Enhancement of Corrosion Resistance Properties of Electrodeposited Ni/nano-TiC Composite Layers
Abstract
:1. Introduction
2. Results and Discussion
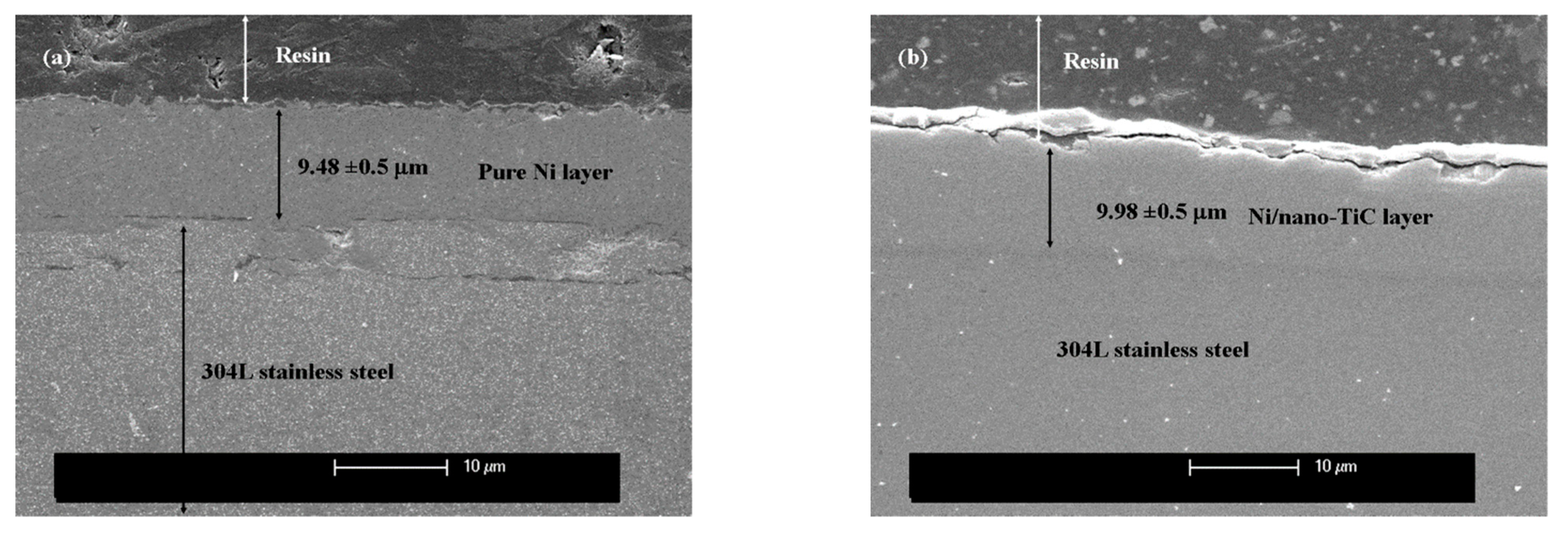
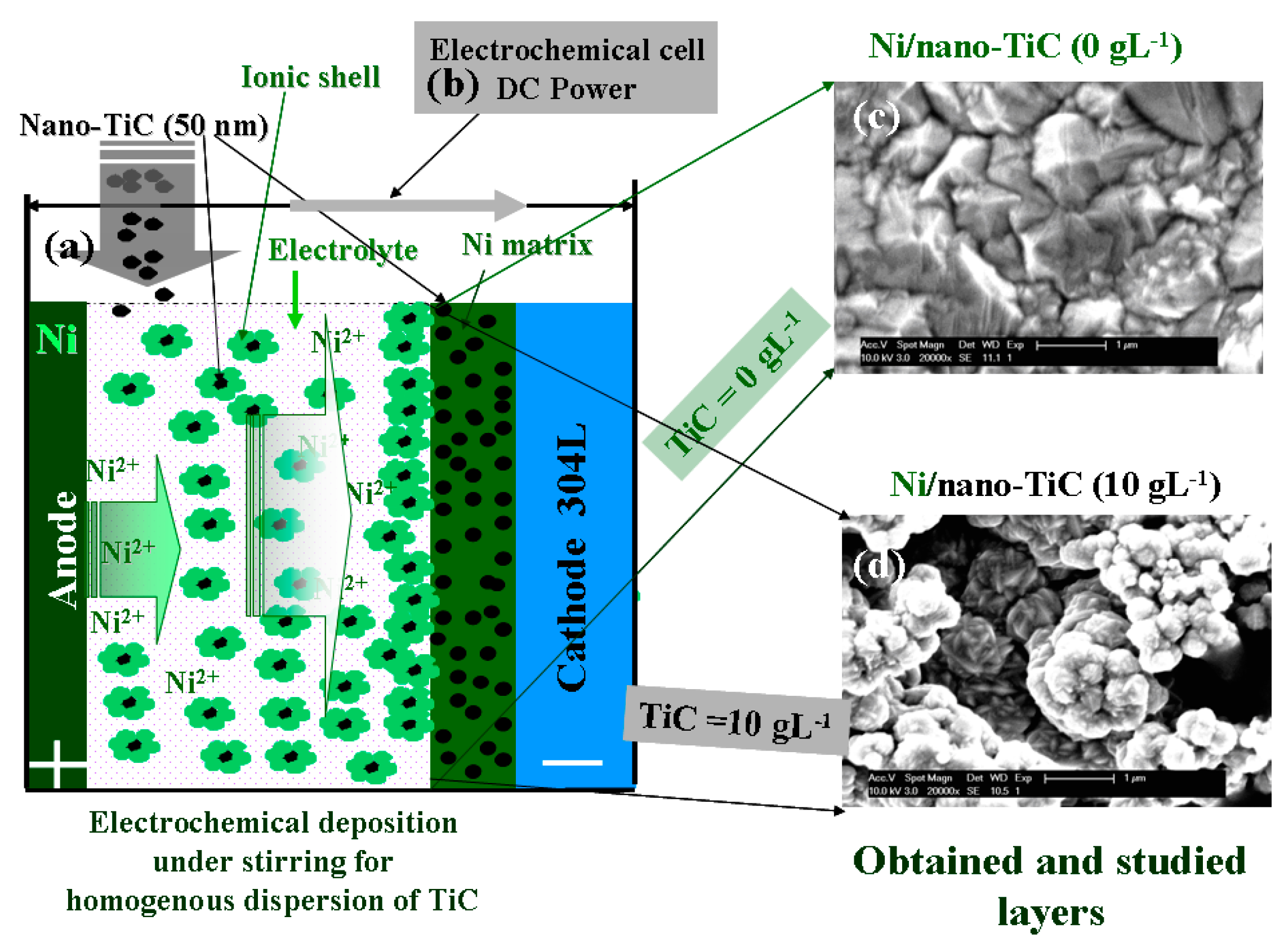
2.1. SEM Surface Morphology and Layer Thicknesses
