Differential Inflammatory Responses in Cultured Endothelial Cells Exposed to Two Conjugated Linoleic Acids (CLAs) under a Pro-Inflammatory Condition
Abstract
:1. Introduction
2. Results
2.1. Viability of EA.hy926 Cells Incubated with TNF-α and FAs
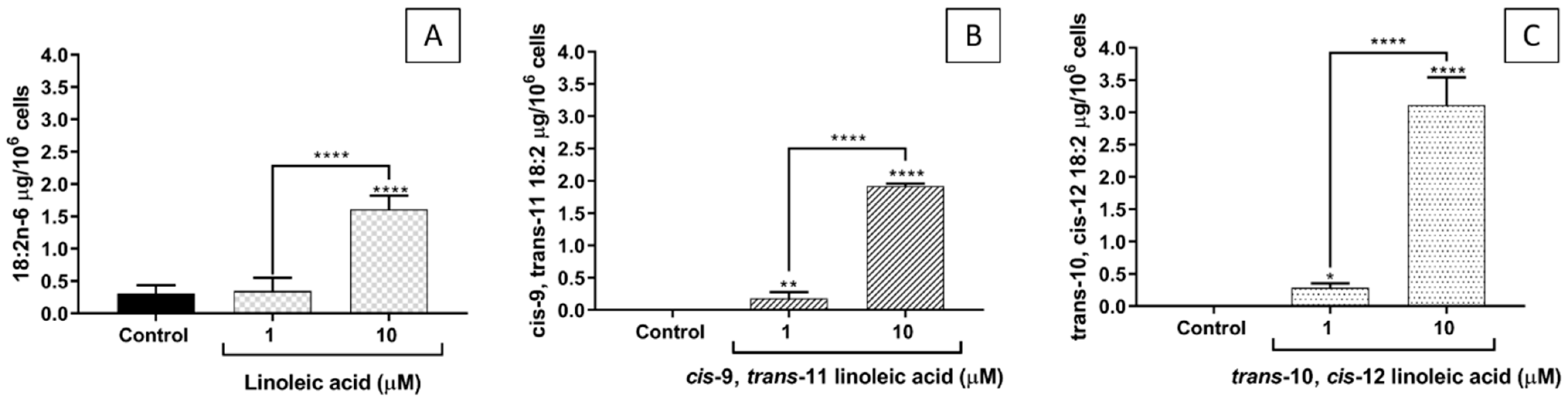
2.2. FA Incorporation into EA.hy926 Cells
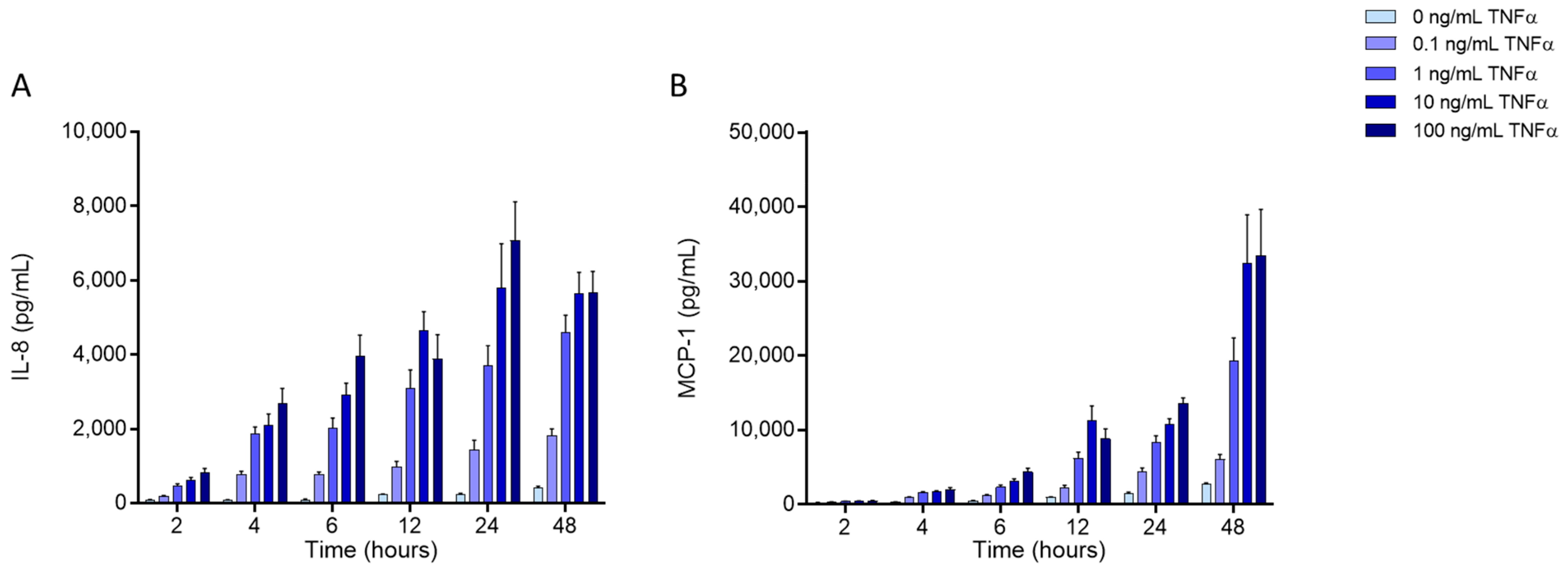
2.3. Effects of FAs on the Levels of Inflammatory Mediators Produced by ECs
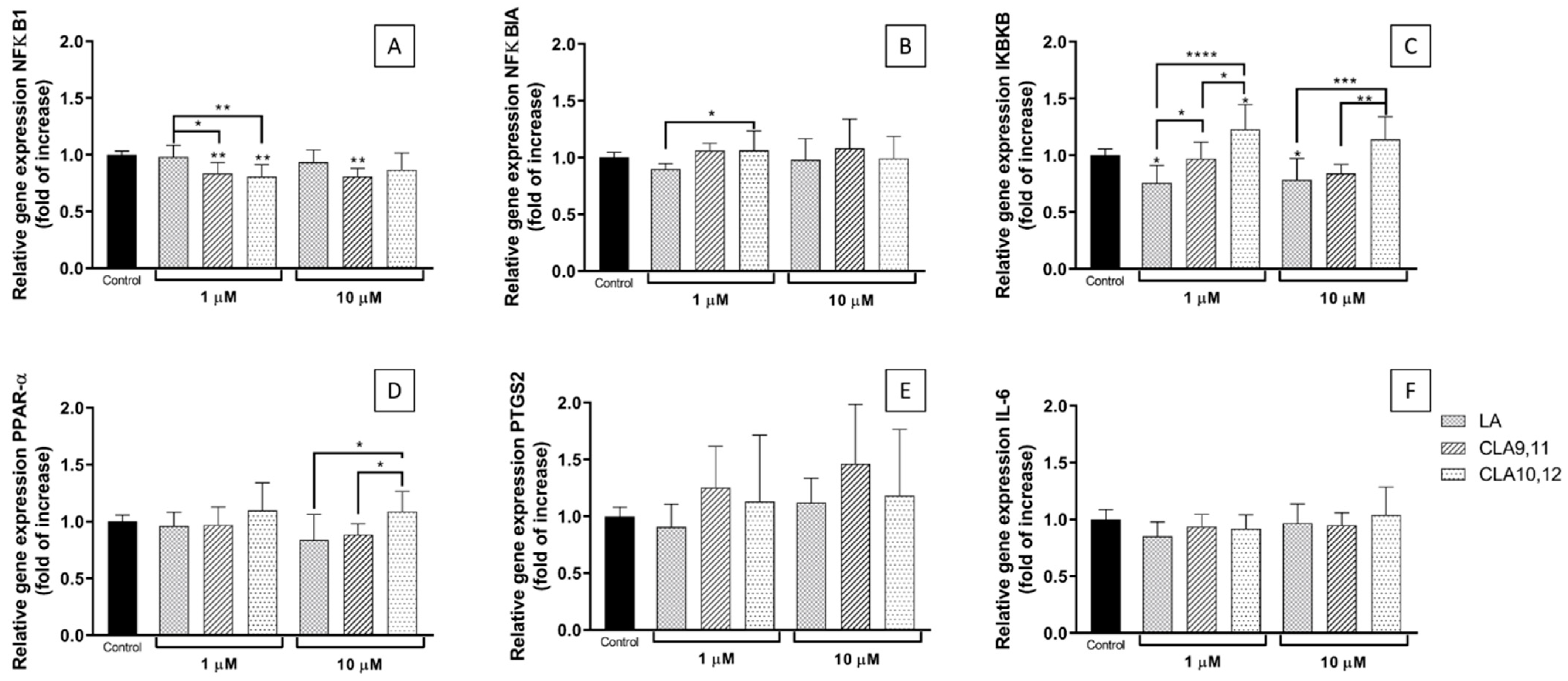
2.4. Effects of FAs on the Expression of Inflammation-Related Genes
2.5. Effects of FAs on THP-1 Adhesion to EA.hy926 Cells
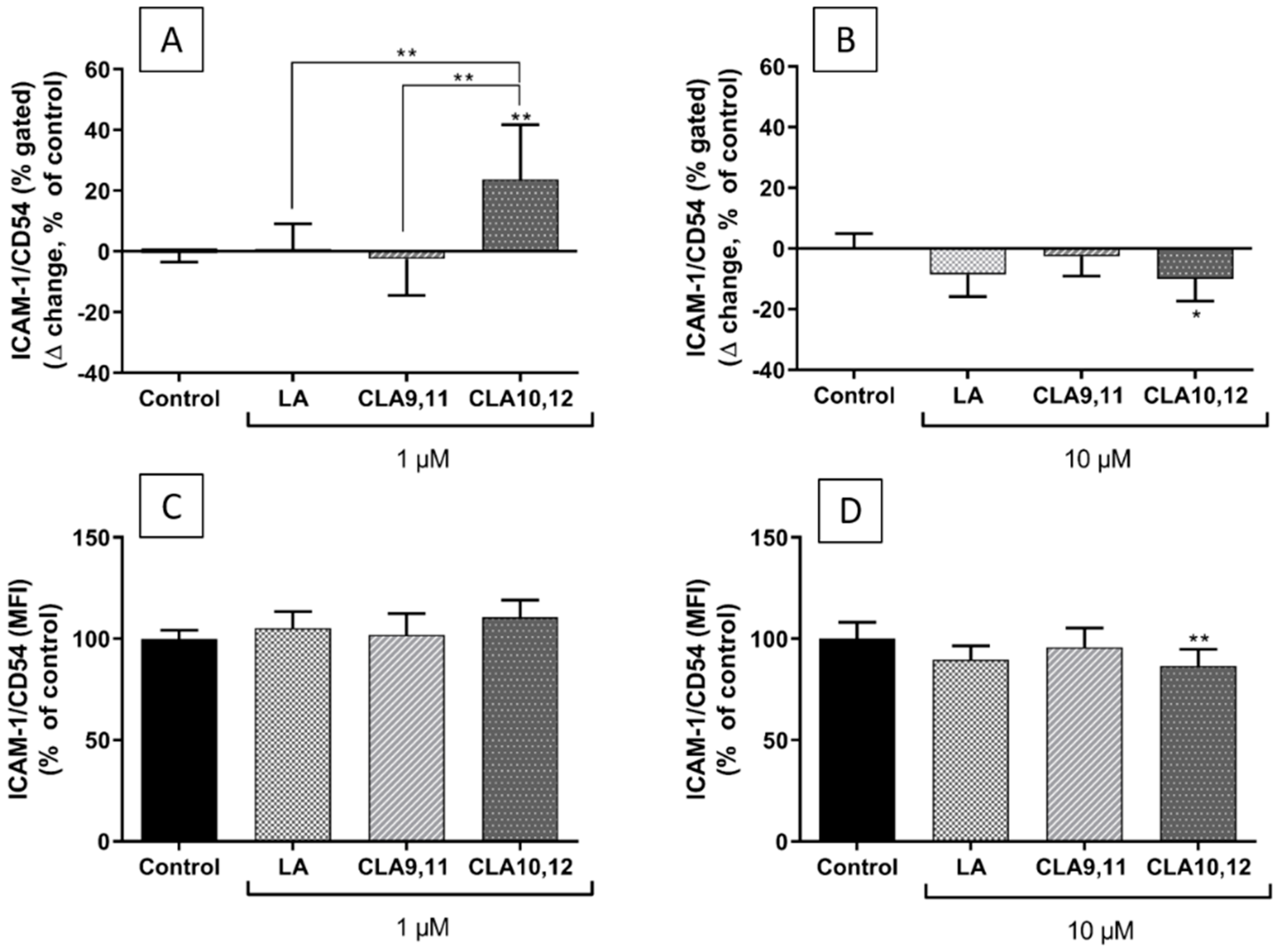
2.6. Effects of FAs on the Expression of ICAM-1 on the Surface of EA.hy926 Cells
3. Discussion
4. Materials and Methods
4.1. Endothelial Cell Model
4.2. Fatty Acid Treatment
4.3. MTT Assay for Cell Viability
4.4. Gas Chromatography
4.5. Multiplex Magnetic ELISA
4.6. RNA Isolation, cDNA Synthesis, and Real-Time PCR
4.7. THP-1 Monocyte Adhesion Assay
4.8. Flow Cytometry
4.9. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganguly, R.; Pierce, G.N. The toxicity of dietary trans fats. Food Chem. Toxicol. 2015, 78, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Degen, C.; Jahreis, G. Evaluation of the impact of ruminant trans fatty acids on human health: Important aspects to consider. Crit. Rev. Food Sci. Nutr. 2015, 56, 1964–1980. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; McKain, N.; Shingfield, K.J.; Devillard, E. Isomers of conjugated linoleic acids are synthesized via different mechanisms in ruminal digesta and bacteria. J. Lipid Res. 2007, 48, 2247–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosley, E.E.; McGuire, M.K.; Williams, J.E.; McGuire, M.A. Cis-9, trans-11 conjugated linoleic acid is synthesized from vaccenic acid in lactating women. J. Nutr. 2006, 136, 2297–2301. [Google Scholar] [CrossRef] [Green Version]
