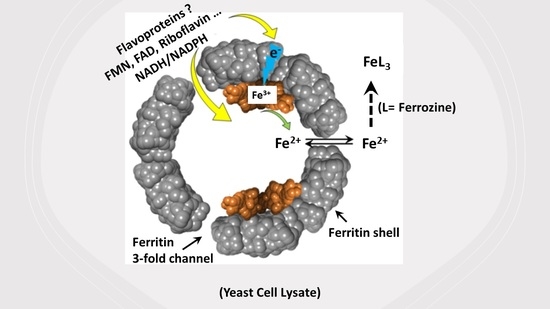
Iron Mobilization from Ferritin in Yeast Cell Lysate and Physiological Implications
Abstract
:1. Introduction
2. Results
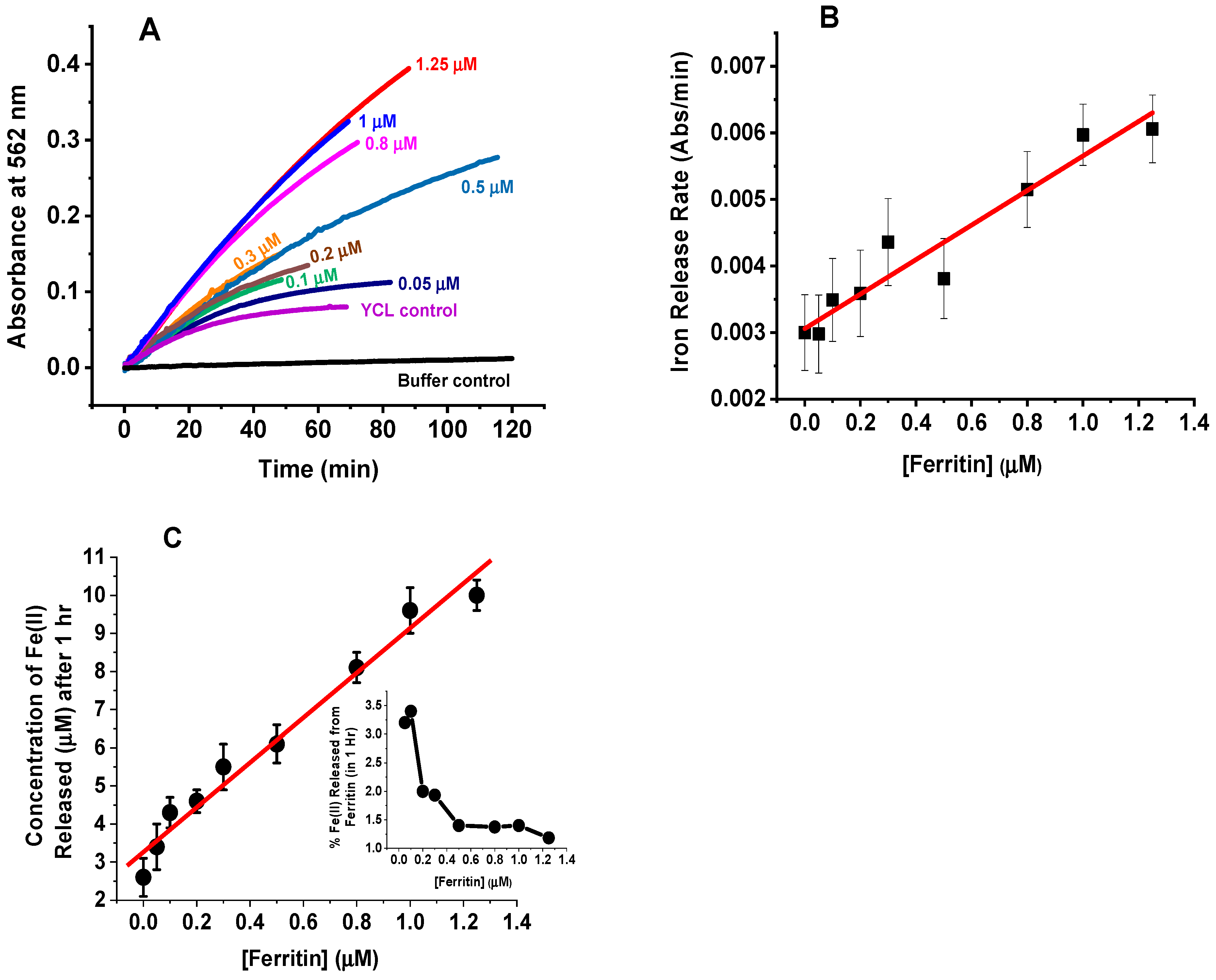
2.1. Iron Mobilization from Ferritin in Cell Lysate
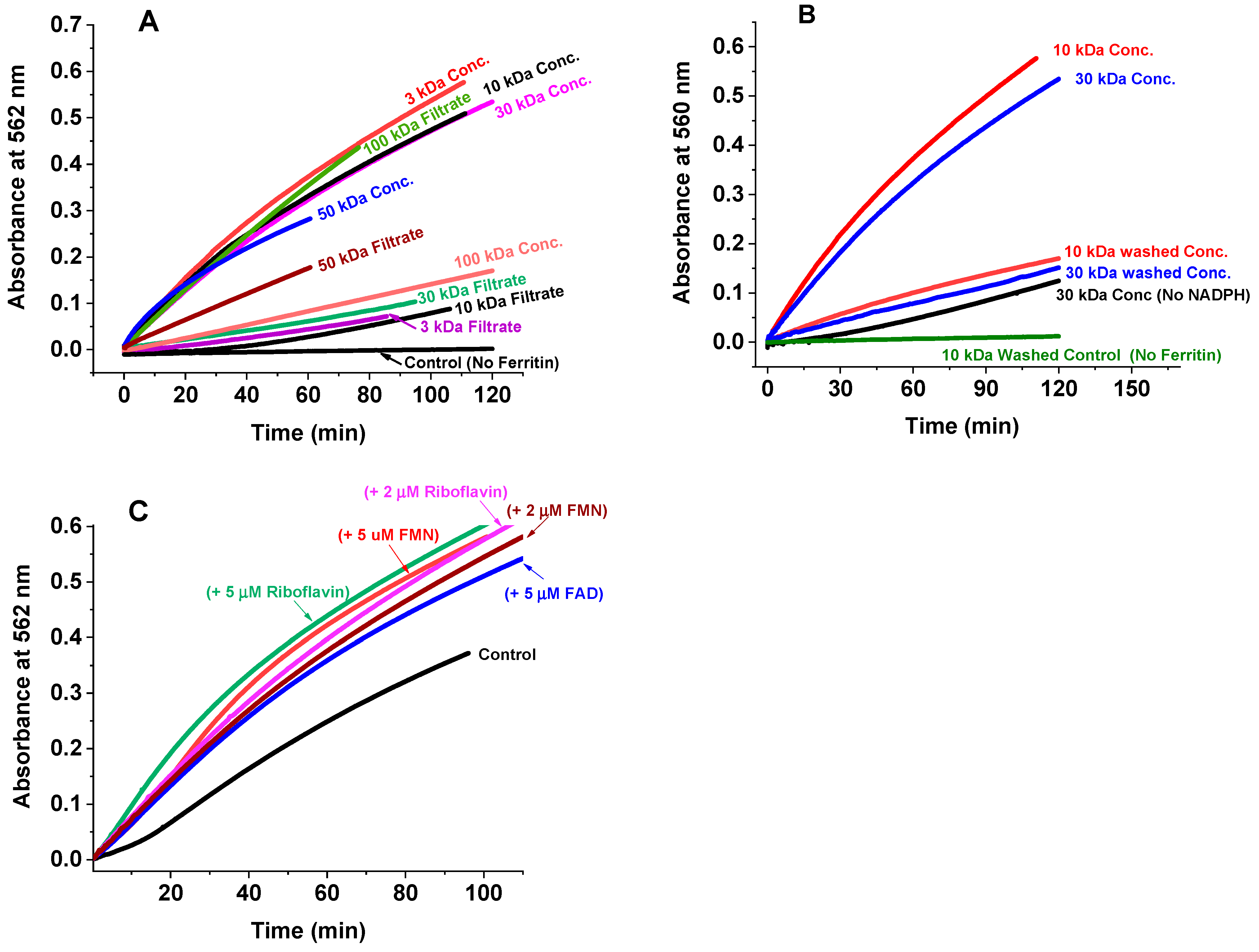
2.2. Iron Release Kinetics and the Effect of Cellular Metabolites
2.3. Effect of Temperature on the Iron Release Kinetics and Differential Scanning Calorimetry (DSC) of Cell Lysate
2.4. Oxygen Concentration and O2 Consumption Kinetics
3. Discussion
4. Materials and Methods
4.1. Ferritin Expression and Purification
4.2. Iron Loading into Ferritin
4.3. Yeast Cell Growth
4.4. Cell Lysate Preparation
4.5. Iron Mobilization Kinetics in Cell Lysate
4.6. Oxygen Microelectrode and O2 Kinetics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, P.M.; Arosio, P. Ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Theil, E.C.; Behera, R.; Tosha, T. Ferritins for chemistry and for life. Coord. Chem. Rev. 2013, 257, 579–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bou-Abdallah, F. The iron redox and hydrolysis chemistry of the ferritins. Biochim. Biophys. Acta 2010, 1800, 719–731. [Google Scholar] [CrossRef]
- Mehlenbacher, M.; Poli, M.; Arosio, P.; Santambrogio, P.; Levi, S.; Chasteen, N.D.; Bou-Abdallah, F. Iron oxidation and core formation in recombinant heteropolymeric human ferritins. Biochemistry 2017, 56, 3900–3912. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; Zhao, G.; Mayne, H.R.; Arosio, P.; Chasteen, N.D. Origin of the unusual kinetics of iron deposition in human H-chain ferritin. J. Am. Chem. Soc. 2005, 127, 3885–3893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Bou-Abdallah, F.; Yang, X.; Arosio, P.; Chasteen, N.D. Is hydrogen peroxide produced during iron(II) oxidation in mammalian apoferritins? Biochemistry 2001, 40, 10832–10838. [Google Scholar] [CrossRef]
- Zhao, G.; Bou-Abdallah, F.; Arosio, P.; Levi, S.; Janus-Chandler, C.; Chasteen, N.D. Multiple pathways for mineral core formation in mammalian apoferritin. The role of hydrogen peroxide. Biochemistry 2003, 42, 3142–3150. [Google Scholar] [CrossRef]