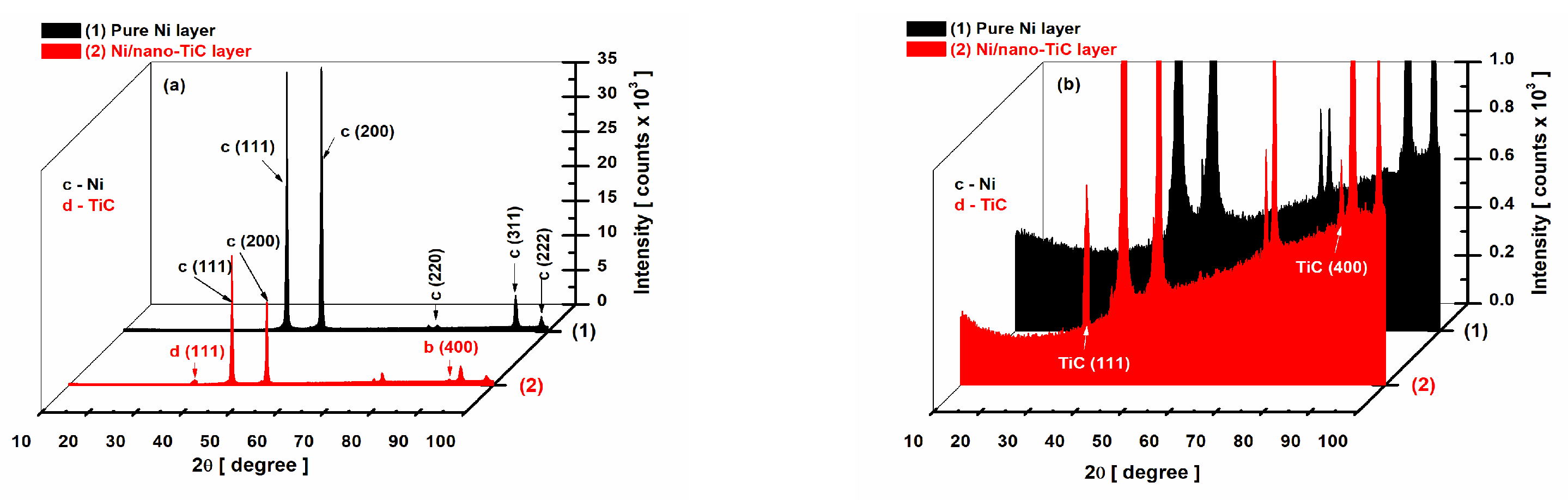
2.2. XRD Diffraction Patterns
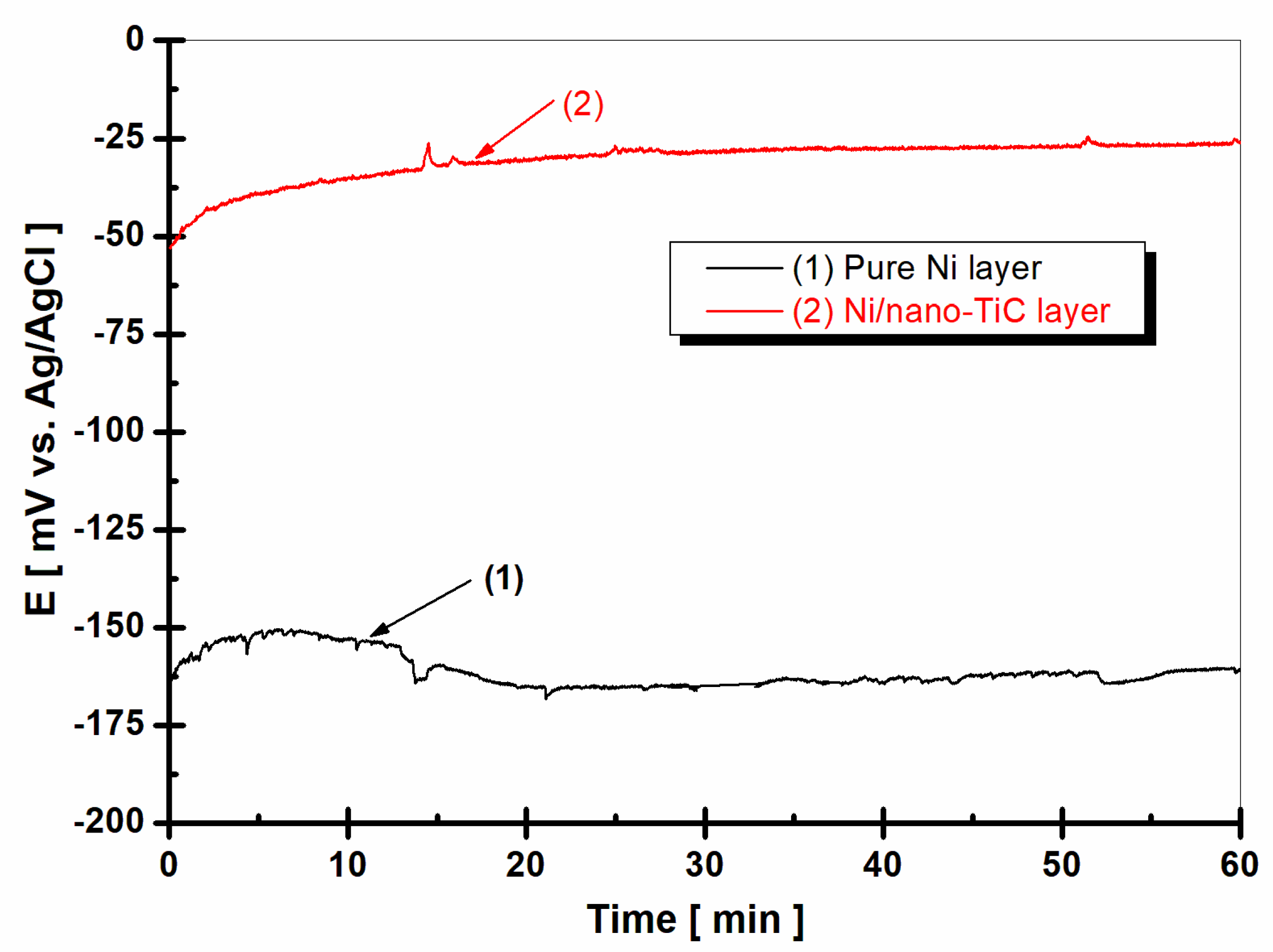
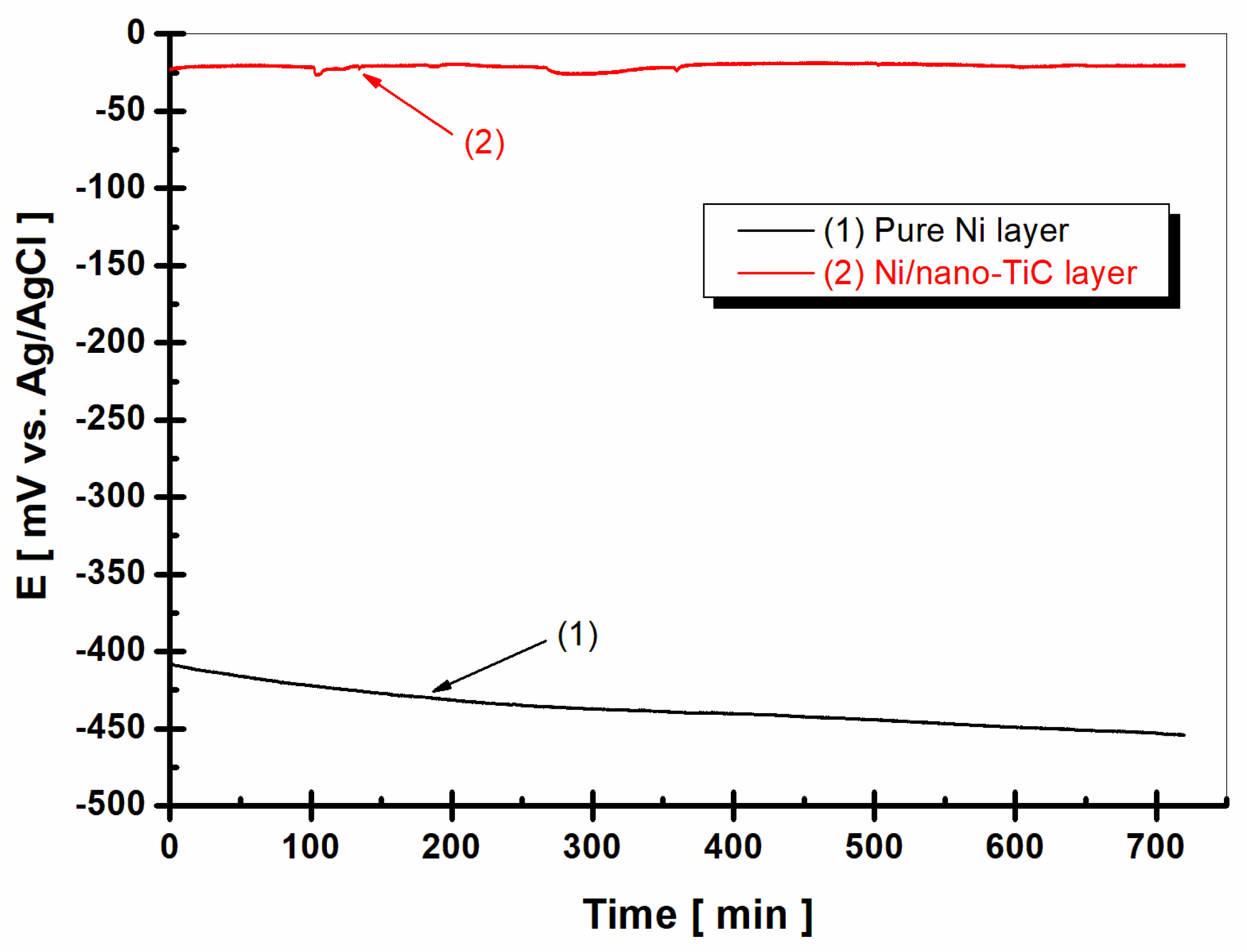
