Prenatal Zinc Deficient Mice as a Model for Autism Spectrum Disorders
Abstract
:1. Introduction
2. Prenatal Zinc-Deficient Mice
2.1. Behavioral Impairments of PZD Mice
| Mouse Model | Shank1 | Shank2 | Shank2 | Shank3 | Shank3 | Shank3 | Shank3 | Shank3 | Shank3 | Neurexin 1 Alpha | PZD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exons 14–15/PDZ | Exons 6–7/PDZ | Exon 7/PDZ | Exons 4–9/ANK repeat (Δex4–9 B) | Exons 4–9/ANK repeat (Δex4–9 J) | Exons 4–7/ANK repeat (Δex4–7) | Exons 13–16/PDZ (Δex13–16) | Exon 11/SH3 (Δex11) | Exon 21 (Δex21) | |||
| Reference | [61,62,85] | [64] | [63] | [65,73] | [66] | [67] | [67] | [63] | [86] | [71] | [53,54] |
| Social Behavior | Reduced sniffing by males in female-male interaction, normal juvenile social interaction | impaired sociability, impaired pup retrieval, impaired nest building | normal sociability, impairments in social novelty, normal initiation of social contact but impairments in maintaining social contact | mild social impairments in reciprocal social interaction (juveniles), normal sociability, normal social novelty, reduced social sniffing in males (male-female interaction | reduced sociability decreased bidirectional social interactions | normal initiation of social interaction, impaired social novelty | decreased frequency of nose-to-nose contact, decreased anogenital sniffing, impaired social novelty | not analyzed | Reduced time sniffing inanimate object in three-chamber test, impairments in social novelty (no preference of novel social target in KO) | increased social approach, increased aggression, reduced nest building | increased social approach, aggression, reduced nest building, decreased time spent in oral-oral contact (females) |
| Ultrasonic vocalizations | reduced number of USV | reduced number of USV during male-female interaction, increased latency to call | no difference in adults during male-male interaction, reduced calls, and longer latency to call during male-female interaction | reduced number of USV (adult mice) | increased number of calls (males), decreased number of calls (females) | not analyzed | not analyzed | not analyzed | No differences in the number of calls or latency to emit the first call in males in free-roaming male-to-female interaction during estrous | not analyzed | reduced number of USVs increased latency to call, reduced number of overtones with harmonics, decreased sound pressure level, increased latency to call during male-male or female-female interaction |
| Repetitive behavior | no increased self-grooming | no increased self-grooming in the home cage but increased in a novel object recognition task, hyperactivity | increased self-grooming, hyperactivity | increased self-grooming, inflexibility in reversal learning Morris water maze | an increased head pokes in hole board test, increased self-grooming | no increase in self-grooming | increase in self-grooming | increase in self-grooming | no increased self-grooming at 9–18 weeks old, increased self-grooming at older age | no increase in self-grooming | impaired marble burying (females), no significant increase in self-grooming |
| Anxiety | partial increased | increased | increased | not determined | not determined | not determined | increased | increased | Avoidance of light in the dark/light task, no differences in elevated plus maze or open field test | increased | increased |
| Learning and Memory | enhanced spatial memory, impaired fear conditioning, reduced motor learning | impaired spatial memory, normal novel object recognition | normal working memory, normal novel object recognition memory | impaired novel object recognition memory, normal spatial memory in Morris Water Maze, normal fear conditioning | impaired short- and long-term memory, impaired spatial learning in Morris Water Maze | not determined | normal spatial learning in Morris Water Maze | not determined | Impaired spatial learning and memory | no impairments in spatial, working, or episodic memory, short term or long memory | normal working memory (trend reduction), impairments in motor learning (rotarod) |
2.2. Extracerebral Pathologies of PZD Mice
2.3. CNS Phenotype of PZD Mice
3. Conclusions
3.1. A Link between Genetic and Non-Genetic Factors in ASD through Zinc Signaling?
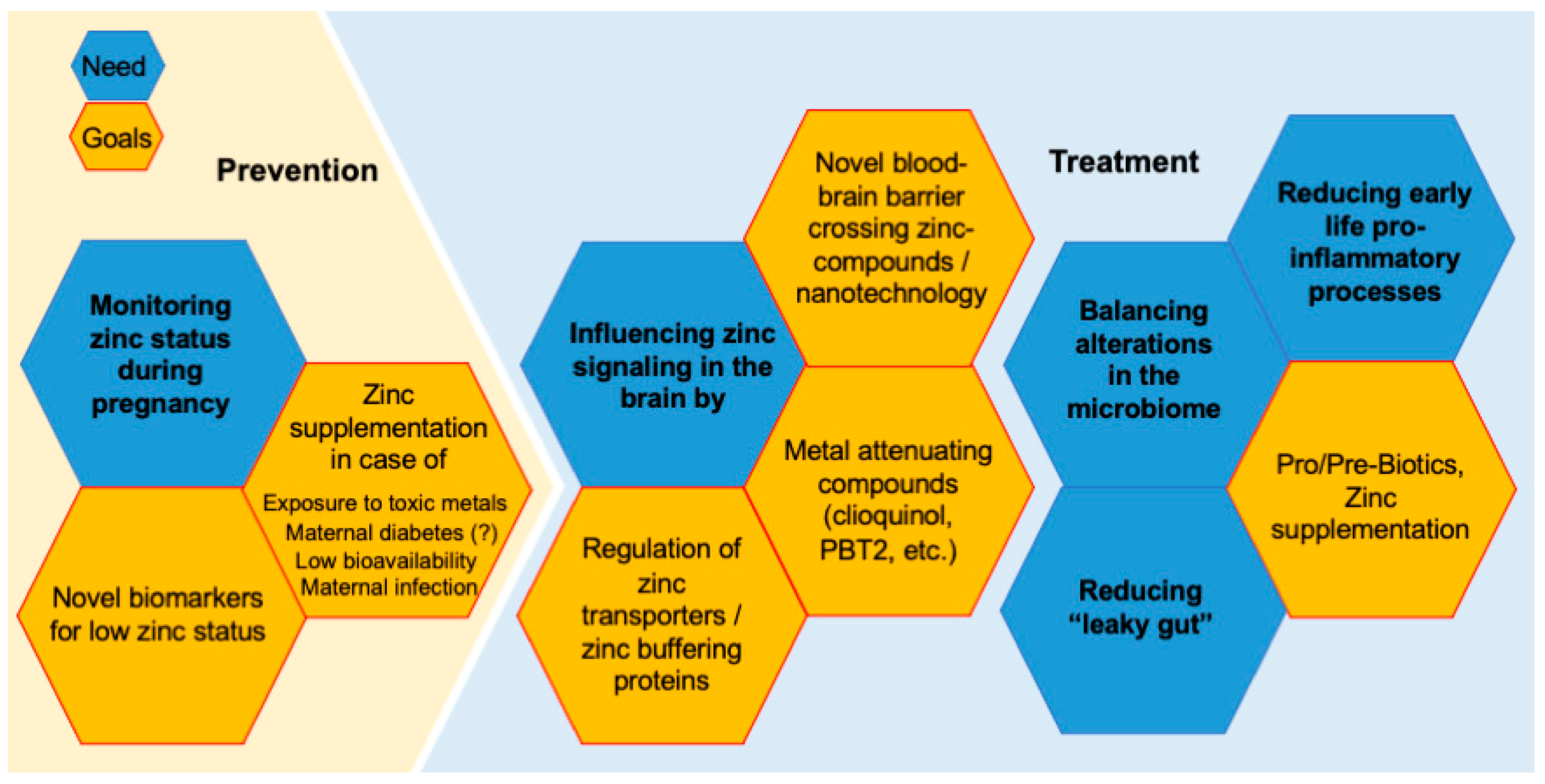
3.2. Future Perspectives—Prevention and Treatment Strategies
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delorme, R.; Ey, E.; Toro, R.; Leboyer, M.; Gillberg, C.; Bourgeron, T. Progress toward treatments for synaptic defects in autism. Nat. Med. 2013, 19, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Huguet, G.; Benabou, M.; Bourgeron, T. The Genetics of Autism Spectrum Disorders. In A Time for Metabolism and Hormones; Sassone-Corsi, P., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 101–129. [Google Scholar]
- Kelleher, R.J., III; Geigenmüller, U.; Hovhannisyan, H.; Trautman, E.; Pinard, R.; Rathmell, B.; Carpenter, R.; Margulies, D. High-throughput sequencing of mGluR signaling pathway genes reveals enrichment of rare variants in autism. PLoS ONE 2012, 7, e35003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guang, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell. Neurosci. 2018, 12, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamain, S.; Quach, H.; Betancur, C.; Råstam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gill-berg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, C.M.; Betancur, C.; Boeckers, T.M.; Bockmann, J.; Chaste, P.; Fauchereau, F.; Nygren, G.; Rastam, M.; Gillberg, I.C.; Anckarsäter, H.; et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2006, 39, 25–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkel, S.; Marshall, C.R.; Weiss, B.; Howe, J.; Roeth, R.; Moog, U.; Endris, V.; Roberts, W.; Szatmari, P.; Pinto, D.; et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010, 42, 489–491. [Google Scholar] [CrossRef]