- Turpeinen, A.M.; Mutanen, M.; Aro, A.; Salminen, I.; Basu, S.; Palmquist, D.L.; Griinari, J.M. Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am. J. Clin. Nutr. 2002, 76, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Ha, Y.; Grimm, N.; Pariza, M. Anticarcinogens from fried ground beef: Heat-altered derivatives of linoleic acid. Carcinogenesis 1987, 8, 1881–1887. [Google Scholar] [CrossRef]
- Lp, C.; Scimeca, J.A.; Thompson, H.J. Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources. Cancer 1994, 74, 1050–1054. [Google Scholar] [CrossRef]
- Lee, K.N.; Kritchevsky, D.; Parizaa, M.W. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 1994, 108, 19–25. [Google Scholar] [CrossRef]
- Yu, Y.; Correll, P.; Heuvel, J.V. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: Evidence for a PPARγ-dependent mechanism. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2002, 1581, 89–99. [Google Scholar] [CrossRef]
- Park, Y.; Storkson, J.M.; Albright, K.J.; Liu, W.; Pariza, M.W. Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 1999, 34, 235–241. [Google Scholar] [CrossRef]
- Tricon, S.; Burdge, G.C.; Williams, C.M.; Calder, P.C.; Yaqoob, P. The effects of conjugated linoleic acid on human health-related outcomes. Proc. Nutr. Soc. 2005, 64, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- WHO. Global Health Estimates 2019: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ross, R. Atherosclerosis is an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Viola, J.; Soehnlein, O. Atherosclerosis—A matter of unresolved inflammation. Semin. Immunol. 2015, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Sneddon, A.A.; McLeod, E.; Wahle, K.W.; Arthur, J.R. Cytokine-induced monocyte adhesion to endothelial cells involves platelet-activating factor: Suppression by conjugated linoleic acid. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2006, 1761, 793–801. [Google Scholar] [CrossRef]
