Nanosecond Pulsed Electric Field (nsPEF): Opening the Biotechnological Pandora’s Box
Abstract
:1. A Brief History on the Development of Electric Pulses Technology
2. nsPEF and Ca2+-Mediated Apoptosis: An Evolutionary Prespective
3. nsPEF Action Mechanism: A Deep Controversy
4. Dissecting the Biophysical Principles behind nsPEF’s Effects
5. It’s All about Pores? In the Shade of Voltage-Gated Ion Channels
5.1. Voltage-Gated Channel (VGC) Activation Mechanism
- Helical screw-sliding model: This simple model proposes that the S4 helix is responsible for maintaining the pore in a closed state during the resting potential. This is achieved by displacement of the positively charged S4 helix attracted by negative charges close to the cytoplasm. During depolarization, this attraction would vanish, and the system would return to a 60 rotation of S4 around its geometric axis. This rotation is accompanied by a vertical displacement of 5 Å to the extracellular side. According to this model, the positive residues of the S4 helix form salt bridges with acidic residues on opposite transmembrane segments. This model was based on the sodium channel transmembrane structure determined for an Electrophorus electricus channel [110,111]. Charge reversal mutagenesis [112] and disulfide linking [113] were used to probe charge interactions within the VSD of different VGCs. These works demonstrated the existence of a sequential ion pair formation involving S4 basic residues, typical on this type of channels. These interactions were key to conformational changes of the VSD upon VGC voltage activation. Subsequent works demonstrated similar key interactions required to characterize VGC activation in a more detailed fashion. Using site mutagenesis, two negatively-charged residues and a highly conserved one were identified as “catalyzers” of the transfer of each of the VSD basic residues across the membrane electric field [114]. This cluster of residues is known as the Charge Transfer Center.
- Kinetic model: In this model, at hyperpolarizing potentials, the basic amino acid residues of S4 are connected with an intracellular water crevice, maintaining the channel in a closed state. Upon depolarization, the S4 helix tilts and rotates 180 around its geometric axis, allowing it to be connected to an extracellular water crevice. This conformational change of the S4 helix pulls the intracellular side of the S5 transmembrane helix, leading to a rotation and pulling of the intracellular section of the S6 helix, which forms the pore, opening the channel. This model was proposed for mammalian ion channels based on the gating mechanism of the prokaryotic KcSa potassium channel [106,115,116].
- Paddle model: The paddle in this model circumscribes to the helix-turn-helix motif between the S3 and S4 helices. The paddle moves its center of mass nearly 20 Å and tilts towards a more vertical orientation. Since each paddle in the four VSDs of the VGC contains four arginine residues, with one electron charge unit per arginine [117,118], their displacement would account for the total gating charge in the Shaker K channel of 12–14 electrons (3.0–3.5 electrons per subunit) [117,118,119]. Ionic interaction with S2 and S3 helices would stabilize the movement of paddle charges. The S4–S5 linker is pulled to open the VGC pore as a result of the paddle movement.