- Bou-Abdallah, F.; Flint, N.; Wilkinson, T.; Salim, S.; Srivastava, A.K.; Poli, M.; Arosio, P.; Melman, A. Ferritin exhibits Michaelis-Menten behavior with oxygen but not with iron during iron oxidation and core mineralization. Metallomics 2019, 11, 774–783. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry. Curr. Opin. Chem. Biol. 2011, 15, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Theil, E.C.; Tosha, T.; Behera, R.K. Solving biology’s iron chemistry problem with ferritin protein nanocages. Acc. Chem. Res. 2016, 49, 784–791. [Google Scholar] [CrossRef]
- Ebrahimi, K.H.; Hagedoorn, P.-L.; Hagen, W.R. Unity in the Biochemistry of the iron-storage proteins ferritin and bacterioferritin. Chem. Rev. 2015, 115, 295–326. [Google Scholar] [CrossRef] [PubMed]
- Hagen, W.R.; Hagedoorn, P.-L.; Ebrahimi, K.H. The workings of ferritin: A crossroad of opinions. Metallomics 2017, 9, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melman, A.; Bou-Abdallah, F. Iron mineralization and core dissociation in mammalian homopolymeric H-ferritin: Current understanding and future perspectives. Biochim. Biophys. Acta 2020, 1864, 129700. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; McNally, J.; Liu, X.X.; Melman, A. Oxygen catalyzed mobilization of iron from ferritin by iron(III) chelate ligands. Chem. Comm. 2011, 47, 731–733. [Google Scholar] [CrossRef]
- Johnson, L.E.; Wilkinson, T.; Arosio, P.; Melman, A.; Bou-Abdallah, F. Effect of chaotropes on the kinetics of iron release from ferritin by flavin nucleotides. Biochim. Biophys. Acta 2017, 1861, 3257–3262. [Google Scholar] [CrossRef]
- Melman, G.; Bou-Abdallah, F.; Vane, E.; Maura, P.; Arosio, P.; Melman, A. Iron release from ferritin by flavin nucleotides. Biochim. Biophys. Acta 2013, 1830, 4669–4674. [Google Scholar] [CrossRef]
- Theil, E.C.; Takagi, H.; Small, G.W.; He, L.; Tipton, A.R.; Danger, D. The ferritin iron entry and exit problem. Inorg. Chim. Acta 2000, 297, 242–251. [Google Scholar] [CrossRef]
- Watt, R.K.; Hilton, R.J.; Graff, D.M. Oxido- reduction is not the only mechanism allowing ions to traverse the ferritin protein shell. Biochim. Biophys. Acta 2010, 1800, 745–759. [Google Scholar] [CrossRef]
- Linder, M.C. Mobilization of stored iron in mammals: A review. Nutrients 2013, 5, 4022–4050. [Google Scholar] [CrossRef] [Green Version]
- Kidane, T.Z.; Sauble, E.; Linder, M.C. Release of iron from ferritin requires lysosomal activity. Am. J. Physiol. Cell Physiol. 2006, 291, C445–C455. [Google Scholar] [CrossRef] [Green Version]