- Goua, M.; Mulgrew, S.; Frank, J.; Rees, D.; Sneddon, A.A.; Wahle, K.W. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: Involvement of the transcription factor NF-kappaB? Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Siennicka, A.; Baśkiewcz-Hałasa, M.; Bober, J.; Machalinski, B.; Chlubek, D. Conjugated linoleic acid isomers may diminish human macrophages adhesion to endothelial surface. Int. J. Food Sci. Nutr. 2011, 63, 30–35. [Google Scholar] [CrossRef]
- Shen, W.; Chuang, C.-C.; Martinez, K.; Reid, T.; Brown, J.M.; Xi, L.; Hixson, L.; Hopkins, R.; Starnes, J.; McIntosh, M. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice. J. Lipid Res. 2013, 54, 909–922. [Google Scholar] [CrossRef] [Green Version]
- Poirier, H.; Shapiro, J.S.; Kim, R.J.; Lazar, M.A. Nutritional supplementation with trans-10, cis-12-conjugated linoleic acid induces inflammation of white adipose tissue. Diabetes 2006, 55, 1634–1641. [Google Scholar] [CrossRef] [Green Version]
- Tholstrup, T.; Raff, M.; Straarup, E.M.; Lund, P.; Basu, S.; Bruun, J.M. An oil mixture with trans-10, cis-12 conjugated linoleic acid increases markers of inflammation and in vivo lipid peroxidation compared with cis-9, trans-11 conjugated linoleic acid in postmenopausal women. J. Nutr. 2008, 138, 1445–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risérus, U.; Basu, S.; Jovinge, S.; Fredrikson, G.N.; Ärnlöv, J.; Vessby, B. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: A potential link to fatty acid-induced insulin resistance. Circulation 2002, 106, 1925–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steck, S.E.; Chalecki, A.M.; Miller, P.; Conway, J.; Austin, G.; Hardin, J.; Albright, C.D.; Thuillier, P. Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. J. Nutr. 2007, 137, 1188–1193. [Google Scholar] [CrossRef] [Green Version]
- Smedman, A.; Basu, S.; Jovinge, S.; Fredrikson, G.N.; Vessby, B. Conjugated linoleic acid increased C-reactive protein in human subjects. Br. J. Nutr. 2005, 94, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Ramakers, J.D.; Plat, J.; Sébédio, J.-L.; Mensink, R.P. Effects of the individual isomers cis-9,trans-11 vs. trans-10,cis-12 of conjugated linoleic acid (CLA) on inflammation parameters in moderately overweight subjects with LDL-phenotype B. Lipids 2005, 40, 909–918. [Google Scholar] [CrossRef]