- Gauthier, J.; Spiegelman, D.; Piton, A.; Lafrenière, R.G.; Laurent, S.; St-Onge, J.; Lapointe, L.; Hamdan, F.F.; Cossette, P.; Mottron, L.; et al. Novel de novo SHANK3 mutation in autistic patients. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 150, 421–424. [Google Scholar] [CrossRef]
- Huber, K.M.; Klann, E.; Costa-Mattioli, M.; Zukin, R.S. Dysregulation of Mammalian Target of Rapamycin Signaling in Mouse Models of Autism. J. Neurosci. 2015, 35, 13836–13842. [Google Scholar] [CrossRef]
- Grabrucker, A.M. Environmental Factors in Autism. Front. Psychiatry 2013, 3, 118. [Google Scholar] [CrossRef] [Green Version]
- Jen, M.; Yan, A.C. Syndromes associated with nutritional deficiency and excess. Clin. Dermatol. 2010, 28, 669–685. [Google Scholar] [CrossRef]
- Yasuda, H.; Yoshida, K.; Yasuda, Y.; Tsutsui, T. Infantile zinc deficiency: Association with autism spectrum disorders. Sci. Rep. 2011, 1, 129. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Tsutsui, T. Assessment of infantile mineral imbalances in autism spectrum disorders (ASDs). Int. J. Environ. Res. Public Health 2013, 10, 6027–6043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, H.; Kobayashi, M.; Yasuda, Y.; Tsutsui, T. Estimation of autistic children by metallomics analysis. Sci. Rep. 2013, 3, 1199, Correction in Sci. Rep. 2013, 3, 2254. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Reichenberg, A.; Willfors, C.; Austin, C.; Gennings, C.; Berggren, S.; Lichtenstein, P.; Anckarsäter, H.; Tammimies, K.; Bölte, S. Fetal and postnatal metal dysregulation in autism. Nat. Commun. 2017, 8, 15493. [Google Scholar] [CrossRef]
- Fiore, M.; Barone, R.; Copat, C.; Grasso, A.; Cristaldi, A.; Rizzo, R.; Ferrante, M. Metal and essential element levels in hair and association with autism severity. J. Trace Elements Med. Biol. 2019, 57, 126409. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Ahangari, N.; Hendi, K.; Saleh, F.; Rezaei, N. Status of essential elements in autism spectrum disorder: Systematic review and meta-analysis. Rev. Neurosci. 2017, 28, 783–809. [Google Scholar] [CrossRef]
- Babaknejad, N.; Sayehmiri, F.; Sayehmiri, K.; Mohamadkhani, A.; Bahrami, S. The Relationship between Zinc Levels and Autism: A Systematic Review and Meta-analysis. Rev. Neurosci. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Sayehmiri, F.; Babaknejad, N.; Bahrami, S.; Sayehmiri, K.; Rezaei-Tavirani, M. Zn/Cu Levels in the Field of Autism Disorders: A Systematic Review and Meta-analysis. Iran. J. Child Neurol. 2015, 9, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Shen, L.; Khan, N.U.; Zhang, X.; Chen, L.; Zhao, H.; Luo, P. Trace elements in children with autism spectrum disorder: A meta-analysis based on case-control studies. J. Trace Elements Med. Biol. 2021, 67, 126782. [Google Scholar] [CrossRef]
- Lakshmi Priya, M.D.; Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 2011, 142, 148–158. [Google Scholar] [CrossRef]
- Faber, S.; Zinn, G.M.; Ii, J.C.K.; Kingston, H.M.S. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers 2009, 14, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Curtin, P.; Austin, C.; Curtin, A.; Gennings, C.; Arora, M.; Tammimies, K.; Willfors, C.; Berggren, S.; Siper, P.; Rai, D.; et al. Dynamical features in fetal and postnatal zinc-copper metabolic cycles predict the emergence of autism spectrum disorder. Sci. Adv. 2018, 4, eaat1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, J.E.; Malijauskaite, S.; McGourty, K.; Grabrucker, A.M. The Metallome as a Link between the “Omes” in Autism Spectrum Disorders. Front. Mol. Neurosci. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.S.; Keen, C.L.; Gershwin, M.E.; Hendrickx, A.G. Developmental Zinc Deficiency and Behavior. J. Nutr. 1995, 125, 2263S–2271S. [Google Scholar] [CrossRef]
- Hagmeyer, S.; Haderspeck, J.C.; Grabrucker, A.M. Behavioral impairments in animal models for zinc deficiency. Front. Behav. Neurosci. 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- A Reid, C.; Hildebrand, M.; A Mullen, S.; Hildebrand, J.M.; Berkovic, S.F.; Petrou, S. Synaptic Zn2+ and febrile seizure susceptibility. J. Cereb. Blood Flow Metab. 2016, 174, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Tuchman, R.; Cuccaro, M. Epilepsy and Autism: Neurodevelopmental Perspective. Curr. Neurol. Neurosci. Rep. 2011, 11, 428–434. [Google Scholar] [CrossRef]
- Tye, C.; Runicles, A.K.; Whitehouse, A.; Alvares, G. Characterizing the Interplay between Autism Spectrum Disorder and Comorbid Medical Conditions: An Integrative Review. Front. Psychiatry 2019, 9, 751. [Google Scholar] [CrossRef] [Green Version]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of Zinc in the Central Nervous System: The Zinc-Containing Neuron. J. Nutr. 2000, 130 (Suppl. S5), 1471S–1483S. [Google Scholar] [CrossRef]
- Frederickson, C.J. Neurobiology of Zinc and Zinc-Containing Neurons. Int. Rev. Neurobiol. 1989, 31, 145–238. [Google Scholar] [CrossRef]
