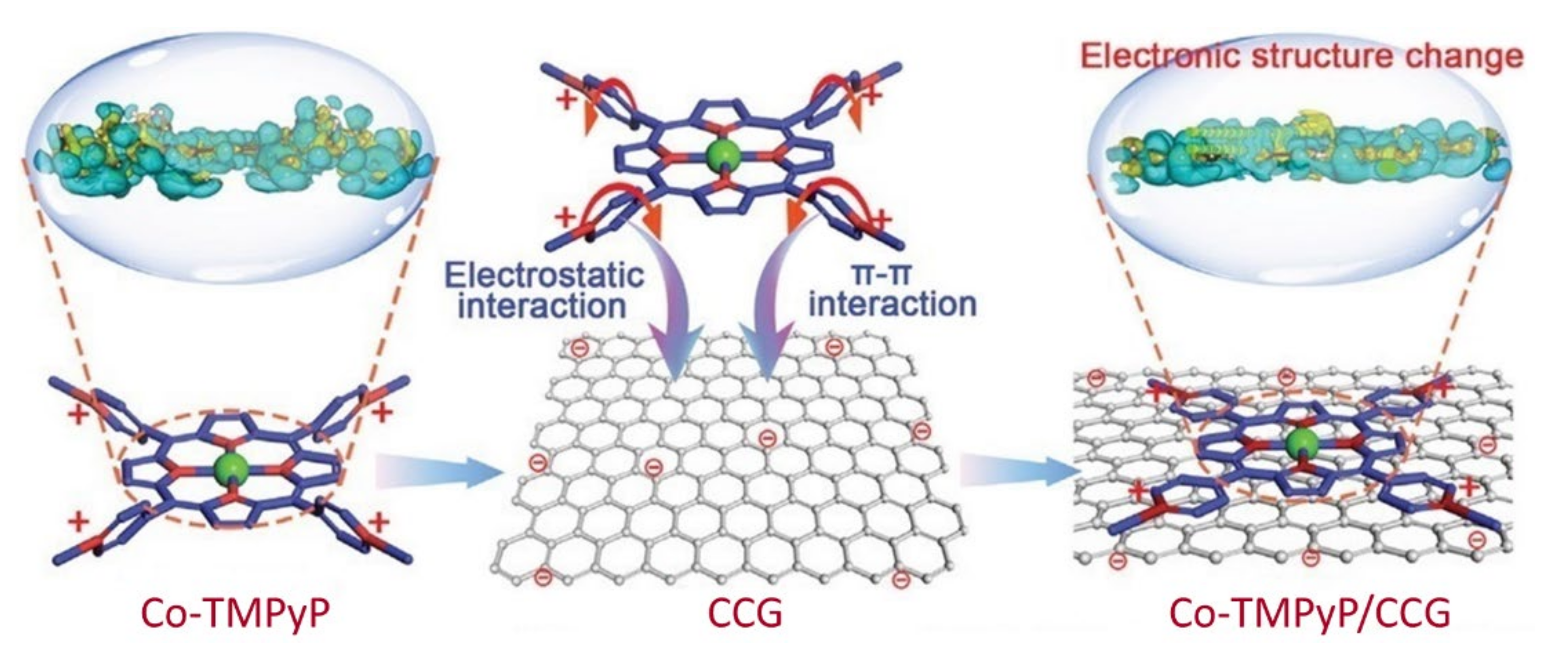
| Co-TMPyP/CCG | 1.0 M KOH | 379 | 62.4 | 0.1 M KOH | 0.824 | 78.5 | — | 225.4 | [93] |
| CoTThP/C | 0.1 M KOH | 584 | 110 | 0.1 M KOH | 0.746 | 54 | — | — | [94] |
| FeEIP/CNT | 0.1 M KOH | 500 | 84 | 0.1 M KOH | 0.84 | — | 0.89 | 132.9 | [96] |
| rGO/(Ni2+/THPP/Co2+/THPP)8 | 1.0 M KOH | 340 | 50.17 | 0.1 M KOH | 0.84 c | — | — | — | [97] |
| (Co–P)0.5(Fe–P)0.5@CNT | 0.1 M KOH | 420 | — | 0.1 M KOH | 0.80 | 70.1 | 0.74 | 174.5 | [98] |
| PCN-224(Co)/MWCNT | borate buffer (pH = 9.2) | 510 a | — | 0.5 M H2SO4 | — | 154 | — | — | [100] |
| PCN-226(Co)/C | 1.0 M KOH | 445 | 111 | 0.1 M KOH | 0.75 | 58.9 | — | 133 | [101] |
| Fe2P/Fe4N@C-800 | 1.0 M KOH | 410 | 177 | 0.1 M KOH | 0.80 | 65 | — | — | [102] |
| CNTs@(Fe,Co,Ni)PP-800 | 1.0 M KOH | 355 | 78.5 | 0.1 M KOH | 0.837 | 63.85 | 0.69 | 155.13 | [103] |
| CoFeNG | 1.0 M KOH | 360 | 68.9 | 0.1 M KOH | 0.777 | 53.5 | — | 53.4 | [104] |
| CNT@POF | — | — | — | — | — | — | 0.71 | 237 | [106] |
| G@POF-Co | 0.1 M KOH | 430 | 161 | 0.1 M KOH | 0.81 | 46.9 | — | — | [107] |
| Co-POC | 0.1 M KOH | 470 | 139 | 0.1 M KOH | 0.83 | 53.5 | 1.04 | 78 | [108] |
| Co3O4@POF | 0.1 M KOH | 390 | 66 | 0.1 M KOH | 0.85 | 38 | 1.00 | 222.2 | [109] |
| LDH-POF | 0.1 M KOH | 250 | 99 | 0.1 M KOH | 0.80 | 46 | 0.74 | 185 | [110] |
| CoTAPP-NA | 1.0 M KOH | 416 | 68 | 1.0 M KOH | 0.84 | — | — | — | [111] |
4. Conclusions and Perspectives
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CC | Carbon Cloth |
| CCG | Chemically Converted Graphene |
| CF | Copper Foam |
| CFP | Carbon Fiber Paper |
| CMPs | Conjugated Mesoporous/Microporous Polymers |
| CNT | Carbon Nanotube |
| CoCOP | Cobalt-porphyrin-based covalent organic polymer |
| Co-P | 5,10,15,20-Tetrakis(pentafluorophenyl) porphyrin Cobalt |
| Co-PDY | Graphdiyne Co-coordinated Porphyrin Covalent Organic Framework |
| CoN3O | 5,10,15,20-Tetraphenyl-21-oxaporphyrin cobalt chloride |
| CoOEP | 2,3,7,8,12,13,17,18-Octaethyl porphyrin cobalt (II) |
| CoTMPP | 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin cobalt(II) |
| CoP | Cobalt Porphyrin |
| CoP-py | 5-(4-Pyridyl)-10,15,20-triphenylporphyrin cobalt (II) |
| CPF | Covalent Porphyrin Framework |
| Fe-P | 5,10,15,20-Tetrakis(pentafluorophenyl) porphyrin iron |
| FeTPyP | 5,10,15,20-Tetra(4-pyridyl) porphyrin iron(III) chloride |
| FTO | Fluorine-doped Tin Oxide |
| GDY | Graphdiyne |
| GC | Glassy Carbon |
| HER | Hydrogen Evolution Reaction |
| ITO | Indium Tin Oxide |
| LBL | Layer-By-Layer |
| LDH | NiFe Layered Double Hydroxides |
| Mn2DPs | manganese porphyrin dimers |
| MOFs | Metal–Organic Frameworks |
| MWCNTs | Multi-Walled Carbon Nanotubes |
| OER | Oxygen Evolution Reaction |
| PCN-224 | Porphyritic MOF by the coordination of Zr(IV) with meso-tetra(4-carboxy-phenyl) porphyrin |
| PIZA-1 | Porphyritic MOF CoT(p-CO2)PPCo1.5 |
| POPs | Porous Organic Polymers |
| rGO | reduced Graphene Oxide |
| SOXPES | Synchrotron-based Photoelectron Spectroscopy |
| TAPP | 5,10,15,20-Tetrakis(4-aminophenyl) porphyrin |
| TCPP | 5,10,15,20-Tetrakis(4-carboxyphenyl) porphyrin |
| THPP | 5,10,15,20-tetrakis(4-hydroxyphenyl) porphyrin |
| TiCP-PCP | Porphyrin-based porous titanium coordination polymer |
| TMPP | 5,10,15,20-Tetrakis(4-methoxyphenyl) porphyrin |
| TMPyP | 5,10,15,20-Tetrakis(4-N-methylpyridinyl) porphyrin |
| TOF | Turnover Frequency |
| TON | Turnover Number |
| TPP | 5,10,15,20-Tetraphenyl porphyrin |
| TPyP | 5,10,15,20-Tetra(4-pyridyl) porphyrin |
| TThP | meso-tetra(4-thiophenephenyl) porphyrin |
| TTP | meso-tetra(thienyl) porphyrin |
| ZrP | Zirconium Phosphate |
| ZIF-8 | Zeolitic Imidazolate Framework-8 |
| η10 | The overpotential required to yield a catalytic density if 10 mA cm−2 |
References