- Tricon, S.; Burdge, G.C.; Jones, E.L.; Russell, J.J.; El-Khazen, S.; Moretti, E.; Hall, W.; Gerry, A.B.; Leake, D.; Grimble, R.F.; et al. Effects of dairy products naturally enriched with cis-9, trans-11 conjugated linoleic acid on the blood lipid profile in healthy middle-aged men. Am. J. Clin. Nutr. 2006, 83, 744–753. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, C.A.; Baker, E.J.; De Souza, C.O.; Miles, E.A.; Calder, P.C. Differential effects of ruminant and industrial 18-carbon trans-monounsaturated fatty acids (trans vaccenic and elaidic) on the inflammatory responses of an endothelial cell line. Molecules 2021, 26, 5834. [Google Scholar] [CrossRef]
- Gebauer, S.K.; Chardigny, J.-M.; Jakobsen, M.U.; Lamarche, B.; Lock, A.L.; Proctor, S.D.; Baer, D.J. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: A comprehensive review of epidemiological, clinical, and mechanistic studies. Adv. Nutr. 2011, 2, 332–354. [Google Scholar] [CrossRef] [Green Version]
- Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 2016, 785, 87–95. [Google Scholar] [CrossRef]
- Tricon, S.; Burdge, G.; Kew, S.; Banerjee, T.; Russell, J.J.; Jones, E.L.; Grimble, R.F.; Williams, C.M.; Yaqoob, P.; Calder, P. Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am. J. Clin. Nutr. 2004, 80, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Tricon, S.; Burdge, G.; Kew, S.; Banerjee, T.; Russell, J.J.; Grimble, R.F.; Williams, C.M.; Calder, P.; Yaqoob, P. Effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on immune cell function in healthy humans. Am. J. Clin. Nutr. 2004, 80, 1626–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raff, M.; Tholstrup, T.; Basu, S.; Nonboe, P.; Sørensen, M.T.; Straarup, E.M. A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory, or diabetic