- Philpott, C.C.; Jadhav, S. The ins and outs of iron: Escorting iron through the mammalian cytosol. Free. Radic. Biol. Med. 2019, 133, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Ryu, M.S.; Frey, A.; Patel, S. Cytosolic iron chaperones: Proteins delivering iron cofactors in the cytosol of mammalian cells. J. Biol. Chem. 2017, 292, 12764–12771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.J.; Protchenko, O.; Shakoury-Elizeh, M.; Baratz, E.; Jadhav, S.; Philpott, C.C. The iron chaperone and nucleic acid–binding activities of poly(rC)-binding protein 1 are separable and independently essential. Proc. Natl. Acad. Sci. USA 2021, 118, e2104666118. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Bencze, K.Z.; Stemmler, T.L.; Philpott, C.C. A cytosolic iron chaperone that delivers iron to ferritin. Science 2008, 320, 1207–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leidgens, S.; Bullough, K.Z.; Shi, H.; Li, F.; Shakoury-Elizeh, M.; Yabe, T.; Subramanian, P.; Hsu, E.; Natarajan, N.; Nandal, A.; et al. Each member of the poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin. J. Biol. Chem. 2013, 288, 17791–17802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-Codina, N.; Mancias, J.D. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals 2018, 11, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryzik, M.; Srivastava, A.K.; Longhi, G.; Bertuzzi, M.; Gianoncelli, A.; Carmona, F.; Poli, M.; Arosio, P. Expression and characterization of the ferritin binding domain of Nuclear Receptor Coactivator-4 (NCOA4). Biochim. Biophys. Acta 2017, 1861, 2710–2716. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Flint, N.; Kreckel, H.; Gryzik, M.; Poli, M.; Arosio, P.; Bou-Abdallah, F. Thermodynamic and kinetic studies of the nuclear receptor coactivator-4 (NCOA4) interaction with human ferritin. Biochemistry 2020, 29, 2707–2717. [Google Scholar] [CrossRef]
- Mancias, J.D.; Pontano Vaites, L.; Nissim, S.; Biancur, D.E.; Kim, A.J.; Wang, X.; Liu, Y.; Goessling, W.; Kimmelman, A.C.; Harper, J.W. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife 2015, 4, e10308. [Google Scholar] [CrossRef]
- La, A.; Nguyen, T.; Tran, K.; Sauble, E.; Tu, D.; Gonzalez, A.; Kidane, T.Z.; Soriano, C.; Morgan, J.; Doan, M. Mobilization of iron from ferritin: New steps and details. Metallomics 2018, 10, 154–168. [Google Scholar] [CrossRef]