- Fang, Y.X.; Hou, Y.D.; Fu, X.Z.; Wang, X.C. Semiconducting polymers for oxygen evolution reaction under light illumination. Chem. Rev. 2022, 122, 4204–4256. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liu, H.F.; Chu, W.S.; Wu, C.Z.; Xie, Y. Structural transformation of heterogeneous materials for electrocatalytic oxygen evolution reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.S.; Shang, L.; Zhang, T.R.; Waterhouse, G.I.N. Recent advances in the development of single-atom catalysts for oxygen electrocatalysis and zinc–air batteries. Adv. Energy Mater. 2020, 10, 2003018. [Google Scholar] [CrossRef]
- Fu, J.; Liang, R.L.; Liu, G.H.; Yu, A.P.; Bai, Z.Y.; Yang, L.; Chen, Z.W. Recent progress in electrically rechargeable zinc-air batteries. Adv. Mater. 2019, 31, e1805230. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.K.; Cui, X.; Sewell, C.D.; Li, J.; Lin, Z.Q. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 8428–8469. [Google Scholar] [CrossRef]
- Yu, M.Q.; Budiyanto, E.; Tüysüz, H. Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, N.X.; Zhao, Y.H.; Zhang, X.L.; Wu, J.S.; Weng, J.N.; Li, S.; Huo, F.W.; Huang, W. Frontiers and structural engineering for building flexible zinc-air batteries. Adv. Sci. 2022, 9, e2103954. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.Y.; Huang, B.T.; Stephens, I.E.L.; Risch, M.; Xu, Z.C.J.; Shao-Horn, Y. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Anantharaj, S.; Aravindan, V. Developments and perspectives in 3d transition-metal-based eectrocatalysts for neutral and near-neutral water electrolysis. Adv. Energy Mater. 2020, 10, 1902666. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.M.; Gray, H.B.; Müller, A.M. Earth-abundant heterogeneous water oxidation catalysts. Chem. Rev. 2016, 116, 14120–14136. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.Z.; Cao, R. Energy-related small molecule activation reactions: Oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin- and corrole-based systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef] [PubMed]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and functionalization of porphyrins through organometallic methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef]
- Jin, L.; Lv, S.B.; Miao, Y.Y.; Liu, D.P.; Song, F.L. Recent development of porous porphyrin–based nanomaterials for photocatalysis. ChemCatChem 2020, 13, 140–152. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, J.P.; Liu, B.C.; Yang, J.K.; Hou, H.J. Recent advances in metalloporphyrins for environmental and energy applications. Chemosphere 2019, 219, 617–635. [Google Scholar] [CrossRef]
- Zhang, X.; Wasson, M.C.; Shayan, M.; Berdichevsky, E.K.; Ricardo-Noordberg, J.; Singh, Z.; Papazyan, E.K.; Castro, A.J.; Marino, P.; Ajoyan, Z.; et al. A historical perspective on porphyrin-based metal-organic frameworks and their applications. Coord. Chem. Rev. 2021, 429, 213615. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, W.A. Synthesis, self-assembly and applications of functional polymers based on porphyrins. Prog. Polym. Sci. 2019, 95, 65–117. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ren, K.; Wang, L.; Wang, L.; Fan, Z.J. Porphyrin-based heterogeneous photocatalysts for solar energy conversion. Chin. Chem. Lett. 2022, 33, 33–60. [Google Scholar] [CrossRef]
- Beyene, B.B.; Hung, C.-H. Recent progress on metalloporphyrin-based hydrogen evolution catalysis. Coord. Chem. Rev. 2020, 410, 213234. [Google Scholar] [CrossRef]
- Ladomenou, K.; Natali, M.; Iengo, E.; Charalampidis, G.; Scandola, F.; Coutsolelos, A.G. Photochemical hydrogen generation with porphyrin-based systems. Coord. Chem. Rev. 2015, 304–305, 38–54. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, J.H.; Jang, W.-D. Applications of porphyrins in emerging energy conversion technologies. Coord. Chem. Rev. 2020, 407, 213157. [Google Scholar]