- Pe’rez-Clausell, J.; Danscher, G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985, 337, 91–98. [Google Scholar] [CrossRef]
- Grabrucker, A.M. A role for synaptic zinc in ProSAP/Shank PSD scaffold malformation in autism spectrum disorders. Dev. Neurobiol. 2013, 74, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao-Cheng, J.-H.; Toy, D.; Winters, C.A.; Reese, T.S.; Dosemeci, A. Zinc Stabilizes Shank3 at the Postsynaptic Density of Hippocampal Synapses. PLoS ONE 2016, 11, e0153979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, S.R.; Semba, K. A heavy metal marker of the developing striatal mosaic. Dev. Brain. Res. 1989, 45, 155–159. [Google Scholar] [CrossRef]
- Grabrucker, S.; Haderspeck, J.C.; Sauer, A.K.; Kittelberger, N.; Asoglu, H.; Abaei, A.; Rasche, V.; Schön, M.; Boeckers, T.M.; Grabrucker, A.M. Brain Lateralization in Mice Is Associated with Zinc Signaling and Altered in Prenatal Zinc Deficient Mice That Display Features of Autism Spectrum Disorder. Front. Mol. Neurosci. 2018, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Sindreu, C.; Storm, D.R. Modulation of Neuronal Signal Transduction and Memory Formation by Synaptic Zinc. Front. Behav. Neurosci. 2011, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Baltaci, A.K.; Yuce, K. Zinc Transporter Proteins. Neurochem. Res. 2017, 43, 517–530. [Google Scholar] [CrossRef]
- Cole, T.B.; Wenzel, H.J.; Kafer, K.E.; Schwartzkroin, P.A.; Palmiter, R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef] [Green Version]
- Linkous, D.H.; Flinn, J.M.; Koh, J.Y.; Lanzirotti, A.; Bertsch, P.; Jones, B.F.; Giblin, L.J.; Frederickson, C.J. Evidence That the ZNT3 Protein Controls the Total Amount of Elemental Zinc in Synaptic Vesicles. J. Histochem. Cytochem. 2007, 56, 3–6. [Google Scholar] [CrossRef]
- McAllister, B.B.; Dyck, R.H. Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 2017, 80, 329–350. [Google Scholar] [CrossRef]
- Yoo, M.H.; Kim, T.-Y.; Yoon, Y.H.; Koh, J.-Y. Autism phenotypes in ZnT3 null mice: Involvement of zinc dyshomeostasis, MMP-9 activation and BDNF upregulation. Sci. Rep. 2016, 6, 28548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval, K.C.; Thackray, S.E.; Wong, A.; Niewinski, N.; Chipak, C.; Rehal, S.; Dyck, R.H. Lack of Vesicular Zinc Does Not Affect the Behavioral Phenotype of Polyinosinic:Polycytidylic Acid-Induced Maternal Immune Activation Mice. Front. Behav. Neurosci. 2022, 16, 769322. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Huang, L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. Eur. J. Physiol. 2003, 447, 744–751. [Google Scholar] [CrossRef]
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. ZnT5 Variant B Is a Bidirectional Zinc Transporter and Mediates Zinc Uptake in Human Intestinal Caco-2 Cells. J. Biol. Chem. 2007, 282, 14389–14393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palumaa, P.; Eriste, E.; Njunkova, O.; Pokras, L.; Jörnvall, H.; Sillard, R. Brain-Specific Metallothionein-3 Has Higher Metal-Binding Capacity than Ubiquitous Metallothioneins and Binds Metals Noncooperatively. Biochemistry 2002, 41, 6158–6163. [Google Scholar] [CrossRef]
- Hagmeyer, S.; Cristóvão, J.S.; Mulvihill, J.J.E.; Boeckers, T.M.; Gomes, C.M.; Grabrucker, A.M. Zinc Binding to S100B Affords Regulation of Trace Metal Homeostasis and Excitotoxicity in the Brain. Front. Mol. Neurosci. 2018, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- O’Roak, B.J.; Deriziotis, P.; Lee, C.; Vives, L.; Schwartz, J.J.; Girirajan, S.; Karakoc, E.; MacKenzie, A.P.; Ng, S.B.; Baker, C.; et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat. Genet. 2011, 43, 585–589. [Google Scholar] [CrossRef]
- Sanders, S.J.; Murtha, M.T.; Gupta, A.R.; Murdoch, J.D.; Raubeson, M.J.; Willsey, A.J.; Ercan-Sencicek, A.G.; DiLullo, N.M.; Parikshak, N.N.; Stein, J.L.; et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 2012, 485, 237–241. [Google Scholar] [CrossRef]
- Egger, G.; Roetzer, K.M.; Noor, A.; Lionel, A.C.; Mahmood, H.; Schwarzbraun, T.; Boright, O.; Mikhailov, A.; Marshall, C.R.; Windpassinger, C.; et al. Identification of risk genes for autism spectrum disorder through copy number variation analysis in Austrian families. Neurogenetics 2014, 15, 117–127. [Google Scholar] [CrossRef]
- Rogers, J.M.; Keen, C.L.; Hurley, L.S. Zinc deficiency in pregnant Long-Evans hooded rats: Teratogenicity and tissue trace elements. Teratology 1985, 31, 89–100. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The role of Fe, Zn, and Cu in pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Grabrucker, S.; Boeckers, T.M.; Grabrucker, A.M. Gender Dependent Evaluation of Autism like Behavior in Mice Exposed to Prenatal Zinc Deficiency. Front. Behav. Neurosci. 2016, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabrucker, S.; Jannetti, L.; Eckert, M.; Gaub, S.; Chhabra, R.; Pfaender, S.; Mangus, K.; Reddy, P.P.; Rankovic, V.; Schmeisser, M.J.; et al. Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain 2013, 137, 137–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawley, J.N. Mouse Behavioral Assays Relevant to the Symptoms of Autism. Brain Pathol. 2007, 17, 448–459. [Google Scholar] [CrossRef]
- Kolk, S.M.; Rakic, P. Development of prefrontal cortex. Neuropsychopharmacology 2021, 47, 41–57. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, T.; Fan, K.; Cai, W.; Liu, H. Astrocyte-Neuron Signaling in Synaptogenesis. Front. Cell Dev. Biol. 2021, 9, 680301. [Google Scholar] [CrossRef]