- Badu-Boateng, C.; Naftalin, R.J. Ascorbate and ferritin interactions: Consequences for iron release in vitro and in vivo and implications for inflammation. Free Rad. Biol. Med. 2019, 133, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; Paliakkara, J.J.; Melman, G.; Melman, A. Reductive mobilization of iron from intact ferritin: Mechanisms and physiological implication. Pharmaceuticals 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehlhase, J.; Sandig, G.; Pantopoulos, K.; Grune, T. Oxidation-induced ferritin turn-over in microglial cells: Role of proteasome. Free Radic. Biol. Med. 2005, 38, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Voogd, A.; Sluiter, W.; van Eijk, H.G.; Koster, J.F. Low molecular weight iron and the oxygen paradox in isolated rat hearts. J. Clin. Investig. 1992, 90, 2050–2055. [Google Scholar] [CrossRef]
- Reif, D.W. Ferritin as a source of iron for oxidative damage. Free Radic. Biol. Med. 1992, 12, 417–427. [Google Scholar] [CrossRef]
- Double, K.L.; Maywald, M.; Schmittel, M.; Riederer, P.; Gerlach, M. In vitro studies of ferritin iron release and neurotoxicity. J. Neurochem. 1998, 70, 2492–2499. [Google Scholar] [CrossRef]
- Puntarulo, S.; Cederbaum, A.I. Role of cytochrome P-450 in the stimulation of microsomal production of reactive oxygen species by ferritin. Biochim. Biophys. Acta 1996, 1289, 238–246. [Google Scholar] [CrossRef]
- Apanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharm. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Resgen, N.; Eckl, P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef]
- Rudeck, M.; Volk, T.; Sitte, N.; Grune, T. Ferritin oxidation in vitro: Implication of iron release and degradation by the 20S proteasome. IUBMB Life 2000, 49, 451–456. [Google Scholar] [CrossRef]
- Smith, G.L.; Reutovich, A.A.; Srivastava, A.K.; Reichard, R.E.; Welsh, C.H.; Melman, A.; Bou-Abdallah, F. Complexation of ferrous ions by ferrozine, 2,2′-bipyridine and 1,10-phenanthroline: Implication for the quantification of iron in biological systems. J. Inorg. Biochem. 2021, 220, 111460. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours: Implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, F.; Palacios, O.; Galvez, N.; Cuesta, R.; Atrian, S.; Capdevila, M.; Dominguez-Vera, J.M. Ferritin iron uptake and release in the presence of metals and metalloproteins: Chemical implications in the brain. Coord. Chem. Rev. 2013, 257, 2752–2764. [Google Scholar] [CrossRef]