- Gopalakrishnan, V.N.; Becerra, J.; Pena, E.F.; Sakar, M.; Béland, F.; Do, T.-O. Porphyrin and single atom featured reticular materials: Recent advances and future perspective of solar-driven CO2 reduction. Green Chem. 2021, 23, 8332–8360. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.F.; Liu, X.Y.; Huang, Y.M.; Pan, Q.; Peng, R.; Du, R.Y.; Zheng, X.X.; Yin, Z.Y.; Li, S.Q.; et al. Porphyrin-based Ti-MOFs conferred with single-atom Pt for enhanced photocatalytic hydrogen evolution and NO removal. Chem. Eng. J. 2022, 428, 132045. [Google Scholar] [CrossRef]
- Jiang, G.M.; Liu, X.Y.; Jian, H.L.; Lu, P.; Bai, J.W.; Zhang, G.Z.; Yun, W.; Li, S.Q.; He, Y.Z. Cu-clusters nodes of 2D metal-organic frameworks as a cost-effective noble-metal-free cocatalyst with high atom-utilization efficiency for efficient photocatalytic hydrogen evolution. Chin. Chem. Lett. 2021, 33, 3049–3052. [Google Scholar] [CrossRef]
- Jiang, G.M.; Zhang, C.H.; Liu, X.Y.; Bai, J.W.; Xu, M.M.; Xu, Q.; Li, Y.; Long, L.J.; Zhang, G.Z.; Li, S.Q.; et al. Electrocatalytic hydrogen evolution of highly dispersed Pt/NC nanoparticles derived from porphyrin MOFs under acidic and alkaline medium. Int. J. Hydrog. Energy 2022, 47, 6631–6637. [Google Scholar] [CrossRef]
- Liang, Z.Z.; Wang, H.Y.; Zheng, H.Q.; Zhang, W.; Cao, R. Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide. Chem. Soc. Rev. 2021, 50, 2540–2581. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhu, Y.F.; Chen, X.J.; Zhang, H.J.; Wang, J. A full-spectrum metal-free porphyrin supramolecular photocatalyst for dual functions of highly efficient hydrogen and oxygen evolution. Adv. Mater. 2019, 31, 1806626. [Google Scholar] [CrossRef]
- Liu, C.Y.; van den Bos, D.; den Hartog, B.; van der Meij, D.; Ramakrishnan, A.; Bonnet, S. Ligand controls the activity of light-driven water oxidation catalyzed by nickel(II) porphyrin complexes in neutral homogeneous aqueous solutions. Angew. Chem. Int. Ed. 2021, 60, 13463–13469. [Google Scholar] [CrossRef]
- Ghobadi, T.G.U.; Yildiz, E.A.; Buyuktemiz, M.; Akbari, S.S.; Topkaya, D.; Isci, U.; Dede, Y.; Yaglioglu, H.G.; Karadas, F. A noble-metal-free heterogeneous photosensitizer-relay catalyst triad that catalyzes water oxidation under visible light. Angew. Chem. Int. Ed. 2018, 57, 17173–17177. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Hetterscheid, D.G.H.; Williams, R.M.; van der Vlugt, J.I.; Reek, J.N.H.; Brouwer, A.M. Platinum(ii)–porphyrin as a sensitizer for visible-light driven water oxidation in neutral phosphate buffer. Energy Environ. Sci. 2015, 8, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Paille, G.; Gomez-Mingot, M.; Roch-Marchal, C.; Lassalle-Kaiser, B.; Mialane, P.; Fontecave, M.; Mellot-Draznieks, C.; Dolbecq, A. A fully noble metal-free photosystem based on cobalt-polyoxometalates immobilized in a porphyrinic metal-organic framework for water oxidation. J. Am. Chem. Soc. 2018, 140, 3613–3618. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Wang, L.; Li, F.S.; Fukushima, T.; Tanaka, K.; Sun, L.C.; Imahori, H. Visible light-driven water oxidation using a covalently-linked molecular catalyst-sensitizer dyad assembled on a TiO2 electrode. Chem. Sci. 2016, 7, 1430–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materna, K.L.; Jiang, J.B.; Regan, K.P.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W. Optimization of photoanodes for photocatalytic water oxidation by combining a heterogenized iridium water-oxidation catalyst with a high-potential porphyrin photosensitizer. ChemSusChem 2017, 10, 4526–4534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, A.; Hu, K.; Roy, S.; Brennaman, M.K.; Shan, B.; Meyer, G.J.; Meyer, T.J. Synthesis and photophysical properties of a covalently linked porphyrin chromophore–Ru(II) water oxidation catalyst assembly on SnO2 electrodes. J. Phys. Chem. C 2018, 122, 13455–13461. [Google Scholar] [CrossRef]