- Transport model: Experimental observations on the Shaker K channel with fluorescent resonance energy transfer (FRET) concluded that the S4 helix does not move during channel activation. To explain this observation, the same author of the kinetic model proposed that the voltage gating is due to a transmembrane field rearrangement. In this rearrangement, VSD’s water crevice plays a key role. In this model, the S4 helix gyrates 45 around its geometric axis and has a vertical shift of less than 2 Å. The S4 turn relocates the S4 charges, reverberating from a deep, internally facing aqueous crevice in the closed state to an external water crevice when it opens. This model is supported by experimental evidence of a proton-conducting pore in a mutant Shaker channel in the closed state [120], the strong dependence of gating charge quantity on intracellular ionic strength, and the measurement of an amplified membrane electric field near the second gating-charge amino acid residue [121].
5.2. The Time-Scale Controversy behind Ion Channel Activation by nsPEF Protocols: The Role of MD Simulations
5.3. Voltage-Gated Calcium Channel
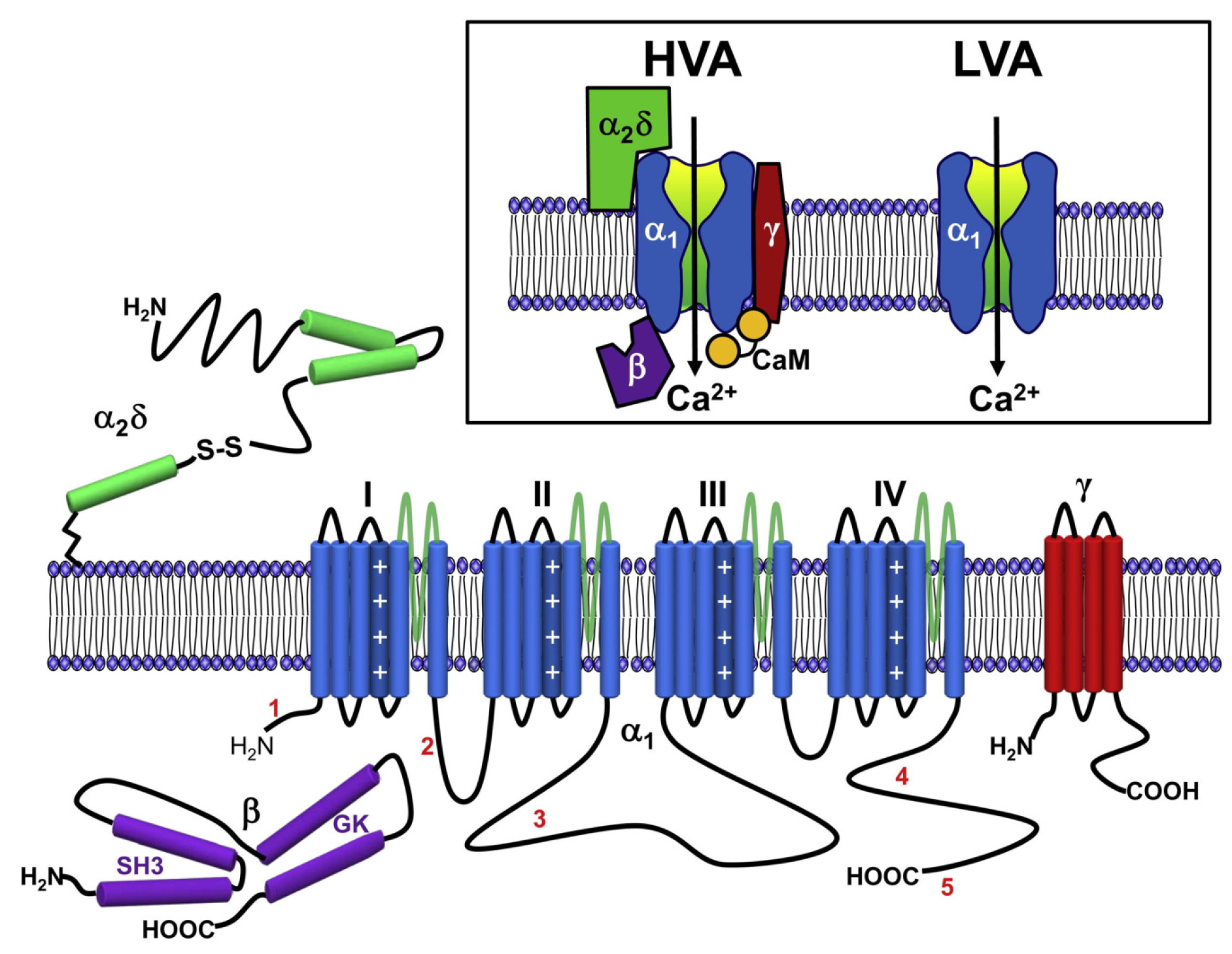
6. Protein-Mediated Electroporation: An Additional nsPEF Effect?
7. nsPEF Applications
7.1. In Human Health
- Activation of excitable cells:Cardiac cells: nsPEF (10–80 kV/cm, 4 ns, 1–20 pulses with 200/400/600 ms intervals) can indirectly lead to cardiac cell excitation. Of note, these results challenge the concept of chronaxie: minimum time required for an electric current to double the strength of the rheobase in order to stimulate a muscle or a neuron. The use of nsPEF technology to excite cardiac cells and mobilize intracellular Ca may prove valuable for cardiac pacing and defibrillation [210]. For other related studies see [211,212,213].Neurons: nsPEF (27.8 kV/cm, 10 ns, single pulse) was sufficient to initiate action potentials. The observed effect was repeatable and stable. These results highlight the potential use of ultrashort pulsed electric fields for stimulation of subcortical structures and suggest they may be used as a wireless alternative for deep brain stimulation [214]. For other related studies see [188,215,216,217].
- Phenotype manipulation:Differentiation: nsPEF (1.5–25 kV/cm, 300 ns, 5 pulses) can induce proliferation and myotubule maturation or nodule formation in myoblasts and osteoblasts, respectively. Myoblasts were isolated from hind-limb skeletal muscle of four-week-old mice Pten, and primary human osteoblasts were obtained from a vendor (Sciencell®) [218].Dedifferentiation: nsPEF (10–20 kV/cm, 100 ns pulse) induces dedifferentiation partially through transient activation of the wnt/–catenin signaling pathway in porcine chondrocytes [219].
- ntiparasitic: Cystic echinococcosis is a widely endemic helminthic disease caused by infection with metacestodes (larval stage) of the Echinococcus granulosus tapeworm. Application of nsPEF (21 KV/cm, 300 ns, 100 pulses) caused a significant increase in the death rate of protoscolices (future heads of the adult worms) [225]. For related studies see [226,227].
- Immune response: Using in vivo experiments, nsPEF (15 kV, 100 ns, 400 pulses) induced translocation of calreticulin in rat tumor cell-surfaces, a molecular pattern associated with damage that is indicative of immunogenic cell death (ICD). The nsPEF also triggered CD8-dependent inhibition of secondary tumor growth, concluded by comparing the tumor size using rats depleted of CD8 cytotoxic T-cells under the same nsPEF treatment. The first group showed an average size of only 3% of the primary tumor size compared with the 54% shown by the CD8-depleted rats. Additionally, with immunohistochemistry it was observed that CD8 T-cells were highly enriched in the first group. Furthermore, it was shown that vaccinating rats with isogenic tumor cells (MCA205 fibrosarcoma cell line) treated with nsPEF (50 kV, 100 ns, 500 pulses) stimulates an immune response that inhibits the growth of secondary tumors in a CD8-dependent manner [231]. This work opens the door to the fabrication of cell-based vaccines using nsPEF stimulation to promote an improved immune response. For other related studies reporting tumor ablation through an antitumor immune response using nsPEF see [232,233,234,235,236].