2.3. Evolution of Open Circuit Potential
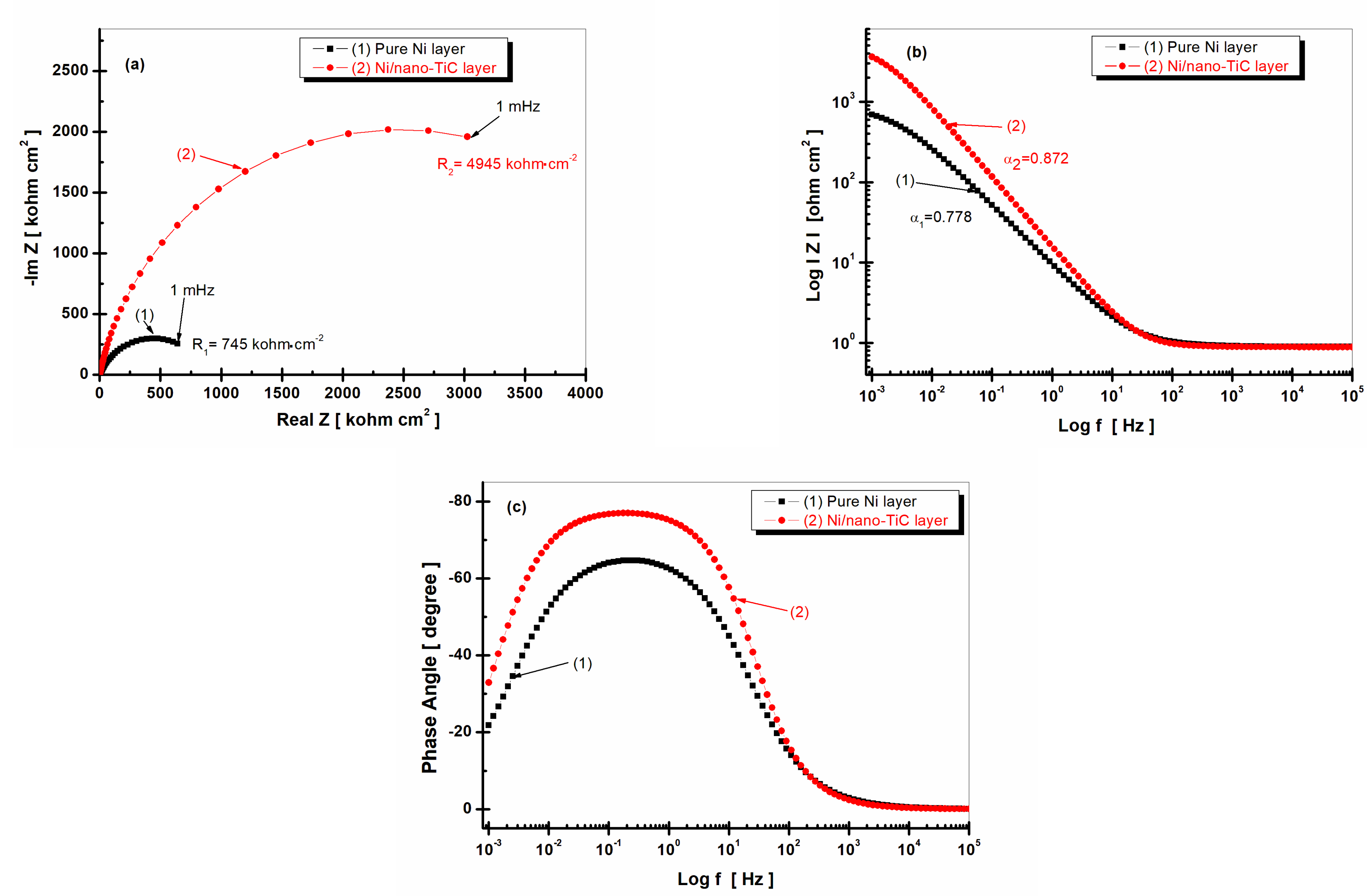
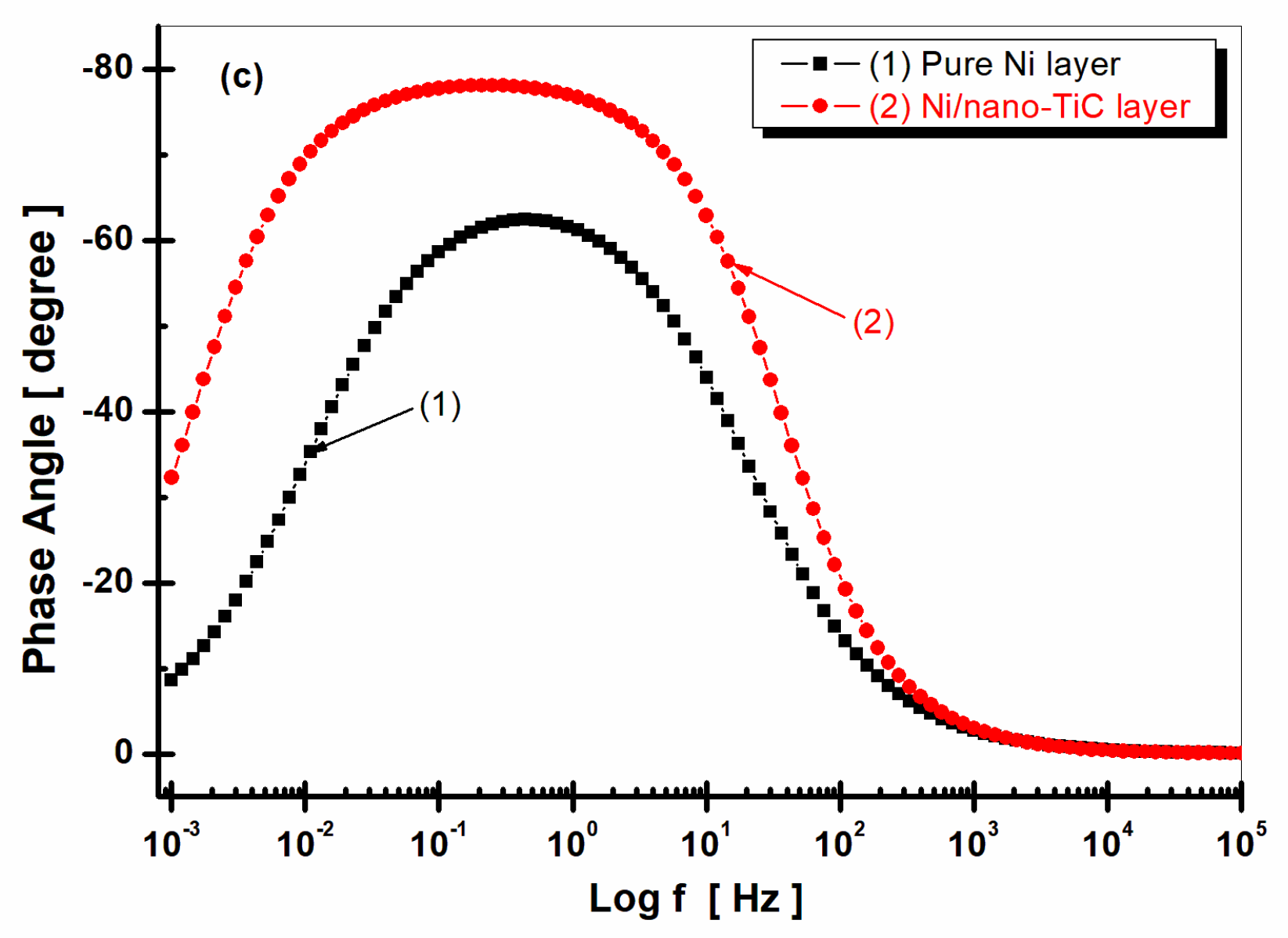
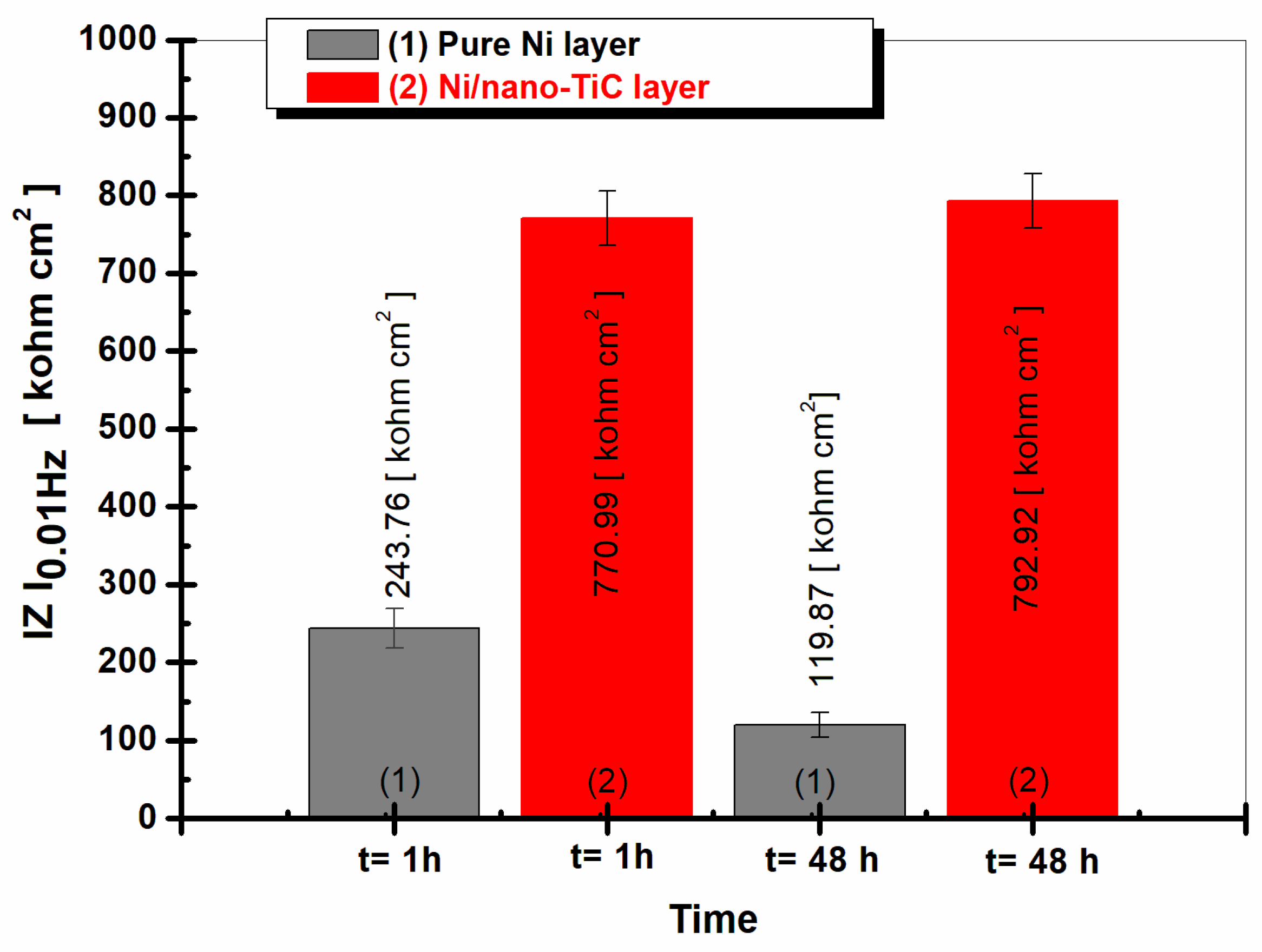