- Naruta, Y.; Sasayama, M.-A.; Sasaki, T. Oxygen evolution by oxidation of water with manganese porphyrin dimers. Angew. Chem. Int. Ed. 1994, 33, 1839–1841. [Google Scholar] [CrossRef]
- Luna, M.A.; Moyano, F.; Sereno, L.; D’Eramo, F. Spectroscopic and electrochemical studies of high-valent water soluble manganese porphyrine. Electrocatalytic water oxidation. Electrochim. Acta 2014, 135, 301–310. [Google Scholar] [CrossRef]
- Wang, D.; Groves, J.T. Efficient water oxidation catalyzed by homogeneous cationic cobalt porphyrins with critical roles for the buffer base. Proc. Natl. Acad. Sci. USA 2013, 110, 15579–15584. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.Z.; Wu, Y.Z.; Lai, W.Z.; Cao, R. Electrocatalytic water oxidation by a water-soluble nickel porphyrin complex at neutral pH with low overpotential. Inorg. Chem. 2015, 54, 5604–5613. [Google Scholar] [CrossRef]
- Liu, Y.J.; Han, Y.Z.; Zhang, Z.Y.; Zhang, W.; Lai, W.Z.; Wang, Y.; Cao, R. Low overpotential water oxidation at neutral pH catalyzed by a copper(II) porphyrin. Chem. Sci. 2019, 10, 2613–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, Y.S.; Magyar, A.P.; Lee, D.; Kim, J.W.; Yun, D.S.; Park, H.; Pollom, T.S., Jr.; Weitz, D.A.; Belcher, A.M. Biologically templated photocatalytic nanostructures for sustained light-driven water oxidation. Nat. Nanotechnol. 2010, 5, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wagner, P.; Tong, L.; Wallace, G.G.; Officer, D.L.; Swiegers, G.F. A porphyrin-doped polymer catalyzes selective, light-assisted water oxidation in seawater. Angew. Chem. Int. Ed. 2012, 51, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Li, J.S.; Zheng, H.F.; Liu, X.; Ye, S.; Du, P.W. Pyrolyzed cobalt porphyrin-modified carbon nanomaterial as an active catalyst for electrocatalytic water oxidation. Int. J. Hydrog. Energy 2015, 40, 6538–6545. [Google Scholar] [CrossRef]
- Matsuda, S.; Mori, S.; Hashimoto, K.; Nakanishi, S. Transition metal complexes with macrocyclic ligands serve as efficient electrocatalysts for aprotic oxygen evolution on Li2O2. J. Phys. Chem. C 2014, 118, 28435–28439. [Google Scholar] [CrossRef]
- Han, A.L.; Jia, H.X.; Ma, H.; Ye, S.F.; Wu, H.T.; Lei, H.T.; Han, Y.Z.; Cao, R.; Du, P.W. Cobalt porphyrin electrode films for electrocatalytic water oxidation. Phys. Chem. Chem. Phys. 2014, 16, 11209–11217. [Google Scholar] [CrossRef]
- Daniel, Q.; Ambre, R.B.; Zhang, B.B.; Philippe, B.; Chen, H.; Li, F.S.; Fan, K.; Ahmadi, S.; Rensmo, H.; Sun, L.C. Re-investigation of cobalt porphyrin for electrochemical water oxidation on FTO surface: Formation of CoOx as active species. ACS Catal. 2017, 7, 1143–1149. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.F.; Wang, D.; Wan, L.J. Molecular evidence for the catalytic process of cobalt porphyrin catalyzed oxygen evolution reaction in alkaline solution. J. Am. Chem. Soc. 2019, 141, 7665–7669. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Wu, H.L.; Liu, W.Q.; Jiang, X.; Li, Z.J.; Chen, B.; Tung, C.H.; Wu, L.Z. Improved photoelectrocatalytic performance for water oxidation by earth-abundant cobalt molecular porphyrin complex-iintegrated BiVO4 photoanode. ACS Appl. Mater. Interfaces 2016, 8, 18577–18583. [Google Scholar] [CrossRef]
- Alvarez, I.B.; Wu, Y.; Sanchez, J.; Ge, Y.L.; Ramos-Garcés, M.V.; Chu, T.; Jaramillo, T.F.; Colón, J.L.; Villagrán, D. Cobalt porphyrin intercalation into zirconium phosphate layers for electrochemical water oxidation. Sustain. Energy Fuels 2021, 5, 430–437. [Google Scholar] [CrossRef]
- Akamine, K.; Morita, K.; Sakai, K.; Ozawa, H. A molecular-based water electrolyzer consisting of two mesoporous TiO2 electrodes modified with metalloporphyrin molecular catalysts showing a quantitative Faradaic efficiency. ACS Appl. Energy Mater. 2020, 3, 4860–4866. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Basu, O.; Das, S.K. ZIF-8 MOF encapsulated Co-porphyrin, an efficient electrocatalyst for water oxidation in a wide pH range: Works better at neutral pH. ChemCatChem 2020, 12, 5430–5438. [Google Scholar] [CrossRef]