risk markers in healthy young men. J. Nutr. 2008, 138, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbonés-Mainar, J.M.; Navarro, M.A.; Guzmán, M.A.; Arnal, C.; Surra, J.C.; Acín, S.; Carnicer, R.; Osada, J.; Roche, H.M. Selective effect of conjugated linoleic acid isomers on atherosclerotic lesion development in apolipoprotein E knockout mice. Atherosclerosis 2006, 189, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Bruen, R.; Curley, S.; Kajani, S.; Lynch, G.; O’Reilly, M.E.; Dillon, E.T.; Fitzsimons, S.; Mthunzi, L.; McGillicuddy, F.C.; Belton, O. Different monocyte phenotypes result in proresolving macrophages in conjugated linoleic acid-induced attenuated progression and regression of atherosclerosis. FASEB J. 2019, 33, 11006–11020. [Google Scholar] [CrossRef] [Green Version]
- Pontis, S.; Ribeiro, A.; Sasso, O.; Piomelli, D. Macrophage-derived lipid agonists of PPAR-α as intrinsic controllers of inflammation. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 PUFA and inflammation: From membrane to nucleus and from bench to bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef]
- Evans, N.P.; Misyak, S.A.; Schmelz, E.M.; Guri, A.J.; Hontecillas, R.; Bassaganya-Riera, J. Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARgamma. J. Nutr. 2010, 140, 515–521. [Google Scholar] [CrossRef]
- Reynolds, C.M.; Draper, E.; Keogh, B.; Rahman, A.; Moloney, A.P.; Mills, K.H.; Loscher, C.E.; Roche, H.M. A conjugated linoleic acid-enriched beef diet attenuates lipopolysaccharide-induced inflammation in mice in part through PPARgamma-mediated suppression of toll like receptor 4. J. Nutr. 2009, 139, 2351–2357. [Google Scholar] [CrossRef] [Green Version]
- Weatherill, A.R.; Lee, J.Y.; Zhao, L.; Lemay, D.; Youn, H.S.; Hwang, D.H. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J. Immunol. 2005, 174, 5390–5397. [Google Scholar] [CrossRef]