- Cancer: This is by far the most-studied nsPEF application, with 46 in vitro studies up to 2016 [27] and over 100 so far. Recently, preclinical animal studies have demonstrated that nsPEF can induce local and systemic CD8 T-cell mediated adaptive immune response against tumors [233,236]. In clinical trials, nsPEF proved to be a safe and effective therapy against basal cell carcinoma [237,238]. There are other novel techniques to combat cancer that also use electric fields, known as electrochemotherapy [239,240], irreversible electroporation [7], and electro-gene therapy [7]. Electrochemotherapy and electro-gene therapy use electroporation to achieve the anti-tumoral effect of other agents. In irreversible electroporation, cytoplasmic membranes of tumor cells cannot recover from permeabilization, causing cell death mainly by necrosis. Unlike the just mentioned electro-technique, nsPEF is cell-dependent. A possible explanation for this may be related to apoptosis (programmed cell death type 1 [241]), which is a tightly controlled cell process and different in each cell type [242]. Thus, if nsPEF induces apoptosis, as seems to be the case, it is expected to exhibit cell-dependent responses. This makes nsPEF an extraordinary tool, with specific responses based on tuning the intensity, duration, and number of pulses. There are several examples of cell dependence and nsPEF. Stacey et al. in 2002 demonstrated that exposing cancer cells to nsPEF with 60 kV/cm could induce DNA damage [243] (Figure 5). Beebe et al. in 2002 studied the antitumor effects of nsPEF on Jurkat cells, with pulses at 60, 150, and 300 kV/cm [139]. Xinh ua Chen et al. in 2012 applied nsPEF with 900 pulses at 68 kV/cm to ablate hepatocellular carcinoma [244]. Nuccitelli et al. in 2013 inhibited human pancreatic carcinoma using 100 pulses of 100 ns duration and 30 kV/cm [245]. More importantly for nsPEF as cancer treatment, tumor cells are more sensitive to nsPEF than normal cells [246]. See Figure 6 for an example of a nsPEF device suitable for use in cancer treatment.
7.2. Industrial
- Cell proliferation: nsPEF (10 kV/cm, 100 ns) can increase Arthrospira platensis SAG 21.99 (a cyanobacteria) cell growth after repeated pulses in the exponential growth phase. The effect was most pronounced five days after treatment. Treatments with nsPEF might improve sustainable and economical microalgae-based biorefineries [24]. For other studies see [218,248,249].
- Fermentation industry: nsPEF (15 kV/cm, 100 ns, 20 pulse) increased avermectin (anthelmintic and insecticidal agent) production in Streptomyces avermitilis by 42% and reduced the time needed for reaching a plateau in the fermentation process from 5 to 7 days [250]. For other related studies see [251].
- Food industry: Microalgae are a novel food ingredient of increasing interest as they can be grown on non-arable lands and fixates CO when grown photoautotrophically. Treatment with nsPEF (5–100 kV/cm, 2–100 ns) reduced total bacterial contamination >log in Chlorella vulgaris cultures without compromising the microalgae. For related studies see [252,253].
- Seed germination: nsPEF (10–30 kV/cm, 100 ns, 20 pulses) application significantly affected seed germination and pre-growth of Haloxylon ammodendron (Figure 7). This is probably due to the exogenous and endogenous NO generated in the nsPEF seed-treatment system [254]. For related studies see [255,256].
8. Challenges and Future Perspectives of nsPEF’s Effect on Cells
8.1. Nomenclature, Abbreviations, and Mathematical Formulas
8.2. Nanopores
Nanopores, Cholesterol, and Cancer
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| nsPEF | Nanosecond Pulsed Electric Field |
| NPS | Nano Pulsed Stimulation |
| IRA | impulse radiating antenna |
| CaBP | calcium-binding proteins |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| electric field | |
| force | |
| voltage difference | |
| d | membrane thickness |
| q | charge |
| MD | Molecular Dynamics |
| MCD | methyl--cyclodextrin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| time-constant | |
| R | resistance |
| C | capacitance |
| t | time |
| voltage at certain time t | |
| voltage difference across the cytoplasmatic membrane | |
| a | cell radius |
| polar angle | |
| membrane relaxation time-constant | |
| membrane capacitance | |
| external conductivity | |
| internal conductivity | |
| VGC | Voltage-Gated Channels |
| VSD | Voltage Sensing Domain |
| FRET | fluorescent resonance energy transfer |
| membrane voltage difference | |
| length of the Z-axis of the simulation Box | |
| TMV | transmembrane voltage |
| VGCC | Voltage-Gated Calcium Channels |
| HVA | high-voltage-activated HVA |
| LVA | low-voltage-activated |
| DHP | dihydropyridine |
| VGNC | Na voltage-gated channel |
References
- Meijerink, M.R.; Scheffer, H.J.; Narayanan, G. Irreversible Electroporation in Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Bowman, A.M.; Ibey, B.L.; Andre, F.M.; Pakhomova, O.N.; Schoenbach, K.H. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem. Biophys. Res. Commun. 2009, 385, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsong, T.Y. On electroporation of cell membranes and some related phenomena. Bioelectrochem. Bioenerg. 1990, 24, 271–295. [Google Scholar] [CrossRef]
- Orlowski, S.; Belehradek, J., Jr.; Paoletti, C.; Mir, L.M. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem. Pharmacol. 1988, 37, 4727–4733. [Google Scholar] [CrossRef]
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davalos, R.V.; Mir, L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223. [Google Scholar] [CrossRef]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-based technologies for medicine: Principles, applications, and challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef] [Green Version]