- Cline, H.T. Dendritic arbor development and synaptogenesis. Curr. Opin. Neurobiol. 2001, 11, 118–126. [Google Scholar] [CrossRef]
- Grzadzinski, R.; Huerta, M.; Lord, C. DSM-5 and autism spectrum disorders (ASDs): An opportunity for identifying ASD subtypes. Mol. Autism. 2013, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021, 51, 4253–4270. [Google Scholar] [CrossRef]
- Wöhr, M.; Roullet, F.I.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Communication Impairments in Mice Lacking Shank1: Reduced Levels of Ultrasonic Vocalizations and Scent Marking Behavior. PLoS ONE 2011, 6, e20631. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.L.; Turner, S.M.; Barkan, C.L.; Tolu, S.S.; Saxena, R.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011, 1380, 120–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Won, H.; Lee, H.-R.; Gee, H.Y.; Mah, W.; Kim, J.-I.; Lee, J.; Ha, S.; Chung, C.; Jung, E.S.; Cho, Y.S.; et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012, 486, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Bozdagi, O.; Sakurai, T.; Papapetrou, D.; Wang, X.; Dickstein, D.L.; Takahashi, N.; Kajiwara, Y.; Yang, M.; Katz, A.M.; Scattoni, M.L.; et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol. Autism 2010, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; McCoy, P.A.; Rodriguiz, R.M.; Pan, Y.; Je, H.S.; Roberts, A.C.; Kim, C.J.; Berrios, J.; Colvin, J.S.; Bousquet-Moore, D.; et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum. Mol. Genet. 2011, 20, 3093–3108. [Google Scholar] [CrossRef] [Green Version]
- Peça, J.; Feliciano, C.; Ting, J.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Peters, D. Effects of prenatal nutritional deficiency on affiliation and aggression in rats. Physiol. Behav. 1978, 20, 359–362. [Google Scholar] [CrossRef]
- Lokken, P.M.; Halas, E.S.; Sandstead, H.H. Influence of Zinc Deficiency on Behavior. Exp. Biol. Med. 1973, 144, 680–682. [Google Scholar] [CrossRef]
- Halas, E.S.; Sandstead, H.H. Some effects of prenatal zinc deficiency on behavior of the adult rat. Pediatr. Res. 1975, 9, 94–97. [Google Scholar] [CrossRef]
- Grayton, H.M.; Missler, M.; Collier, D.A.; Fernandes, C. Altered Social Behaviours in Neurexin 1α Knockout Mice Resemble Core Symptoms in Neurodevelopmental Disorders. PLoS ONE 2013, 8, e67114. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, E.C.; Caruso, A.; Servadio, M.; Andreae, L.; Trezza, V.; Scattoni, M.L.; Fernandes, C. Assessing the developmental trajectory of mouse models of neurodevelopmental disorders: Social and communication deficits in mice with Neurexin 1α deletion. Genes Brain Behav. 2019, 19, e12630. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Bozdagi, O.; Scattoni, M.L.; Wöhr, M.; Roullet, F.I.; Katz, A.M.; Abrams, D.N.; Kalikhman, D.; Simon, H.; Woldeyohannes, L.; et al. Reduced Excitatory Neurotransmission and Mild Autism-Relevant Phenotypes in Adolescent Shank3 Null Mutant Mice. J. Neurosci. 2012, 32, 6525–6541. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Chen, Q.; Van Der Goes, M.-S.; Hawrot, J.; Yao, A.Y.; Gao, X.; Lu, C.; Zang, Y.; Zhang, Q.; et al. Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism. J. Clin. Investig. 2017, 127, 1978–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, S.W.; Lerner, M.; McLeod, B.D.; Wood, J.J.; Ginsburg, G.S.; Kerns, C.; Ollendick, T.; Kendall, P.C.; Piacentini, J.; Walkup, J.; et al. Anxiety in Youth with and without Autism Spectrum Disorder: Examination of Factorial Equivalence. Behav. Ther. 2014, 46, 40–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollocks, M.J.; Lerh, J.W.; Magiati, I.; Meiser-Stedman, R.; Brugha, T.S. Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychol. Med. 2018, 49, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Bouwknecht, J.A.; Teague, R.; Paylor, R.; Zoghbi, H.Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet. 2004, 14, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Etherton, M.R.; Blaiss, C.A.; Powell, C.M.; Su, T.C. Mouse neurexin-1alfa deletion causes correlated electrophysiological and behavioral changes. Proc. Natl. Acad. Sci. USA 2009, 106, 17998–18003. [Google Scholar] [CrossRef] [Green Version]
- Peñagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 Leads to Epilepsy, Neuronal Migration Abnormalities, and Core Autism-Related Deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Halas, E.S.; Hunt, C.D.; Eberhardt, M.J. Learning and memory disabilities in young adult rats from mildly zinc deficient dams. Physiol. Behav. 1986, 37, 451–458. [Google Scholar] [CrossRef]
- Boroujeni, S.T.; Naghdi, N.; Shahbazi, M.; Farrokhi, A.; Bagherzadeh, F.; Kazemnejad, A.; Javadian, M. The Effect of Severe Zinc Deficiency and Zinc Supplement on Spatial Learning and Memory. Biol. Trace Element Res. 2009, 130, 48–61. [Google Scholar] [CrossRef]
- Yu, X.; Jin, L.; Zhang, X.; Yu, X. Effects of maternal mild zinc deficiency and zinc supplementation in offspring on spatial memory and hippocampal neuronal ultrastructural changes. Nutrition 2013, 29, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.S.; Gershwin, M.; Vijayan, V.K. Passive avoidance performance of mice fed marginally or severely zinc deficient diets during post-embryonic brain development. Physiol. Behav. 1983, 30, 409–413. [Google Scholar] [CrossRef]