2.4. Electrochemical Impedance Spectroscopy (EIS)
3. Materials and Methods
- -
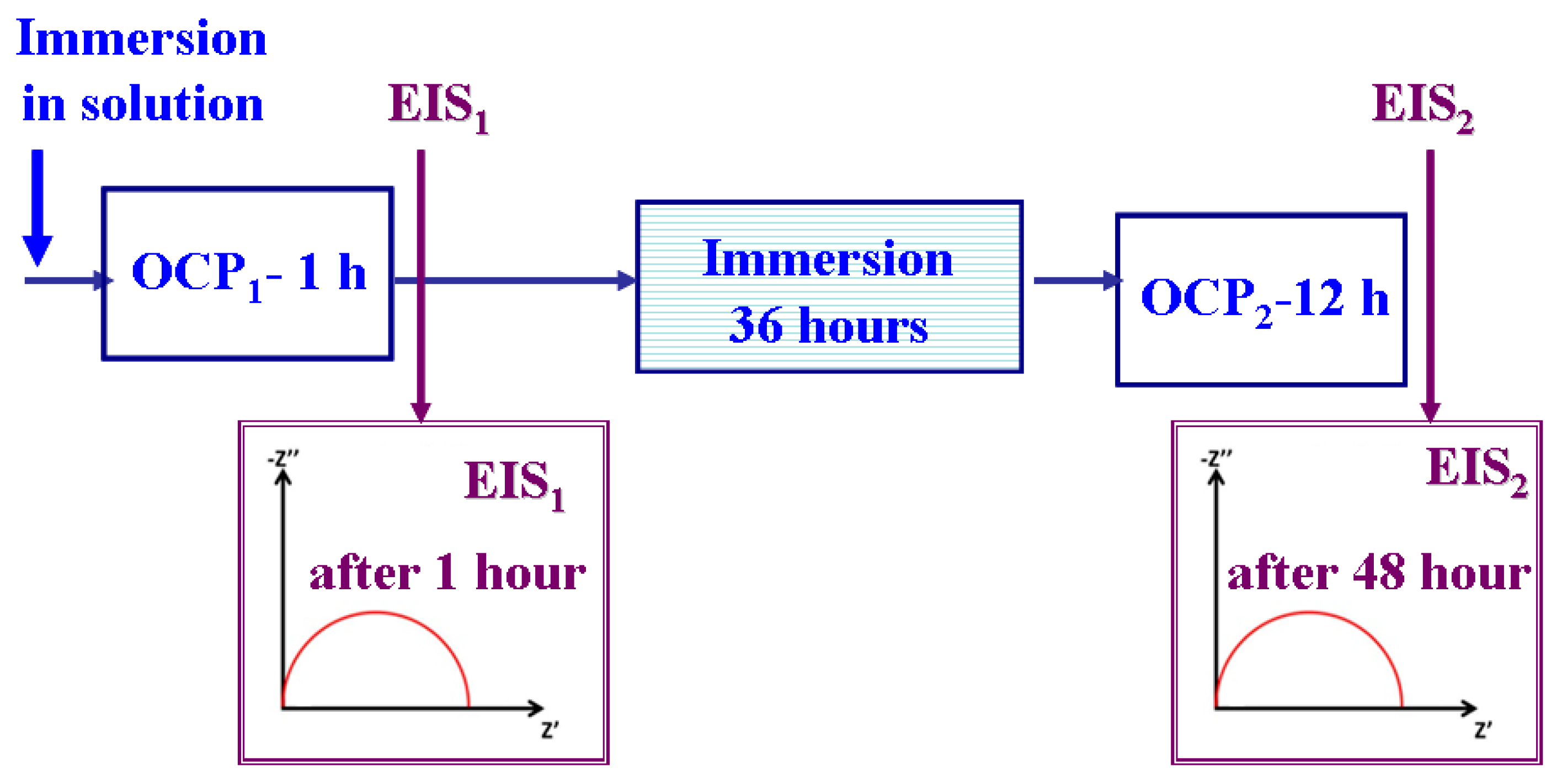
- Measuring the evolution of the free potential E(t), which represents the variation in time of the electrode potential (WE) in an open circuit (OCP), over 60 min.