- Kotnik, T.; Frey, W.; Sack, M.; Meglič, S.H.; Peterka, M.; Miklavčič, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- Mahnič-Kalamiza, S.; Vorobiev, E.; Miklavčič, D. Electroporation in food processing and biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Saldaña, G.; Álvarez, I.; Condón, S.; Raso, J. Microbiological aspects related to the feasibility of PEF technology for food pasteurization. Crit. Rev. Food Sci. Nutr. 2014, 54, 1415–1426. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Alden, R.W.; Fox, T.J. Biofouling prevention with pulsed electric fields. In Proceedings of the 1996 International Power Modulator Symposium, Boca Raton, FL, USA, 25–27 June 1996; pp. 75–78. [Google Scholar]
- Baum, C.E. Producing large transient electromagnetic fields in a small region: An electromagnetic implosion. In Ultra-Wideband Short-Pulse Electromagnetics 8; Springer: Berlin/Heidelberg, Germany, 2007; pp. 97–104. [Google Scholar]
- Heeren, T.; Camp, J.T.; Kolb, J.F.; Schoenbach, K.H.; Katsuki, S.; Akiyama, H. 250 kV sub-nanosecond pulse generator with adjustable pulse-width. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 884–888. [Google Scholar] [CrossRef]
- Plattner, H.; Verkhratsky, A. The ancient roots of calcium signalling evolutionary tree. Cell Calcium 2015, 57, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazmierczak, J.; Kempe, S.; Kremer, B. Calcium in the early evolution of living systems: A biohistorical approach. Curr. Org. Chem. 2013, 17, 1738–1750. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Burnstock, G. Biology of purinergic signalling: Its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays 2014, 36, 697–705. [Google Scholar] [CrossRef]
- Haeseleer, F.; Sokal, I.; Verlinde, C.L.; Erdjument-Bromage, H.; Tempst, P.; Pronin, A.N.; Benovic, J.L.; Fariss, R.N.; Palczewski, K. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J. Biol. Chem. 2000, 275, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Kinjo, T.G.; Schnetkamp, P.P. Ca2+ chemistry, storage and transport in biologic systems: An overview. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Jaiswal, J. Calcium—How and why? J. Biosci. 2001, 26, 357–363. [Google Scholar] [CrossRef]
- Williams, R.J. The evolution of calcium biochemistry. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 1139–1146. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.; Martin-Almedina, S.; Iglesias, J.; Gonzalez-Florez, M.; Fernandez, M. Evolutionary perspective on annexin calcium-binding domains. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1742, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Haynes, L.P.; McCue, H.V.; Burgoyne, R.D. Evolution and functional diversity of the Calcium Binding Proteins (CaBPs). Front. Mol. Neurosci. 2012, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Buchmann, L.; Frey, W.; Gusbeth, C.; Ravaynia, P.S.; Mathys, A. Effect of nanosecond pulsed electric field treatment on cell proliferation of microalgae. Bioresour. Technol. 2019, 271, 402–408. [Google Scholar] [CrossRef]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napotnik, T.B.; Reberšek, M.; Vernier, P.T.; Mali, B.; Miklavčič, D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): A systematic review. Bioelectrochemistry 2016, 110, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beebe, S.J.; White, J.; Blackmore, P.F.; Deng, Y.; Somers, K.; Schoenbach, K.H. Diverse effects of nanosecond pulsed electric fields on cells and tissues. DNA Cell Biol. 2003, 22, 785–796. [Google Scholar] [CrossRef]
- Thompson, G.L.; Roth, C.C.; Dalzell, D.R.; Kuipers, M.A.; Ibey, B.L. Calcium influx affects intracellular transport and membrane repair following nanosecond pulsed electric field exposure. J. Biomed. Opt. 2014, 19, 055005. [Google Scholar] [CrossRef]
- Zhang, J.; Blackmore, P.F.; Hargrave, B.Y.; Xiao, S.; Beebe, S.J.; Schoenbach, K.H. Nanosecond pulse electric field (nanopulse): A novel non-ligand agonist for platelet activation. Arch. Biochem. Biophys. 2008, 471, 240–248. [Google Scholar] [CrossRef]
- Beebe, S.J.; Fox, P.M.; Rec, L.J.; Willis, E.L.K.; Schoenbach, K.H. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003, 17, 1493–1495. [Google Scholar] [CrossRef] [Green Version]
- Semenov, I.; Xiao, S.; Pakhomov, A.G. Primary pathways of intracellular Ca2+ mobilization by nanosecond pulsed electric field. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 981–989. [Google Scholar] [CrossRef] [Green Version]
- Vernier, P.T.; Ziegler, M.J.; Sun, Y.; Gundersen, M.A.; Tieleman, D.P. Nanopore-facilitated, voltage-driven phosphatidylserine translocation in lipid bilayers—In cells and in silico. Phys. Biol. 2006, 3, 233. [Google Scholar] [CrossRef]
- Semenov, I.; Xiao, S.; Kang, D.; Schoenbach, K.H.; Pakhomov, A.G. Cell stimulation and calcium mobilization by picosecond electric pulses. Bioelectrochemistry 2015, 105, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Craft, C.M.; Gundersen, M.A. Nanoelectropulse-induced phosphatidylserine translocation. Biophys. J. 2004, 86, 4040–4048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Craft, C.M.; Gundersen, M.A. Nanosecond pulsed electric fields perturb membrane phospholipids in T lymphoblasts. FEBS Lett. 2004, 572, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenov, I.; Casciola, M.; Ibey, B.L.; Xiao, S.; Pakhomov, A.G. Electropermeabilization of cells by closely spaced paired nanosecond-range pulses. Bioelectrochemistry 2018, 121, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Semenov, I.; Xiao, S.; Pakhomova, O.N.; Pakhomov, A.G. Recruitment of the intracellular Ca2+ by ultrashort electric stimuli: The impact of pulse duration. Cell Calcium 2013, 54, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, H.; Zhou, C.; Gianulis, E.; Xiao, S. Non-Invasive nsPEF Stimulation for Biomodulation. Available online: https://www.researchgate.net/profile/Hollie-Ryan-2/publication/324223510_Non-Invasive_nsPEF_Stimulation_for_Biomodulation/links/5ac61c0fa6fdcc051db012fc/Non-Invasive-nsPEF-Stimulation-for-Biomodulation.pdf (accessed on 27 April 2022).