- Wei, P.C.; Lin, K.F.; Meng, D.D.; Xie, T.F.; Na, Y. Photoelectrochemical performance for water oxidation improved by molecular Nickel porphyrin-integrated WO3/TiO2 photoanode. ChemSusChem 2018, 11, 1746–1750. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.J.; Sun, W.J.; Dong, Y.J.; Dong, C.Z.; Hu, Q.Y.; Ma, B.C.; Ding, Y. A graphene oxide–molecular Cu porphyrin-integrated BiVO4 photoanode for improved photoelectrochemical water oxidation performance. J. Mater. Chem. A 2020, 8, 4062–4072. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Zahran, Z.N.; Naruta, Y. Covalent bonds immobilization of cofacial Mn porphyrin dimers on an ITO electrode for efficient water oxidation in aqueous solutions. J. Catal. 2017, 352, 293–299. [Google Scholar] [CrossRef]
- Jin, X.T.; Li, X.L.; Lei, H.T.; Guo, K.; Lv, B.; Guo, H.B.; Chen, D.D.; Zhang, W.; Cao, R. Comparing electrocatalytic hydrogen and oxygen evolution activities of first-row transition metal complexes with similar coordination environments. J. Energy Chem. 2021, 63, 659–666. [Google Scholar] [CrossRef]
- Younis, S.A.; Lim, D.K.; Kim, K.H.; Deep, A. Metalloporphyrinic metal-organic frameworks: Controlled synthesis for catalytic applications in environmental and biological media. Adv. Colloid Interface Sci. 2020, 277, 102108. [Google Scholar] [CrossRef]
- Harvey, P.D. Porphyrin-based metal- and covalent-organic frameworks as heterogeneous nanosized photocatalysts in organic synthesis. J. Mater. Chem. C 2021, 9, 16885–16910. [Google Scholar] [CrossRef]
- Wurster, B.; Grumelli, D.; Hotger, D.; Gutzler, R.; Kern, K. Driving the oxygen evolution reaction by nonlinear cooperativity in bimetallic coordination catalysts. J. Am. Chem. Soc. 2016, 138, 3623–3626. [Google Scholar] [CrossRef]
- Wang, A.J.; Cheng, L.X.; Shen, X.L.; Zhu, W.H.; Li, L.H. Mechanistic insight on porphyrin based porous titanium coordination polymer as efficient bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. Dye. Pigment 2020, 181, 108568. [Google Scholar] [CrossRef]
- Wang, A.J.; Cheng, L.X.; Shen, X.L.; Chen, X.D.; Zhu, W.H.; Zhao, W.; Lv, C.C. Porphyrin coordination polymer/Co1−xS composite electrocatalyst for efficient oxygen evolution reaction. Chem. Eng. J. 2020, 400, 125975. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.C.; Jiang, H.L. Metal-organic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Park, I.H.; Medishetty, R.; Vittal, J.J. Two-dimensional metal-organic framework materials: Synthesis, structures, properties and applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.N.; Fan, W.D.; Bi, J.H.; Jiang, P.; Liu, D.D.; Zhang, X.R.; Lin, H.; Gong, C.F.; Wang, R.M.; Zhang, L.L.; et al. A lead-porphyrin metal-organic framework: Gas adsorption properties and electrocatalytic activity for water oxidation. Dalton Trans. 2016, 45, 61–65. [Google Scholar] [CrossRef]
- Usov, P.M.; Ahrenholtz, S.R.; Maza, W.A.; Stratakes, B.; Epley, C.C.; Kessinger, M.C.; Zhu, J.; Morris, A.J. Cooperative electrochemical water oxidation by Zr nodes and Ni–porphyrin linkers of a PCN-224 MOF thin film. J. Mater. Chem. A 2016, 4, 16818–16823. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-J.; Gu, Z.-G.; Zhang, W.H.; Kang, Y.; Zhang, J. Epitaxial encapsulation of homodispersed CeO2 in a cobalt–porphyrin network derived thin film for the highly efficient oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 20126–20130. [Google Scholar] [CrossRef]