- -
- Plotting the electrochemical impedance spectroscopy (EIS) diagrams at free potential and in alternating current, with an amplitude of AC = 10 mV in the frequency range from 100 kHz to 1 mHz, with 20 frequencies per decade.
- -
- Immersing the samples in solution for 36 h.
- -
- Measuring the evolution of the free potential E (t), or the OCP, over 12 h.
- -
- Plotting the electrochemical impedance spectroscopy (EIS) diagrams after 48 h of immersion.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, M. Nanocomposite materials. In Nanotechnology and the Environment; Sen, M., Ed.; IntechOpen: ondon, UK, 2020; pp. 1–12. [Google Scholar]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for food packaging applications: An overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Gendelberg, N.; Bird, D.; Ravindra, N.M. Nanocomposites for biomedical applications. In Polymer-Based Multifunctional Nanocomposites and Their Applications; Kenan, S., Chuntai, L., Guo, J.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–199. [Google Scholar]
- Benea, L.; Dănăilă, E.; Ponthiaux, P.; Celis, J.P. Improving Tribocorroson behaviour by electro-codeposition of TiC nano-dispersed particles with nickel as hybrid layers for energy applications. In Proceedings of the 13th International Conference on Tribology, ROTRIB’16, Galati, Romania, 22–24 September 2016; Volume 174, p. 012045. [Google Scholar]
- Benea, L.; Caron, N.; Raquet, O. Improving tribological behavior of a Ni matrix hybrid nanocomposite reinforced by titanium carbide nanoparticles during electro-codeposition. RSC Adv. 2016, 6, 59775–59783. [Google Scholar] [CrossRef]
- Benea, L.; Celis, J.P. Effect of nano-TiC dispersed particles and electro-codeposition parameters on morphology and structure of hybrid Ni/TiC nanocomposite layers. Materials 2016, 9, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dănăilă, E.; Benea, L.; Caron, N.; Raquet, O. Titanium carbide nanoparticles reinforcing nickel matrix for improving nanohardness and fretting wear properties in wet conditions. Met. Mater. Int. 2016, 22, 924–934. [Google Scholar] [CrossRef]
- Bhat, A.; Budholiya, S.; Aravind Raj, S.; Sultan, M.T.H.; Hui, D.; Md Shah, A.U.; Safri, S.N.A. Review on nanocomposites based on aerospace applications. Nanotechnol. Rev. 2021, 10, 237–253. [Google Scholar] [CrossRef]
- Nobel, M.L.; Picken, S.J.; Mendes, E. Waterborne nanocomposite resins for automotive coating applications. Prog. Org. Coat. 2007, 58, 96–104. [Google Scholar] [CrossRef]
- Podsiadły, B.; Walter, P.; Kamiński, M.; Skalski, A.; Słoma, M. Electrically conductive nanocomposite fibers for flexible and structural electronics. Appl. Sci. 2022, 12, 941. [Google Scholar] [CrossRef]
- Tripathy, J. Polymer Nanocomposites for biomedical and biotechnology applications. In Properties and Applications of Polymer Nanocomposites; Tripathy, D., Sahoo, B., Eds.; Springer: Berlin, Germany, 2017; pp. 57–76. [Google Scholar]
- Raja, M.; Ramesh Bapu, G.N.K.; Maharaja, J.; Sekar, R. Electrodeposition and characterisation of Ni–TiC nanocomposite using Watts bath. Surf. Eng. 2014, 30, 697–701. [Google Scholar] [CrossRef]
- Benea, L.; Dănăilă, E.; Celis, J.P. Influence of Electro-co-deposition parameters on nano-TiO2 inclusion into nickel matrix and properties characterization of nanocomposite coatings obtained. Mater. Sci. Eng. A 2014, 610, 106–115. [Google Scholar] [CrossRef]
- SimioNeScu-Bogatu, N.; BeNea, L. Effect of the applied current density and deposition time on electro-codeposition proces of cobalt matrix reinforced with nano-CeO2. Arch. Metall. Mater. 2022, 67, 615–622. [Google Scholar]
- Ciubotariu, A.C.; Benea, L.; Lakatos-Varsanyi, M.; Dragan, V. Electrochemical impedance spectroscopy and corrosion behaviour of Al2O3-Ni nano composite coatings. Electrochim. Acta 2008, 53, 4557–4563. [Google Scholar] [CrossRef]
- Gül, H.; Kılıç, F.; Aslan, S.; Alp, A.; Akbulut, H. Characteristics of electro-co-deposited Ni-Al2O3 nano-particle reinforced metal matrix composite (MMC) coatings. Wear 2009, 267, 976–990. [Google Scholar] [CrossRef]
- Srivastava, M.; Grips, V.K.W.; Rajam, K.S. Electrodeposition of Ni-Co composites containing nano-CeO2 and their structure, properties. Appl. Surf. Sci. 2010, 257, 717–722. [Google Scholar] [CrossRef]
- Qu, N.S.; Zhu, D.; Chan, K.C. Fabrication of Ni-CeO2 nanocomposite by electrodeposition. Scr. Mater. 2006, 54, 1421–1425. [Google Scholar] [CrossRef]