- Beebe, S.J.; Chen, X.; Kolb, J.; Schoenbach, K.H. Non-ionizing radiation generated by nanosecond pulsed electric fields (nspefs) induce apoptosis in cancer in vivo. In Proceedings of the 2008 IEEE 35th International Conference on Plasma Science, Karlsruhe, Germany, 15–19 June 2008; p. 1. [Google Scholar]
- Napotnik, T.B.; Wu, Y.H.; Gundersen, M.A.; Miklavčič, D.; Vernier, P.T. Nanosecond electric pulses cause mitochondrial membrane permeabilization in Jurkat cells. Bioelectromagnetics 2012, 33, 257–264. [Google Scholar] [CrossRef]
- Beebe, S.J.; Sain, N.M.; Ren, W. Induction of cell death mechanisms and apoptosis by nanosecond pulsed electric fields (nsPEFs). Cells 2013, 2, 136–162. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Joshi, R. Model evaluation of changes in electrorotation spectra of biological cells after nsPEF electroporation. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1888–1894. [Google Scholar] [CrossRef]
- Schoenbach, K. Bioelectric effect of intense nanosecond pulses. In Advanced Electroporation Techniques in Biology and Medicine; Pakhomov, A.G., Miklavcic, D., Markov, M.S., Eds.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Gowrishankar, T.; Weaver, J.C. Electrical behavior and pore accumulation in a multicellular model for conventional and supra-electroporation. Biochem. Biophys. Res. Commun. 2006, 349, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Kotnik, T.; Miklavčič, D. Theoretical evaluation of voltage inducement on internal membranes of biological cells exposed to electric fields. Biophys. J. 2006, 90, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Schoenbach, K.H.; Abou-Ghazala, A.; Vithoulkas, T.; Alden, R.W.; Turner, R.; Beebe, S. The effect of pulsed electrical fields on biological cells. In Digest of Technical Papers, Proceedings of the 11th IEEE International Pulsed Power Conference (Cat. No. 97CH36127), Baltimore, MD, USA, 29 June–2 July 1997; IEEE: Piscataway, NJ, USA, 1997; Volume 1, pp. 73–78. [Google Scholar]
- Gowrishankar, T.R.; Esser, A.T.; Vasilkoski, Z.; Smith, K.C.; Weaver, J.C. Microdosimetry for conventional and supra-electroporation in cells with organelles. Biochem. Biophys. Res. Commun. 2006, 341, 1266–1276. [Google Scholar] [CrossRef]
- Müller, K.; Sukhorukov, V.; Zimmermann, U. Reversible electropermeabilization of mammalian cells by high-intensity, ultra-short pulses of submicrosecond duration. J. Membr. Biol. 2001, 184, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Schoenbach, K.H.; Beebe, S.J.; Buescher, E.S. Intracellular effect of ultrashort electrical pulses. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2001, 22, 440–448. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Blackmore, P.F.; Schoenbach, K.H.; Beebe, S.J. Stimulation of capacitative calcium entry in HL-60 cells by nanosecond pulsed electric fields. J. Biol. Chem. 2004, 279, 22964–22972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernier, P.T.; Sun, Y.; Chen, M.T.; Gundersen, M.A.; Craviso, G.L. Nanosecond electric pulse-induced calcium entry into chromaffin cells. Bioelectrochemistry 2008, 73, 1–4. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Kolb, J.F.; White, J.A.; Joshi, R.P.; Xiao, S.; Schoenbach, K.H. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2007, 28, 655–663. [Google Scholar] [CrossRef]
- Cooper, R.A. Influence of increased membrane cholesterol on membrane fluidity and cell function in human red blood cells. J. Supramol. Struct. 1978, 8, 413–430. [Google Scholar] [CrossRef]
- Minto, R.E.; Adhikari, P.R.; Lorigan, G.A. A 2H solid-state NMR spectroscopic investigation of biomimetic bicelles containing cholesterol and polyunsaturated phosphatidylcholine. Chem. Phys. Lipids 2004, 132, 55–64. [Google Scholar] [CrossRef]
- Kusumi, A.; Tsuda, M.; Akino, T.; Ohnishi, S.; Terayama, Y. Protein-phospholipid-cholesterol interaction in the photolysis of invertebrate rhodopsin. Biochemistry 1983, 22, 1165–1170. [Google Scholar] [CrossRef]
- McMullen, T.P.; McElhaney, R.N. Physical studies of cholesterol-phospholipid interactions. Curr. Opin. Colloid Interface Sci. 1996, 1, 83–90. [Google Scholar] [CrossRef]
- Lu, J.X.; Caporini, M.A.; Lorigan, G.A. The effects of cholesterol on magnetically aligned phospholipid bilayers: A solid-state NMR and EPR spectroscopy study. J. Magn. Reson. 2004, 168, 18–30. [Google Scholar] [CrossRef]
- Tiburu, E.K.; Dave, P.C.; Lorigan, G.A. Solid-state 2H NMR studies of the effects of cholesterol on the acyl chain dynamics of magnetically aligned phospholipid bilayers. Magn. Reson. Chem. 2004, 42, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Tulenko, T.N.; Jacob, R.F. Direct evidence for cholesterol crystalline domains in biological membranes: Role in human pathobiology. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1610, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Lund-Katz, S.; Laboda, H.M.; McLean, L.R.; Phillips, M.C. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry 1988, 27, 3416–3423. [Google Scholar] [CrossRef]