- Wong, S.W.; Kwon, M.-J.; Choi, A.M.; Kim, H.-P.; Nakahira, K.; Hwang, D.H. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 2009, 284, 27384–27392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babcock, T.A.; Novak, T.; Ong, E.; Jho, D.H.; Helton, W.S.; Espat, N.J. Modulation of lipopolysaccharide-stimulated macrophage tumor necrosis factor-a production by w−3 fatty acid is associatedwith differential cyclooxygenase-2 protein expression and is independent of interleukin-10. J. Surg. Res. 2002, 107, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.E.; Babcock, T.A.; Jho, D.H.; Helton, W.S.; Espat, N.J. NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am. J. Physiol. 2003, 284, L84–L89. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 through Toll-like receptor. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, P.; Miles, E.; Calder, P.C. Comparative anti-inflammatory effects of plant- and marine-derived omega-3 fatty acids explored in an endothelial cell line. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2020, 1865, 158662. [Google Scholar] [CrossRef]
- Calder, P.C. Docosahexaenoic acid. Ann. Nutr. Metab. 2016, 69, 8–21. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Gould, I.G.; Lal, S.; Aizezi, B. Incorporation profiles of conjugated linoleic acid isomers in cell membranes and their positional distribution in phospholipids. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2011, 1811, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Grądzka, I.; Sochanowicz, B.; Brzóska, K.; Wójciuk, G.; Sommer, S.; Wojewódzka, M.; Gasińska, A.; Degen, C.; Jahreis, G.; Szumiel, I. Cis-9,trans-11-conjugated linoleic acid affects lipid raft composition and sensitizes human colorectal adenocarcinoma HT-29 cells to X-radiation. Biochim. Biophys. Acta BBA-Gen. Subj. 2013, 1830, 2233–2242. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, B.; Deng, Z.; Fan, Y.; Li, J.; Li, H. Lipid rafts promote trans fatty acid-induced inflammation in human umbilical vein endothelial cells. Lipids 2016, 52, 27–35. [Google Scholar] [CrossRef]