- Benea, L.; Wenger, F.; Ponthiaux, P.; Celis, J.P. Tribocorrosion behaviour of Ni-SiC nano-structured composite coatings obtained by electrodeposition. Wear 2009, 266, 398–405. [Google Scholar] [CrossRef]
- Benea, L.; Bonora, P.L.; Borello, A.; Martelli, S. Effect of SiC size dimensions on the corrosion wear resistance of the electrodeposited composite coating. Mater. Corros. 2002, 53, 23–29. [Google Scholar] [CrossRef]
- Singh, D.K.; Singh, V.B. Electrodeposition and characterization of Ni-TiC composite using N-methylformamide bath. Mater. Sci. Eng. A 2012, 532, 493–499. [Google Scholar] [CrossRef]
- Singh, D.K.; Tripathi, M.K.; Singhz, V.B. Preparation of Ni-TiC nanocomposites by electrolytic codeposition from a non aqueous bath and their characterization. J. Electrochem. Soc. 2012, 159, D469–D472. [Google Scholar] [CrossRef]
- Karbasi, M.; Yazdian, N.; Vahidian, A. Development of electro-co-deposited Ni-TiC nanoparticle reinforced nanocomposite coatings. Surf. Coat. Technol. 2012, 207, 587–593. [Google Scholar] [CrossRef]
- Thiemig, D.; Bund, A. Characterization of electrodeposited Ni-TiO2 nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 2976–2984. [Google Scholar] [CrossRef]
- Benea, L. Electrodeposition and tribocorrosion behaviour of ZrO2-Ni composite coatings. J. Appl. Electrochem. 2009, 39, 1671–1681. [Google Scholar] [CrossRef]
- Wang, W.; Hou, F.Y.; Wang, H.; Guo, H.T. Fabrication and characterization of Ni-ZrO2 composite nano-coatings by pulse electrodeposition. Scr. Mater. 2005, 53, 613–618. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu, N. Corrosion behavior of Ni/WC nano-structured composite layers synthesized by electrochemical method. In Proceedings of the International Conference on Innovative Research—ICIR EUROINVENT 2019, Iasi, Romania, 16–17 May 2019; Volume 572, p. 012004. [Google Scholar]
- Durán-Olvera, J.M.; Orozco-Cruz, R.; Galván-Martínez, R.; León, C.A.; Contreras, A. Characterization of TiC/Ni composite immersed in synthetic seawater. MRS Adv. 2017, 2, 2865–2873. [Google Scholar] [CrossRef]
- Matveev, V.V.; Baranov, D.A.; Yurkov, G.Y.; Akatiev, N.G.; Dotsenko, I.P.; Gubin, S.P. Cobalt nanoparticles with preferential hcp structure: A confirmation by X-ray diffraction and NMR. Chem. Phys. Lett. 2006, 422, 402–405. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu-Bogatu, N. Reactivity and corrosion behaviors of Ti6Al4V alloy implant biomaterial under metabolic perturbation conditions in physiological solutions. Materials 2021, 14, 7404. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, O.; Tran, M.T.T.; Tribollet, B.; Turmine, M.; Vivier, V. Revisiting cyclic voltammetry and electrochemical impedance spectroscopy analysis for capacitance measurements. Electrochim. Acta 2020, 343, 136109. [Google Scholar] [CrossRef]
- Benea, L.; Simionescu-Bogatu, N.; Chiriac, R. Electrochemically obtained Al2O3 nanoporous layers with increased anticorrosive properties of aluminum alloy. J. Mater. Res. Technol. 2022, 17, 2636–2647. [Google Scholar] [CrossRef]



| Chemical Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Ni | Cr | N | Cu | Mo |
| 0.013 | 0.490 | 1.140 | 0.031 | 0.006 | 8.030 | 18.21 | 0.027 | 0.240 | 0.140 |
| Characteristics | Technical Specifications |
|---|---|
| Color | Black |
| Morphology | Powder |
| Purity | >99.0% |
| Crystalline structure | Cubic |
| The specific size of the surface | 23 m2/g |
| Particle dimension | 50 nm |
| Density at 20 °C | 4.93 g/cm3 |
| Melting point | 3050–3230 °C |
| Boiling point | 4800 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogatu, N.; Benea, L.; Axente, E.R.; Celis, J.P. Enhancement of Corrosion Resistance Properties of Electrodeposited Ni/nano-TiC Composite Layers. Int. J. Mol. Sci. 2022, 23, 6069. https://doi.org/10.3390/ijms23116069
Bogatu N, Benea L, Axente ER, Celis JP. Enhancement of Corrosion Resistance Properties of Electrodeposited Ni/nano-TiC Composite Layers. International Journal of Molecular Sciences. 2022; 23(11):6069. https://doi.org/10.3390/ijms23116069
Chicago/Turabian StyleBogatu, Nicoleta, Lidia Benea, Elena Roxana Axente, and Jean Pierre Celis. 2022. "Enhancement of Corrosion Resistance Properties of Electrodeposited Ni/nano-TiC Composite Layers" International Journal of Molecular Sciences 23, no. 11: 6069. https://doi.org/10.3390/ijms23116069
APA StyleBogatu, N., Benea, L., Axente, E. R., & Celis, J. P. (2022). Enhancement of Corrosion Resistance Properties of Electrodeposited Ni/nano-TiC Composite Layers. International Journal of Molecular Sciences, 23(11), 6069. https://doi.org/10.3390/ijms23116069