- Smaby, J.M.; Brockman, H.L.; Brown, R.E. Cholesterol’s interfacial interactions with sphingomyelins and-phosphatidylcholines: Hydrocarbon chain structure determines the magnitude of condensation. Biochemistry 1994, 33, 9135–9142. [Google Scholar] [CrossRef]
- Anderson, T.G.; McConnell, H.M. Condensed complexes and the calorimetry of cholesterol-phospholipid bilayers. Biophys. J. 2001, 81, 2774–2785. [Google Scholar] [CrossRef] [Green Version]
- Okonogi, T.; McConnell, H. Contrast inversion in the epifluorescence of cholesterol-phospholipid monolayers. Biophys. J. 2004, 86, 880–890. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.; Needham, D. Physical properties of surfactant bilayer membranes: Thermal transitions, elasticity, rigidity, cohesion and colloidal interactions. J. Phys. Chem. 1987, 91, 4219–4228. [Google Scholar] [CrossRef]
- Needham, D.; McIntosh, T.; Evans, E. Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers. Biochemistry 1988, 27, 4668–4673. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Yin, J.J.; Hyde, J.S.; Kusumi, A. Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry 1994, 33, 7670–7681. [Google Scholar] [CrossRef]
- Bloom, M.; Mouritsen, O.G. The evolution of membranes. Can. J. Chem. 1988, 66, 706–712. [Google Scholar] [CrossRef]
- Smondyrev, A.M.; Berkowitz, M.L. Structure of dipalmitoylphosphatidylcholine/cholesterol bilayer at low and high cholesterol concentrations: Molecular dynamics simulation. Biophys. J. 1999, 77, 2075–2089. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.; Nunn, R.S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 1990, 58, 997–1009. [Google Scholar] [CrossRef] [Green Version]
- Portet, T.; Dimova, R. A new method for measuring edge tensions and stability of lipid bilayers: Effect of membrane composition. Biophys. J. 2010, 99, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Mattei, B.; Lira, R.B.; Perez, K.R.; Riske, K.A. Membrane permeabilization induced by Triton X-100: The role of membrane phase state and edge tension. Chem. Phys. Lipids 2017, 202, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Waugh, R.E. Bending rigidity of SOPC membranes containing cholesterol. Biophys. J. 1993, 64, 1967–1970. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.; Rawicz, W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 1990, 64, 2094. [Google Scholar] [CrossRef]
- Petelska, A.D.; Naumowicz, M.; Figaszewski, Z.A. The interfacial tension of the lipid membrane formed from lipid-cholesterol and lipid-lipid systems. Cell Biochem. Biophys. 2006, 44, 205–211. [Google Scholar] [CrossRef]
- Naumowicz, M.; Petelska, A.D.; Figaszewski, Z.A. Capacitance and resistance of the bilayer lipid membrane formed of phosphatidylcholine and cholesterol. Cell. Mol. Biol. Lett. 2003, 8, 5–18. [Google Scholar]
- Shmygol, A.; Noble, K.; Wray, S. Depletion of membrane cholesterol eliminates the Ca2+-activated component of outward potassium current and decreases membrane capacitance in rat uterine myocytes. J. Physiol. 2007, 581, 445–456. [Google Scholar] [CrossRef]
- Levitan, I.; Christian, A.E.; Tulenko, T.N.; Rothblat, G.H. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 2000, 115, 405–416. [Google Scholar] [CrossRef] [Green Version]
- Naumowicz, M.; Figaszewski, Z.A. Pore formation in lipid bilayer membranes made of phosphatidylcholine and cholesterol followed by means of constant current. Cell Biochem. Biophys. 2013, 66, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncharenko, M.; Katkov, I. Effect of cholesterol on the stability of human erythrocyte membranes to electric breakdown. Biofizika 1985, 30, 441–445. [Google Scholar] [PubMed]
- Raffy, S.; Teissie, J. Control of lipid membrane stability by cholesterol content. Biophys. J. 1999, 76, 2072–2080. [Google Scholar] [CrossRef] [Green Version]
- Needham, D.; Hochmuth, R. Electro-mechanical permeabilization of lipid vesicles. Role of membrane tension and compressibility. Biophys. J. 1989, 55, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Koronkiewicz, S.; Kalinowski, S. Influence of cholesterol on electroporation of bilayer lipid membranes: Chronopotentiometric studies. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1661, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Casciola, M.; Bonhenry, D.; Liberti, M.; Apollonio, F.; Tarek, M. A molecular dynamic study of cholesterol rich lipid membranes: Comparison of electroporation protocols. Bioelectrochemistry 2014, 100, 11–17. [Google Scholar] [CrossRef]
- Koronkiewicz, S.; Kalinowski, S.; Bryl, K. Programmable chronopotentiometry as a tool for the study of electroporation and resealing of pores in bilayer lipid membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1561, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, S.; Ibron, G.; Bryl, K.; Figaszewski, Z. Chronopotentiometric studies of electroporation of bilayer lipid membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1998, 1369, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Coster, H.G. Dielectric and Electrical Properties of Lipid Bilayers in Relation to their Structure. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 7, pp. 75–108. [Google Scholar]