- Sirivech, S.; Frieden, E.; Osaki, S. The release of iron from horse spleen ferritin by reduced flavins. Biochem. J. 1974, 143, 311–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, Q.H.; Hastings, J.W. The oxidation of reduced flavin mononucleotide by molecular oxygen. Biochem. J. 1962, 83, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Spencer, R.; Walsh, C. Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogs. Biochemistry 1978, 17, 4011–4017. [Google Scholar] [CrossRef]
- Satoh, J.; Kimata, S.; Nakamoto, S.; Ishii, T.; Tanaka, E.; Yumoto, S.; Takeda, K.; Yoshimura, E.; Kanesaki, Y.; Ishige, T.; et al. Free flavins accelerate release of ferrous iron from iron storage proteins by both free flavin-dependent and -independent ferric reductases in Escherichia coli. J. Gen. Appl. Microbiol. 2019, 65, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Romero, E.; Gómez Castellanos, J.R.; Gadda, G.; Fraaije, M.W.; Mattevi, A. Same Substrate, Many reactions: Oxygen activation in flavoenzymes. Chem. Rev. 2018, 118, 1742–1769. [Google Scholar] [CrossRef] [Green Version]
- Koochana, P.K.; Mohanty, A.; Parida, A.; Behera, N.; Behera, P.M.; Dixit, A.; Rabindra, K.B. Flavin-mediated reductive iron mobilization from frog M and mycobacterial ferritins: Impact of their size, charge and reactivities with NADH/O2. J. Biol. Inorg. Chem. 2021, 26, 265–281. [Google Scholar] [CrossRef]
- Kimata, S.; Mochizuki, D.; Satoh, J.; Kitano, K.; Kanesaki, Y.; Takeda, K.; Abe, A.; Kawasaki, S.; Niimura, Y. Intracellular free flavin and its associated enzymes participate in oxygen and iron metabolism in Amphibacillus xylanus lacking a respiratory chain. FEBS Open Bio 2018, 8, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Coves, J.; Fontecave, M. Reduction and mobilization of iron by a NAD(P)H:flavin oxidoreductase from Escherichia coli. Eur. J. Biochem. 1993, 211, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Topham, R.; Goger, M.; Pearce, K.; Schultz, P. The mobilization of ferritin iron by liver cytosol: A comparison of xanthine and NADH as reducing substrates. Biochem. J. 1989, 261, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, G.D.; Jacobs, D.; Frankel, R.B. Redox reactivity of bacterial and mammalian ferritin: Is reductant entry into the ferritin interior a necessary step for iron release? Proc. Natl. Acad. Sci. USA 1988, 85, 7457–7461. [Google Scholar] [CrossRef] [Green Version]