- Jiang, Y.-G.; Wang, Y.-H.; Zhang, H.; Wang, Z.-Y.; Liu, Y.-Q. Effects of early-life zinc deficiency on learning and memory in offspring and the changes in DNA methylation patterns. Nutr. Neurosci. 2020, 25, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.Y.; Futai, K.; Sala, C.; Valtschanoff, J.G.; Ryu, J.; Woodworth, M.A.; Kidd, F.L.; Sung, C.C.; Miyakawa, T.; Bear, M.F.; et al. Smaller Dendritic Spines, Weaker Synaptic Transmission, but Enhanced Spatial Learning in Mice Lacking Shank1. J. Neurosci. 2008, 28, 1697–1708. [Google Scholar] [CrossRef] [Green Version]
- Kouser, M.; Speed, H.; Dewey, C.M.; Reimers, J.M.; Widman, A.J.; Gupta, N.; Liu, S.; Jaramillo, T.C.; Bangash, M.; Xiao, B.; et al. Loss of Predominant Shank3 Isoforms Results in Hippocampus-Dependent Impairments in Behavior and Synaptic Transmission. J. Neurosci. 2013, 33, 18448–18468. [Google Scholar] [CrossRef]
- Horvath, K.; Perman, J.A. Autistic disorder and gastrointestinal disease. Curr. Opin. Pediatr. 2002, 14, 583–587. [Google Scholar] [CrossRef]
- Nikolov, R.N.; Bearss, K.E.; Lettinga, J.; Erickson, C.; Rodowski, M.; Aman, M.G.; McCracken, J.T.; McDougle, C.J.; Tierney, E.; Vitiello, B.; et al. Gastrointestinal Symptoms in a Sample of Children with Pervasive Developmental Disorders. J. Autism Dev. Disord. 2008, 39, 405–413. [Google Scholar] [CrossRef]
- Chakraborty, P.; Carpenter, K.L.H.; Major, S.; Deaver, M.; Vermeer, S.; Herold, B.; Franz, L.; Howard, J.; Dawson, G. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism 2020, 25, 405–415. [Google Scholar] [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs, G.J., 3rd; Furuta, G.T.; Levy, J.; Vandewater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, Diagnosis, and Treatment of Gastrointestinal Disorders in Individuals With ASDs: A Consensus Report. Pediatrics 2010, 125 (Suppl. 1), S1–S18. [Google Scholar] [CrossRef] [Green Version]
- Molloy, C.a.; Manning-Courtney, P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism 2003, 7, 165–171. [Google Scholar] [CrossRef]
- De Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vela, G.; Stark, P.; Socha, M.; Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc in Gut-Brain Interaction in Autism and Neurological Disorders. Neural Plast. 2015, 2015, 972791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M.; et al. Gastrointestinal Microflora Studies in Late-Onset Autism. Clin. Infect. Dis. 2002, 35, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.R.T.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef]
- Sauer, A.K.; Dooley, L.; Vaughan, A.; Grabrucker, A.M. Altered gut–brain signaling in autism spectrum disorders—From biomarkers to possible intervention strategies. In Neural Engineering Techniques for Autism Spectrum Disorder; Elsevier: Amsterdam, The Netherlands, 2021; pp. 127–149. [Google Scholar]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism–Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Yi, X.; Zhang, X.; Wang, H.; Liu, H.; Mou, W.-W. Imbalance in the Gut Microbiota of Children with Autism Spectrum Disorders. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Sauer, A.K.; Malijauskaite, S.; Meleady, P.; Boeckers, T.M.; McGourty, K.; Grabrucker, A.M. Zinc is a key regulator of gastrointestinal development, microbiota composition and inflammation with relevance for autism spectrum disorders. Cell Mol. Life Sci. 2021, 79, 46. [Google Scholar] [CrossRef]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Díaz, J.; Gómez-Fernández, A.; Chueca, N.; De La Torre-Aguilar, M.J.; Gil, Á.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; et al. Autism Spectrum Disorder (ASD) with and without Mental Regression is Associated with Changes in the Fecal Microbiota. Nutrients 2019, 11, 337. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, G.G.; Zago, M.P.; Aimo, L.; Oteiza, P.I. Zinc deficiency in neuronal biology. IUBMB Life 2007, 59, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.M.; Zago, M.P.; Mackenzie, G.G.; Aimo, L.; Keen, C.L.; Keenan, A.; Oteiza, P.I. The Role of Zinc in the Modulation of Neuronal Proliferation and Apoptosis. Neurotox. Res. 2009, 17, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabrucker, A.M.; Knight, M.J.; Proepper, C.; Bockmann, J.; Joubert, M.; Rowan, M.; Nienhaus, G.U.; Garner, C.C.; Bowie, J.U.; Kreutz, M.R.; et al. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. EMBO J. 2011, 30, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.; Hwang, J.-J.; Han, S.-H.; Chung, S.J.; Koh, J.-Y.; Lee, J.-Y. Endogenous Zinc Mediates Apoptotic Programmed Cell Death in the Developing Brain. Neurotox. Res. 2009, 17, 156–166. [Google Scholar] [CrossRef]
- Adamo, A.M.; Liu, X.; Mathieu, P.; Nuttall, J.R.; Supasai, S.; Oteiza, P.I. Early Developmental Marginal Zinc Deficiency Affects Neurogenesis Decreasing Neuronal Number and Altering Neuronal Specification in the Adult Rat Brain. Front. Cell. Neurosci. 2019, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Kumar, A.; Singh, K.; Avasthi, K.; Kim, J.-J. Neurobiology of zinc and its role in neurogenesis. Eur. J. Nutr. 2021, 60, 55–64. [Google Scholar] [CrossRef]
- Wang, F.; Di Bian, W.; Kong, L.W.; Zhao, F.J.; Guo, J.S.; Jing, N.H. Maternal zinc deficiency impairs brain nestin expression in prenatal and postnatal mice. Cell Res. 2001, 11, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.-L.; Zheng, W.; Xin, N.; Chi, Z.-H.; Wang, Z.-Y.; Chen, J.; Wang, Z.-Y. Zinc Deficiency Reduces Neurogenesis Accompanied by Neuronal Apoptosis Through Caspase-Dependent and -Independent Signaling Pathways. Neurotox. Res. 2009, 16, 416–425. [Google Scholar] [CrossRef]