- Mirza, S.; Chen, H.; Chen, S.-M.; Gu, Z.-G.; Zhang, J. Insight into Fe(Salen) encapsulated Co-porphyrin famework derived thin film for efficient oxygen evolution reaction. Cryst. Growth Des. 2018, 18, 7150–7157. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.-F.; Lai, W.-Y.; Huang, W. Porous organic polymers as promising electrode materials for energy storage devices. Adv. Mater. Technol. 2020, 5, 2000154. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Riduana, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef]
- Ji, W.Y.; Wang, T.-X.; Ding, X.S.; Lei, S.B.; Han, B.-H. Porphyrin- and phthalocyanine-based porous organic polymers: From synthesis to application. Coord. Chem. Rev. 2021, 439, 213875. [Google Scholar] [CrossRef]
- Lee, J.M.; Cooper, A.I. Advances in conjugated microporous polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.H.; Zeng, Z.T.; Wang, H.; Xiong, W.P.; Song, B.; Zhou, C.Y.; Duan, A.B.; Tan, X.F.; He, Q.Y.; Zeng, G.M.; et al. Recent progress in conjugated microporous polymers for clean energy: Synthesis, modification, computer simulations, and applications. Prog. Polym. Sci. 2021, 115, 101374. [Google Scholar] [CrossRef]
- Jia, H.X.; Yao, Y.C.; Gao, Y.Y.; Lu, D.P.; Du, P.W. Pyrolyzed cobalt porphyrin-based conjugated mesoporous polymers as bifunctional catalysts for hydrogen production and oxygen evolution in water. Chem. Commun. 2016, 52, 13483–13486. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.S.; Qian, M.M.; Liu, X.; Sun, Z.J.; Du, P.W. A copper porphyrin-based conjugated mesoporous polymer-derived bifunctional electrocatalyst for hydrogen and oxygen evolution. ChemSusChem 2016, 9, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Bhunia, K.; Patra, B.C.; Das, S.K.; Pradhan, D.; Bhaumik, A.; Pradhan, A.; Bhattacharya, S. Efficacious electrochemical oxygen evolution from a novel Co(II) porphyrin/pyrene-based conjugated microporous polymer. ACS Appl. Mater. Interfaces 2019, 11, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Xu, Z.; Li, H.X.; Young, D.J.; Hu, C.J.; Yang, Y.G. Porphyrin-based NiFe porous organic polymer catalysts for the oxygen evolution reaction. ChemCatChem 2021, 13, 1396–1402. [Google Scholar] [CrossRef]
- Geng, K.Y.; He, T.; Liu, R.Y.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.S.; Gong, Y.F.; Jiang, Q.H.; Jiang, D.L. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Zhao, X.J.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef]
- Schlachter, A.; Asselin, P.; Harvey, P.D. Porphyrin-Containing MOFs and COFs as Heterogeneous Photosensitizers for Singlet Oxygen-Based Antimicrobial Nanodevices. ACS Appl. Mater. Interfaces 2021, 13, 26651–26672. [Google Scholar] [CrossRef]
- Chen, M.H.; Li, H.R.; Liu, C.X.; Liu, J.Y.; Feng, Y.Q.; Wee, A.G.H.; Zhang, B. Porphyrin- and porphyrinoid-based covalent organic frameworks (COFs): From design, synthesis to applications. Coord. Chem. Rev. 2021, 435, 213778. [Google Scholar] [CrossRef]
- Wang, A.J.; Cheng, L.X.; Zhao, W.; Shen, X.L.; Zhu, W.H. Electrochemical hydrogen and oxygen evolution reactions from a cobalt-porphyrin-based covalent organic polymer. J. Colloid Interface Sci. 2020, 579, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, X.-D.; Li, T.; Zhang, W.-D.; Fu, Q.-T.; Lu, H.-S.; Wang, X.; Gu, Z.-G. Three-dimensional porphyrin-based covalent organic frameworks with tetrahedral building blocks for single-site catalysis. New J. Chem. 2019, 43, 16907–16914. [Google Scholar] [CrossRef]
- Huang, C.S.; Li, Y.J.; Wang, N.; Xue, Y.R.; Zuo, Z.C.; Liu, H.B.; Li, Y.L. Progress in research into 2D graphdiyne-based materials. Chem. Rev. 2018, 118, 7744–7803. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, H.B.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, X.; Zhu, L.; Ghazzal, M.N.; Zhang, J.; Tung, C.-H.; Wu, L.-Z. Graphdiyne for crucial gas involved catalytic reactions in energy conversion applications. Energy Environ. Sci. 2020, 13, 1326–1346. [Google Scholar] [CrossRef]