- Koochanaa, P.K.; Mohantya, A.; Dasa, S.; Subhadarshaneea, B.; Satpatic, S.; Dixitc, A.; Sabatc, S.C.; Beheraa, R.K. Releasing iron from ferritin protein nanocage by reductive method: The role of electron transfer mediator. Biochim. Biophys. Acta 2018, 1862, 1190–1198. [Google Scholar] [CrossRef]
- Carmona, U.; Li, L.; Zhang, L.; Knez, M. Ferritin light-chain subunits: Key elements for the electron transfer across the protein cage. Chem. Commun. 2014, 50, 15358–15361. [Google Scholar] [CrossRef]
- Bradley, J.M.; Svistunenko, D.A.; Lawson, T.L.; Hemmings, A.M.; Moore, G.R.; LeBrun, N.E. Three aromatic residues are required for electron transfer during iron mineralization in bacterioferritin. Angew. Chem. 2015, 54, 14763–14767. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.K.; Arosio, P.; Poli, M.; Bou-Abdallah, F. A Novel approach for the synthesis of human heteropolymer ferritins of different H to L subunit ratios. J. Mol. Biol. 2021, 433, 167198. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, G.L.; Srivastava, A.K.; Reutovich, A.A.; Hunter, N.J.; Arosio, P.; Melman, A.; Bou-Abdallah, F. Iron Mobilization from Ferritin in Yeast Cell Lysate and Physiological Implications. Int. J. Mol. Sci. 2022, 23, 6100. https://doi.org/10.3390/ijms23116100
Smith GL, Srivastava AK, Reutovich AA, Hunter NJ, Arosio P, Melman A, Bou-Abdallah F. Iron Mobilization from Ferritin in Yeast Cell Lysate and Physiological Implications. International Journal of Molecular Sciences. 2022; 23(11):6100. https://doi.org/10.3390/ijms23116100
Chicago/Turabian StyleSmith, Gideon L., Ayush K. Srivastava, Aliaksandra A. Reutovich, Nathan J. Hunter, Paolo Arosio, Artem Melman, and Fadi Bou-Abdallah. 2022. "Iron Mobilization from Ferritin in Yeast Cell Lysate and Physiological Implications" International Journal of Molecular Sciences 23, no. 11: 6100. https://doi.org/10.3390/ijms23116100
APA StyleSmith, G. L., Srivastava, A. K., Reutovich, A. A., Hunter, N. J., Arosio, P., Melman, A., & Bou-Abdallah, F. (2022). Iron Mobilization from Ferritin in Yeast Cell Lysate and Physiological Implications. International Journal of Molecular Sciences, 23(11), 6100. https://doi.org/10.3390/ijms23116100