- Nuttall, J.R.; Supasai, S.; Kha, J.; Vaeth, B.M.; Mackenzie, G.G.; Adamo, A.M.; Oteiza, P.I. Gestational marginal zinc deficiency impaired fetal neural progenitor cell proliferation by disrupting the ERK1/2 signaling pathway. J. Nutr. Biochem. 2015, 26, 1116–1123. [Google Scholar] [CrossRef] [Green Version]
- Schoen, M.; Asoglu, H.; Bauer, H.F.; Müller, H.-P.; Abaei, A.; Sauer, A.K.; Zhang, R.; Song, T.-J.; Bockmann, J.; Kassubek, J.; et al. Shank3 Transgenic and Prenatal Zinc-Deficient Autism Mouse Models Show Convergent and Individual Alterations of Brain Structures in MRI. Front. Neural Circuits 2019, 13, 6. [Google Scholar] [CrossRef]
- Baecker, T.; Mangus, K.; Pfaender, S.; Chhabra, R.; Boeckers, T.M.; Grabrucker, A.M. Loss of COMMD1 and copper overload disrupt zinc homeostasis and influence an autism-associated pathway at glutamatergic synapses. BioMetals 2014, 27, 715–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeckers, T.M.; Bockmann, J.; Kreutz, M.R.; Gundelfinger, E.D. ProSAP/Shank proteins—A family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem. 2002, 81, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.K.; Boeckers, T.M.; Vaida, B.; Faham, S.; Gingery, M.; Sawaya, M.R.; Salyer, D.; Gundelfinger, E.D.; Bowie, J.U. An Architectural Framework That May Lie at the Core of the Postsynaptic Density. Science 2006, 311, 531–535. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Vaida, B.; Bockmann, J.; Boeckers, T.M. Efficient targeting of proteins to post-synaptic densities of excitatory synapses using a novel pSDTarget vector system. J. Neurosci. Methods 2009, 181, 227–234. [Google Scholar] [CrossRef]
- Boeckers, T.M.; Liedtke, T.; Spilker, C.; Dresbach, T.; Bockmann, J.; Kreutz, M.R.; Gundelfinger, E.D. C-terminal synaptic targeting elements for postsynaptic density proteins ProSAP1/Shank2 and ProSAP2/Shank3. J. Neurochem. 2005, 92, 519–524. [Google Scholar] [CrossRef]
- Ha, H.T.T.; Leal-Ortiz, S.; Lalwani, K.; Kiyonaka, S.; Hamachi, I.; Mysore, S.; Montgomery, J.M.; Garner, C.; Huguenard, J.; Kim, S.A. Shank and Zinc Mediate an AMPA Receptor Subunit Switch in Developing Neurons. Front. Mol. Neurosci. 2018, 11, 405. [Google Scholar] [CrossRef] [Green Version]
- Arons, M.H.; Thynne, C.J.; Grabrucker, A.M.; Li, D.; Schoen, M.; Cheyne, J.E.; Boeckers, T.M.; Montgomery, J.M.; Garner, C.C. Autism-associated mutations in ProSAP2/Shank3 impair synaptic transmission and neurexin–neuroligin-mediated transsynaptic signaling. J. Neurosci. 2012, 32, 14966–14978. [Google Scholar] [CrossRef]
- Arons, M.H.; Lee, K.; Thynne, C.J.; Kim, S.A.; Schob, C.; Kindler, S.; Montgomery, J.M.; Garner, C. Shank3 Is Part of a Zinc-Sensitive Signaling System That Regulates Excitatory Synaptic Strength. J. Neurosci. 2016, 36, 9124–9134. [Google Scholar] [CrossRef] [Green Version]
- Fourie, C.; Vyas, Y.; Lee, K.; Jung, Y.; Garner, C.; Montgomery, J.M. Dietary Zinc Supplementation Prevents Autism Related Behaviors and Striatal Synaptic Dysfunction in Shank3 Exon 13–16 Mutant Mice. Front. Cell. Neurosci. 2018, 12, 374. [Google Scholar] [CrossRef] [Green Version]
- Vyas, Y.; Lee, K.; Jung, Y.; Montgomery, J.M. Influence of maternal zinc supplementation on the development of autism-associated behavioural and synaptic deficits in offspring Shank3-knockout mice. Mol. Brain 2020, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Canitano, R.; Pallagrosi, M. Autism Spectrum Disorders and Schizophrenia Spectrum Disorders: Excitation/Inhibition Imbalance and Developmental Trajectories. Front. Psychiatry 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Spooren, W.; Lindemann, L.; Ghosh, A.; Santarelli, L. Synapse dysfunction in autism: A molecular medicine approach to drug discovery in neurodevelopmental disorders. Trends Pharmacol. Sci. 2012, 33, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Fabio, R.A.; La Briola, F.; Giannatiempo, S.; Antonietti, A.; Maggiolini, S.; Canevini, M.P. Correlations between neurophysiological, behavioral, and cognitive function in Rett syndrome. Epilepsy Behav. 2010, 17, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chung, C.; Ha, S.; Lee, D.; Kim, D.-Y.; Kim, H.; Kim, E. Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Front. Cell. Neurosci. 2015, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- D’Antoni, S.; Spatuzza, M.; Bonaccorso, C.M.; Musumeci, S.A.; Ciranna, L.; Nicoletti, F.; Huber, K.M.; Catania, M.V. Dysregulation of group-I metabotropic glutamate (mGlu) receptor mediated signalling in disorders associated with Intellectual Disability and Autism. Neurosci. Biobehav. Rev. 2014, 46, 228–241. [Google Scholar] [CrossRef] [Green Version]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef]
- Brašić, J.R.; Nandi, A.; Russell, D.S.; Jennings, D.; Barret, O.; Martin, S.D.; Slifer, K.; Sedlak, T.; Seibyl, J.P.; Wong, D.F.; et al. Cerebral expression of metabotropic glutamate receptor subtype 5 in idiopathic autism spectrum disorder and fragile x syndrome: A pilot study. Int. J. Mol. Sci. 2021, 22, 2863. [Google Scholar] [CrossRef]