- Huang, H.; Li, F.M.; Zhang, Y.; Chen, Y. Two-dimensional graphdiyne analogue Co-coordinated porphyrin covalent organic framework nanosheets as a stable electrocatalyst for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 5575–5582. [Google Scholar] [CrossRef]
- Pan, Q.Y.; Chen, X.S.; Liu, H.; Gan, W.J.; Ding, N.X.; Zhao, Y.J. Crystalline porphyrin-based graphdiyne for electrochemical hydrogen and oxygen evolution reactions. Mater. Chem. Front. 2021, 5, 4596–4603. [Google Scholar] [CrossRef]
- Jia, H.X.; Sun, Z.J.; Jiang, D.C.; Du, P.W. Covalent cobalt porphyrin framework on multiwalled carbon nanotubes for efficient water oxidation at low overpotential. Chem. Mater. 2015, 27, 4586–4593. [Google Scholar] [CrossRef]
- Wu, X.; Tang, C.J.; Cheng, Y.; Min, X.B.; Jiang, S.P.; Wang, S.Y. Bifunctional catalysts for reversible oxygen evolution reaction and oxygen reduction reaction. Chem. Eur. J. 2020, 26, 3906–3929. [Google Scholar] [CrossRef]
- Attatsi, I.K.; Zhu, W.H.; Liang, X. Noncovalent immobilization of Co(ii)porphyrin through axial coordination as an enhanced electrocatalyst on carbon electrodes for oxygen reduction and evolution. New J. Chem. 2020, 44, 4340–4345. [Google Scholar] [CrossRef]
- Qin, H.N.; Wang, Y.Z.; Wang, B.; Duan, X.G.; Lei, H.T.; Zhang, X.P.; Zheng, H.Q.; Zhang, W.; Cao, R. Cobalt porphyrins supported on carbon nanotubes as model catalysts of metal-N4/C sites for oxygen electrocatalysis. J. Energy Chem. 2021, 53, 77–81. [Google Scholar] [CrossRef]
- Lv, H.Y.; Guo, H.B.; Guo, K.; Lei, H.T.; Zhang, W.; Zheng, H.Q.; Liang, Z.Z.; Cao, R. Substituent position effect of Co porphyrin on oxygen electrocatalysis. Chin. Chem. Lett. 2021, 32, 2841–2845. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Q.T.; Bian, Z.N.; Wang, G.M.; Xu, Y.X. Supramolecular modulation of molecular conformation of metal porphyrins toward remarkably enhanced multipurpose electrocatalysis and ultrahigh-performance zinc–air batteries. Adv. Energy Mater. 2021, 11, 2102062. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, L.-J.; Chen, W.-C.; Xu, X.; Peng, W.-Y.; Xiao, X.-Y.; Liu, H.-Y.; Si, L.-P. The S-doped cobalt porphyrin-based molecular catalysts with large conjugated meso-substituents for enhanced electrocatalytic oxygen reduction and evolution reaction. J. Electrochem. Soc. 2021, 168, 116502. [Google Scholar] [CrossRef]
- Marianov, A.N.; Jiang, Y.J. Effect of manganese porphyrin covalent immobilization on electrocatalytic water oxidation and oxygen reduction reactions. ACS Sustain. Chem. Eng. 2019, 7, 3838–3848. [Google Scholar] [CrossRef]
- Xie, L.S.; Zhang, X.-P.; Zhao, B.; Li, P.; Qi, J.; Guo, X.A.; Wang, B.; Lei, H.T.; Zhang, W.; Apfel, U.-P.; et al. Enzyme-inspired iron porphyrins for improved electrocatalytic oxygen reduction and evolution reactions. Angew. Chem. Int. Ed. 2021, 60, 7576–7581. [Google Scholar] [CrossRef]
- Sun, J.Q.; Yin, H.J.; Liu, P.; Wang, Y.; Yao, X.D.; Tang, Z.Y.; Zhao, H.J. Molecular engineering of Ni-/Co-porphyrin multilayers on reduced graphene oxide sheets as bifunctional catalysts for oxygen evolution and oxygen reduction reactions. Chem. Sci. 2016, 7, 5640–5646. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.T.; Zhang, Q.X.; Wang, Y.B.; Gao, Y.M.; Wang, Y.Z.; Liang, Z.Z.; Zhang, W.; Cao, R. Significantly boosted oxygen electrocatalysis with cooperation between cobalt and iron porphyrins. Dalton Trans. 2021, 50, 5120–5123. [Google Scholar] [CrossRef]
- Hotger, D.; Etzkorn, M.; Morchutt, C.; Wurster, B.; Dreiser, J.; Stepanow, S.; Grumelli, D.; Gutzler, R.; Kern, K. Stability of metallo-porphyrin networks under oxygen reduction and evolution conditions in alkaline media. Phys. Chem. Chem. Phys. 2019, 21, 2587–2594. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, S.; Dehghanpour, S.; Ghalkhani, M. A cobalt porphyrin-based metal organic framework/multi-walled carbon nanotube composite electrocatalyst for oxygen reduction and evolution reactions. J. Mater. Sci. 2017, 53, 3624–3639. [Google Scholar] [CrossRef]