- Li, J.; Rao, H.; Bin, Q.; Fan, Y.; Li, H.; Deng, Z. Linolelaidic acid induces apoptosis, cell cycle arrest and inflammation stronger than elaidic acid in human umbilical vein endothelial cells through lipid rafts. Eur. J. Lipid Sci. Technol. 2016, 119, 1600374. [Google Scholar] [CrossRef]
- Baker, E.J.; Yusof, H.M.; Yaqoob, P.; Miles, E.; Calder, P.C. Omega-3 fatty acids and leukocyte-endothelium adhesion: Novel anti-atherosclerotic actions. Mol. Asp. Med. 2018, 64, 169–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdge, G.C.; Lupoli, B.; Russell, J.J.; Tricon, S.; Kew, S.; Banerjee, T.; Shingfield, K.J.; Beever, D.E.; Grimble, R.F.; Williams, C.M.; et al. Incorporation of cis-9,trans-11 or trans-10,cis-12 conjugated linoleic acid into plasma and cellular lipids in healthy men. J. Lipid Res. 2004, 45, 736–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisk, H.L.; West, A.L.; Childs, C.E.; Burdge, G.C.; Calder, P.C. The Use of Gas Chromatography to Analyze Compositional Changes of Fatty Acids in Rat Liver Tissue during Pregnancy. J. Vis. Exp. 2014, 85, e51445. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Calder, P.C. Differential Inflammatory Responses in Cultured Endothelial Cells Exposed to Two Conjugated Linoleic Acids (CLAs) under a Pro-Inflammatory Condition. Int. J. Mol. Sci. 2022, 23, 6101. https://doi.org/10.3390/ijms23116101
Valenzuela CA, Baker EJ, Miles EA, Calder PC. Differential Inflammatory Responses in Cultured Endothelial Cells Exposed to Two Conjugated Linoleic Acids (CLAs) under a Pro-Inflammatory Condition. International Journal of Molecular Sciences. 2022; 23(11):6101. https://doi.org/10.3390/ijms23116101
Chicago/Turabian StyleValenzuela, Carina A., Ella J. Baker, Elizabeth A. Miles, and Philip C. Calder. 2022. "Differential Inflammatory Responses in Cultured Endothelial Cells Exposed to Two Conjugated Linoleic Acids (CLAs) under a Pro-Inflammatory Condition" International Journal of Molecular Sciences 23, no. 11: 6101. https://doi.org/10.3390/ijms23116101