- Daini, E.; Hagmeyer, S.; De Benedictis, C.A.; Cristóvão, J.S.; Bodria, M.; Ross, A.M.; Raab, A.; Boeckers, T.M.; Feldmann, J.; Gomes, C.M.; et al. S100B dysregulation during brain development affects synaptic SHANK protein networks via alteration of zinc homeostasis. Transl. Psychiatry 2021, 11, 562. [Google Scholar] [CrossRef]
- Kleijer, K.T.E.; Schmeisser, M.J.; Krueger, D.D.; Boeckers, T.M.; Scheiffele, P.; Bourgeron, T.; Brose, N.; Burbach, J.P.H. Neurobiology of autism gene products: Towards pathogenesis and drug targets. Psychopharmacology 2014, 231, 1037–1062. [Google Scholar] [CrossRef]
- Bourgeron, T. A synaptic trek to autism. Curr. Opin. Neurobiol. 2009, 19, 231–234. [Google Scholar] [CrossRef]
- Van Spronsen, M.; Hoogenraad, C.C. Synapse Pathology in Psychiatric and Neurologic Disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Bonnycastle, K.; Davenport, E.C.; Cousin, M.A. Presynaptic dysfunction in neurodevelopmental disorders: Insights from the synaptic vesicle life cycle. J. Neurochem. 2020, 157, 179–207. [Google Scholar] [CrossRef]
- Pfaender, S.; Sauer, A.K.; Hagmeyer, S.; Mangus, K.; Linta, L.; Liebau, S.; Bockmann, J.; Huguet, G.; Bourgeron, T.; Boeckers, T.M.; et al. Zinc deficiency and low enterocyte zinc transporter expression in human patients with autism related mutations in SHANK3. Sci. Rep. 2017, 7, 45190. [Google Scholar] [CrossRef] [Green Version]
- Sauer, A.K.; Bockmann, J.; Steinestel, K.; Boeckers, T.M.; Grabrucker, A.M. Altered Intestinal Morphology and Microbiota Composition in the Autism Spectrum Disorders Associated SHANK3 Mouse Model. Int. J. Mol. Sci. 2019, 20, 2134. [Google Scholar] [CrossRef] [Green Version]
- Kirsten, T.B.; Queiroz-Hazarbassanov, N.; Bernardi, M.M.; Felicio, L. Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sci. 2015, 130, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- Al-Ayadhi, L.Y.; Mostafa, G.A. Elevated serum levels of interleukin-17A in children with autism. J. Neuroinflamm. 2012, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Tomova, A.; Keményová, P.; Filčíková, D.; Szapuová, Ž.; Kováč, A.; Babinská, K.; Ostatníková, D. Plasma levels of glial cell marker S100B in children with autism. Physiol. Res. 2019, 68, S315–S323. [Google Scholar] [CrossRef]
- Xiang, A.; Wang, X.; Martinez, M.; Walthall, J.C.; Curry, E.S.; Page, K.; Buchanan, T.A.; Coleman, K.J.; Getahun, D. Association of Maternal Diabetes With Autism in Offspring. JAMA 2015, 313, 1425–1434. [Google Scholar] [CrossRef] [Green Version]
- Xiang, A.H.; Wang, X.; Martinez, M.; Page, K.; Buchanan, T.A.; Feldman, R.K. Maternal Type 1 Diabetes and Risk of Autism in Offspring. JAMA 2018, 320, 89–91. [Google Scholar] [CrossRef]
- Cezar, L.C.; Kirsten, T.B.; da Fonseca, C.C.N.; de Lima, A.P.N.; Bernardi, M.M.; Felicio, L.F. Zinc as a therapy in a rat model of autism prenatally induced by valproic acid. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 173–180. [Google Scholar] [CrossRef]
- Liu, F.; Horton-Sparks, K.; Hull, V.; Li, R.W.; Martínez-Cerdeño, V. The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Mol. Autism 2018, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- De Theije, C.G.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen, J.; Kraneveld, A.D.; Oozeer, R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav. Immun. 2014, 37, 197–206. [Google Scholar] [CrossRef]
- Caulfield, L.E.; Zavaleta, N.; Shankar, A.H.; Merialdi, M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am. J. Clin. Nutr. 1998, 68, 499S–508S. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.L.; Grieger, J.A.; Bianco-Miotto, T.; Roberts, C.T. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients 2016, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Donangelo, C.M.; King, J.C. Maternal Zinc Intakes and Homeostatic Adjustments during Pregnancy and Lactation. Nutrients 2012, 4, 782–798. [Google Scholar] [CrossRef]
- Gallaher, D.D.; E Johnson, P.; Hunt, J.R.; I Lykken, G.; Marchello, M.J. Bioavailability in humans of zinc from beef: Intrinsic vs. extrinsic labels. Am. J. Clin. Nutr. 1988, 48, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [Green Version]
- Lönnerdal, B. Dietary Factors Influencing Zinc Absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Foster, M.; Herulah, U.N.; Prasad, A.; Petocz, P.; Samman, S. Zinc Status of Vegetarians during Pregnancy: A Systematic Review of Observational Studies and Meta-Analysis of Zinc Intake. Nutrients 2015, 7, 4512–4525. [Google Scholar] [CrossRef] [Green Version]
- King, J.C. Determinants of maternal zinc status during pregnancy. Am. J. Clin. Nutr. 2000, 71, 1334S–1343S. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.J.; Zheng, J.J. High dietary calcium intakes reduce zinc absorption and balance in humans. Am. J. Clin. Nutr. 1997, 65, 1803–1809. [Google Scholar] [CrossRef]
- Argiratos, V.; Samman, S. The effect of calcium carbonate and calcium citrate on the absorption of zinc in healthy female subjects. Eur. J. Clin. Nutr. 1994, 48, 198–204. [Google Scholar]
- O’Brien, K.O.; Zavaleta, N.; Caulfield, L.E.; Wen, J.; Abrams, S. Prenatal Iron Supplements Impair Zinc Absorption in Pregnant Peruvian Women. J. Nutr. 2000, 130, 2251–2255. [Google Scholar] [CrossRef] [Green Version]
- Ghishan, F.K.; Said, H.M.; Wilson, P.C.; E Murrell, J.; Greene, H.L. Intestinal transport of zinc and folic acid: A mutual inhibitory effect. Am. J. Clin. Nutr. 1986, 43, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Simmer, K.; Iles, C.A.; James, C.; Thompson, R.P. Are iron-folate supplements harmful? Am. J. Clin. Nutr. 1987, 45, 122–125. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef]