- Cichocka, M.O.; Liang, Z.Z.; Feng, D.W.; Back, S.; Siahrostami, S.; Wang, X.; Samperisi, L.; Sun, Y.J.; Xu, H.Y.; Hedin, N.; et al. A porphyrinic zirconium metal-organic framework for oxygen reduction reaction: Tailoring the spacing between active-sites through chain-based inorganic building units. J. Am. Chem. Soc. 2020, 142, 15386–15395. [Google Scholar] [CrossRef]
- Fan, X.H.; Kong, F.T.; Kong, A.G.; Chen, A.L.; Zhou, Z.Q.; Shan, Y.K. Covalent porphyrin framework-derived Fe2P@Fe4N-coupled nanoparticles embedded in N-doped carbons as efficient trifunctional electrocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 32840–32850. [Google Scholar] [CrossRef]
- Mei, G.X.; Cui, L.L.; Dou, Z.Y.; He, X.Q. Heat-treated multi-walled carbon nanotubes-supported (Fe,Co,Ni)-coordinated polyporphyrin: A robust air cathode catalyst for rechargeable zinc-air batteries. Electrochim. Acta 2020, 358, 136918. [Google Scholar] [CrossRef]
- Li, Y.; Tao, X.S.; Wei, J.C.; Lv, X.L.; Wang, H.G. Metal phthalocyanine-porphyrin-based conjugated microporous polymer-derived bifunctional electrocatalysts for Zn-air batteries. Chem. Asian J. 2020, 15, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Liu, J.N.; Wang, J.; Ren, D.; Li, B.Q.; Zhang, Q. Recent advances of noble-metal-free bifunctional oxygen reduction and evolution electrocatalysts. Chem. Soc. Rev. 2021, 50, 7745–7778. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Q.; Zhang, S.-Y.; Wang, B.; Xia, Z.-J.; Tang, C.; Zhang, Q. A porphyrin covalent organic framework cathode for flexible Zn–air batteries. Energy Environ. Sci. 2018, 11, 1723–1729. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhang, S.Y.; Chen, X.; Chen, C.Y.; Xia, Z.J.; Zhang, Q. One-pot synthesis of framework porphyrin materials and their applications in bifunctional oxygen electrocatalysis. Adv. Funct. Mater. 2019, 29, 1901301. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhao, C.X.; Chen, S.M.; Liu, J.N.; Chen, X.; Song, L.; Zhang, Q. Framework-porphyrin-derived single-atom bifunctional oxygen electrocatalysts and their applications in Zn-air batteries. Adv. Mater. 2019, 31, 1900592. [Google Scholar] [CrossRef]
- Liu, J.-N.; Li, B.-Q.; Zhao, C.-X.; Yu, J.; Zhang, Q. A composite bifunctional oxygen electrocatalyst for high-performance rechargeable zinc–air batteries. ChemSusChem 2020, 13, 1529–1536. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Li, B.Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale construction of bifunctional electrocatalysts for long-lifespan rechargeable zinc–air batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Cai, G.M.; Zeng, L.H.; He, L.Q.; Sun, S.J.; Tong, Y.X.; Zhang, J.Y. Imine gels based on ferrocene and porphyrin and their electrocatalytic property. Chem. Asian J. 2020, 15, 1963–1969. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, B.; He, Y.; Wang, S.; Sun, H.; Liu, X. Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction. Int. J. Mol. Sci. 2022, 23, 6036. https://doi.org/10.3390/ijms23116036
Yao B, He Y, Wang S, Sun H, Liu X. Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction. International Journal of Molecular Sciences. 2022; 23(11):6036. https://doi.org/10.3390/ijms23116036
Chicago/Turabian StyleYao, Bin, Youzhou He, Song Wang, Hongfei Sun, and Xingyan Liu. 2022. "Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction" International Journal of Molecular Sciences 23, no. 11: 6036. https://doi.org/10.3390/ijms23116036
APA StyleYao, B., He, Y., Wang, S., Sun, H., & Liu, X. (2022). Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction. International Journal of Molecular Sciences, 23(11), 6036. https://doi.org/10.3390/ijms23116036