- McIntosh, T.J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 1978, 513, 43–58. [Google Scholar] [CrossRef]
- Cantu, J.C.; Tarango, M.; Beier, H.T.; Ibey, B.L. The biological response of cells to nanosecond pulsed electric fields is dependent on plasma membrane cholesterol. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2636–2646. [Google Scholar] [CrossRef]
- Ullery, J.C.; Beier, H.T.; Ibey, B.L. Sensitivity of Cells to Nanosecond Pulsed Electric Fields is Dependent on Membrane Lipid Microdomains. In Proceedings of the 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies, Portorož, Slovenia, 6–10 September 2015; pp. 239–242. [Google Scholar]
- Hu, Q.; Joshi, R.; Schoenbach, K. Simulations of nanopore formation and phosphatidylserine externalization in lipid membranes subjected to a high-intensity, ultrashort electric pulse. Phys. Rev. E 2005, 72, 031902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, U.; Neil, G.A. Electromanipulation of Cells; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Kolb, J.F.; Kono, S.; Schoenbach, K.H. Nanosecond pulsed electric field generators for the study of subcellular effects. Bioelectromagnetics 2006, 27, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Schoenbach, K.H.; Joshi, R.P.; Beebe, S.J.; Baum, C.E. A scaling law for membrane permeabilization with nanopulses. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1224–1235. [Google Scholar] [CrossRef]
- Vernier, P.T.; Sun, Y.; Marcu, L.; Salemi, S.; Craft, C.M.; Gundersen, M.A. Calcium bursts induced by nanosecond electric pulses. Biochem. Biophys. Res. Commun. 2003, 310, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Rapp, M.; Segev, I. A brief history of time (constants). Cereb. Cortex 1996, 6, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Molitor, S.C.; Manis, P.B. Voltage-gated Ca2+ conductances in acutely isolated guinea pig dorsal cochlear nucleus neurons. J. Neurophysiol. 1999, 81, 985–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hille, B. Ion Channels of Excitable Membranes; Sinauer: Sunderland, MA, USA, 2001; Volume 507. [Google Scholar]
- Zagotta, W.N.; Hoshi, T.; Aldrich, R.W. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J. Gen. Physiol. 1994, 103, 321–362. [Google Scholar] [CrossRef]
- Baker, O.; Larsson, H.; Mannuzzu, L.; Isacoff, E. Three transmembrane conformations and sequence-dependent displacement of the S4 domain in shaker K+ channel gating. Neuron 1998, 20, 1283–1294. [Google Scholar] [CrossRef] [Green Version]
- Sigg, D.; Bezanilla, F.; Stefani, E. Fast gating in the Shaker K+ channel and the energy landscape of activation. Proc. Natl. Acad. Sci. USA 2003, 100, 7611–7615. [Google Scholar] [CrossRef] [Green Version]
- Blaustein, R.O.; Miller, C. Ion channels: Shake, rattle or roll? Nature 2004, 427, 499. [Google Scholar] [CrossRef]
- Horn, R. Conversation between voltage sensors and gates of ion channels. Biochemistry 2000, 39, 15653–15658. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-dependent gating of sodium channels: Correlating structure and function. Trends Neurosci. 1986, 9, 7–10. [Google Scholar] [CrossRef]
- GuY, H.R.; Seetharamulu, P. Molecular model of the action potential sodium channel. Proc. Natl. Acad. Sci. USA 1986, 83, 508–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezanilla, F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 2000, 80, 555–592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ruta, V.; Chen, J.; Lee, A.; MacKinnon, R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature 2003, 423, 42–48. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, A.; Chen, J.; Ruta, V.; Cadene, M.; Chait, B.T.; MacKinnon, R. X-ray structure of a voltage-dependent K+ channel. Nature 2003, 423, 33. [Google Scholar] [CrossRef]
- Chanda, B.; Asamoah, O.K.; Blunck, R.; Roux, B.; Bezanilla, F. Gating charge displacement in voltage-gated ion channels involves limited transmembrane movement. Nature 2005, 436, 852–856. [Google Scholar] [CrossRef]
- Auld, V.; Marshall, J.; Goldin, A.; Dowsett, A.; Catterall, W.; Davidson, N.; Dunn, R. Cloning and characterization of the gene for alpha-subunit of the mammalian voltage-gated sodium-channel. J. Gen. Physiol. 1985, 86, A10–A11. [Google Scholar]
- Noda, M.; Shimizu, S.; Tanabe, T.; Takai, T.; Kayano, T.; Ikeda, T.; Takahashi, H.; Nakayama, H.; Kanaoka, Y.; Minamino, N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature 1984, 312, 121–127. [Google Scholar] [CrossRef]
- Wu, D.; Delaloye, K.; Zaydman, M.A.; Nekouzadeh, A.; Rudy, Y.; Cui, J. State-dependent electrostatic interactions of S4 arginines with E1 in S2 during Kv7. 1 activation. J. Gen. Physiol. 2010, 135, 595–606. [Google Scholar] [CrossRef] [Green Version]