- Sauer, A.K.; Pfaender, S.; Hagmeyer, S.; Tarana, L.; Mattes, A.-K.; Briel, F.; Küry, S.; Boeckers, T.M.; Grabrucker, A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hiPSC. BioMetals 2017, 30, 643–661. [Google Scholar] [CrossRef] [Green Version]
- Sauer, A.K.; Grabrucker, A.M. Zinc Deficiency during Pregnancy Leads to Altered Microbiome and Elevated Inflammatory Markers in Mice. Front. Neurosci. 2019, 13, 1295. [Google Scholar] [CrossRef]
- Pfaender, S.; Grabrucker, A.M. Characterization of biometal profiles in neurological disorders. Metallomics 2014, 6, 960–977. [Google Scholar] [CrossRef]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of assessment of zinc status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2040S–2051S. [Google Scholar] [CrossRef] [Green Version]
- Wieringa, F.T.; Dijkhuizen, M.A.; Fiorentino, M.; Laillou, A.; Berger, J. Determination of Zinc Status in Humans: Which Indicator Should We Use? Nutrients 2015, 7, 3252–3263. [Google Scholar] [CrossRef] [Green Version]
- Pluhator, M.M.; Thomson, A.B.; Fedorak, R.N. Clinical Aspects of Trace Elements: Zinc in Human Nutrition—Assessment of Zinc Status. Can. J. Gastroenterol. 1996, 10, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Hagmeyer, S.; Sauer, A.K.; Grabrucker, A.M. Prospects of Zinc Supplementation in Autism Spectrum Disorders and Shankopathies Such as Phelan McDermid Syndrome. Front. Synaptic Neurosci. 2018, 10, 11. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Coury, D.L.; et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef]
- Gundelfinger, E.D.; Boeckers, T.M.; Baron, M.K.; Bowie, J.U. A role for zinc in postsynaptic density asSAMbly and plasticity? Trends Biochem. Sci. 2006, 31, 366–373. [Google Scholar] [CrossRef]
- Vergnano, A.M.; Rebola, N.; Savtchenko, L.P.; Pinheiro, P.; Casado, M.; Kieffer, B.L.; Rusakov, D.; Mulle, C.; Paoletti, P. Zinc Dynamics and Action at Excitatory Synapses. Neuron 2014, 82, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Vyas, Y.; Jung, Y.; Lee, K.; Garner, C.C.; Montgomery, J.M. In vitro zinc supplementation alters synaptic deficits caused by autism spectrum disorder-associated Shank2 point mutations in hippocampal neurons. Mol. Brain 2021, 14, 95. [Google Scholar] [CrossRef]
- Lee, E.-J.; Lee, H.; Huang, T.-N.; Chung, C.; Shin, W.; Kim, K.; Koh, J.-Y.; Hsueh, Y.-P.; Kim, E. Trans-synaptic zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nat. Commun. 2015, 6, 7168. [Google Scholar] [CrossRef] [Green Version]
- Sampson, E.L.; Jenagaratnam, L.; McShane, R. Metal protein attenuating compounds for the treatment of Alzheimer’s dementia. In Cochrane Database of Systematic Reviews; Sampson, E.L., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; p. CD005380. [Google Scholar]


| Protein | Effect | Induction of ZnD by | Method of Detection | Model System | Reference | |
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| SHANK2 | reduced | TPEN, CaEDTA, High S100B | ICC, WB | x | [54,105,131] | |
| reduced (PD3) # | PZD, Prenatal S100B | IHC, WB | x | [54,131] | ||
| SHANK3 | reduced | TPEN, CaEDTA, High S100B | ICC, WB | x | [54,105,131] | |
| reduced (PD3) # | PZD | IHC, WB | x | [54] † | ||
| SHANK1 | reduced | CaEDTA | ICC | x | [54] † | |
| reduced (PD3) | PZD | IHC, WB | x | [54] † | ||
| HOMER1b/c | reduced | TPEN, CaEDTA | ICC, WB | x | [105] [54] † [121] ** | |
| reduced (PD3) # | PZD | WB | x | [54] † | ||
| VGLUT1 | reduced | TPEN | ICC | x | [121] ** | |
| mGluR5 | blocked increase after HiK+ stimulation | TPEN, CaEDTA | ICC, WB | x | [54] | |
| reduced (PD3) | PZD | WB | x | [54] | ||
| GluA1 | reduced (PD3) | PZD | WB | x | [54] | |
| GluN1 | reduced only in Shank1 deficient synapses | TPEN | ICC | x | [105] | |
| reduced (PD3) | PZD | WB | x | [54] | ||
| GluN2B | reduced (PD3) # | PZD | WB | x | [54] | |
| PSD 95 | reduced only in Shank1 deficient synapses | TPEN | ICC | x | [105] | |
| no change | TPEN, CaEDTA | ICC, WB | [54] | |||
| no change | PZD | WB | x | [54] | ||
| GluN2A | reduced (PD3) | PZD | WB | x | [54] | |
| GluA3 * | reduced (PD3) # | PZD | WB | x | [54] | |
| GluA2 * | reduced (PD3) # | PZD | WB | x | [54] | |
| GKAP * | reduced (PD3) # | PZD | WB | x | [54] | |
| ARRB2 * | loss of lateralized expression | PZD | qRT-PCR, WB * | x | [36] | |
| FEZ1 * | loss of lateralized expression | PZD | qRT-PCR, WB * | x | [105] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Prenatal Zinc Deficient Mice as a Model for Autism Spectrum Disorders. Int. J. Mol. Sci. 2022, 23, 6082. https://doi.org/10.3390/ijms23116082
Sauer AK, Hagmeyer S, Grabrucker AM. Prenatal Zinc Deficient Mice as a Model for Autism Spectrum Disorders. International Journal of Molecular Sciences. 2022; 23(11):6082. https://doi.org/10.3390/ijms23116082
Chicago/Turabian StyleSauer, Ann Katrin, Simone Hagmeyer, and Andreas M. Grabrucker. 2022. "Prenatal Zinc Deficient Mice as a Model for Autism Spectrum Disorders" International Journal of Molecular Sciences 23, no. 11: 6082. https://doi.org/10.3390/ijms23116082
APA StyleSauer, A. K., Hagmeyer, S., & Grabrucker, A. M. (2022). Prenatal Zinc Deficient Mice as a Model for Autism Spectrum Disorders. International Journal of Molecular Sciences, 23(11), 6082. https://doi.org/10.3390/ijms23116082