- DeCaen, P.G.; Yarov-Yarovoy, V.; Sharp, E.M.; Scheuer, T.; Catterall, W.A. Sequential formation of ion pairs during activation of a sodium channel voltage sensor. Proc. Natl. Acad. Sci. USA 2009, 106, 22498–22503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, X.; Lee, A.; Limapichat, W.; Dougherty, D.A.; MacKinnon, R. A gating charge transfer center in voltage sensors. Science 2010, 328, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perozo, E.; Cortes, D.M.; Cuello, L.G. Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nat. Struct. Biol. 1998, 5, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Perozo, E.; Marien, D.; Cuello, L.G. Structural rearrangements underlying K+-channel activation gating. Science 1999, 285, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Seoh, S.A.; Sigg, D.; Papazian, D.M.; Bezanilla, F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron 1996, 16, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.K.; MacKinnon, R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron 1996, 16, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Schoppa, N.E.; McCormack, K.; Tanouye, M.A.; Sigworth, F.J. The size of gating charge in wild-type and mutant Shaker potassium channels. Science 1992, 255, 1712–1715. [Google Scholar] [CrossRef]
- Starace, D.M.; Bezanilla, F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 2004, 427, 548–553. [Google Scholar] [CrossRef]
- Asamoah, O.K.; Wuskell, J.P.; Loew, L.M.; Bezanilla, F. A fluorometric approach to local electric field measurements in a voltage-gated ion channel. Neuron 2003, 37, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.; Jogini, V.; Borhani, D.W.; Leffler, A.E.; Dror, R.O.; Shaw, D.E. Mechanism of voltage gating in potassium channels. Science 2012, 336, 229–233. [Google Scholar] [CrossRef]
- Payandeh, J.; Scheuer, T.; Zheng, N.; Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 2011, 475, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ren, W.; DeCaen, P.; Yan, C.; Tao, X.; Tang, L.; Wang, J.; Hasegawa, K.; Kumasaka, T.; He, J.; et al. Crystal structure of an orthologue of the NaChBac voltage gated sodium channel. Nature 2012, 486, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Yarov-Yarovoy, V.; Baker, D.; Catterall, W.A. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K+ channels. Proc. Natl. Acad. Sci. USA 2006, 103, 7292–7297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarov-Yarovoy, V.; DeCaen, P.G.; Westenbroek, R.E.; Pan, C.Y.; Scheuer, T.; Baker, D.; Catterall, W.A. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc. Natl. Acad. Sci. USA 2012, 109, E93–E102. [Google Scholar] [CrossRef] [Green Version]
- Pathak, M.M.; Yarov-Yarovoy, V.; Agarwal, G.; Roux, B.; Barth, P.; Kohout, S.; Tombola, F.; Isacoff, E.Y. Closing in on the resting state of the Shaker K+ channel. Neuron 2007, 56, 124–140. [Google Scholar] [CrossRef] [Green Version]
- Delemotte, L.; Treptow, W.; Klein, M.L.; Tarek, M. Effect of sensor domain mutations on the properties of voltage-gated ion channels: Molecular dynamics studies of the potassium channel Kv1. 2. Biophys. J. 2010, 99, L72–L74. [Google Scholar] [CrossRef] [Green Version]
- Khalili-Araghi, F.; Jogini, V.; Yarov-Yarovoy, V.; Tajkhorshid, E.; Roux, B.; Schulten, K. Calculation of the gating charge for the Kv1. 2 voltage-activated potassium channel. Biophys. J. 2010, 98, 2189–2198. [Google Scholar] [CrossRef] [Green Version]
- Henrion, U.; Renhorn, J.; Börjesson, S.I.; Nelson, E.M.; Schwaiger, C.S.; Bjelkmar, P.; Wallner, B.; Lindahl, E.; Elinder, F. Tracking a complete voltage-sensor cycle with metal-ion bridges. Proc. Natl. Acad. Sci. USA 2012, 109, 8552–8557. [Google Scholar] [CrossRef] [Green Version]
- Baukrowitz, T.; Yellen, G. Two functionally distinct subsites for the binding of internal blockers to the pore of voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA 1996, 93, 13357–13361. [Google Scholar] [CrossRef] [Green Version]
- Baukrowitz, T.; Yellen, G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science 1996, 271, 653–656. [Google Scholar] [CrossRef] [Green Version]
- Ray, E.C.; Deutsch, C. A trapped intracellular cation modulates K+ channel recovery from slow inactivation. J. Gen. Physiol. 2006, 128, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Payandeh, J.; El-Din, T.M.G.; Scheuer, T.; Zheng, N.; Catterall, W.A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 2012, 486, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Sachs, J.N.; Crozier, P.S.; Woolf, T.B. Atomistic simulations of biologically realistic transmembrane potential gradients. J. Chem. Phys. 2004, 121, 10847–10851. [Google Scholar] [CrossRef]
- Delemotte, L.; Dehez, F.; Treptow, W.; Tarek, M. Modeling membranes under a transmembrane potential. J. Phys. Chem. B 2008, 112, 5547–5550. [Google Scholar] [CrossRef]
- Roux, B. The membrane potential and its representation by a constant electric field in computer simulations. Biophys. J. 2008, 95, 4205–4216. [Google Scholar] [CrossRef] [Green Version]
- Dzubiella, J.; Allen, R.; Hansen, J.P. Electric field-controlled water permeation coupled to ion transport through a nanopore. J. Chem. Phys. 2004, 120, 5001–5004. [Google Scholar] [CrossRef] [Green Version]
- Beebe, S.J.; Fox, P.; Rec, L.; Somers, K.; Stark, R.H.; Schoenbach, K.H. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. IEEE Trans. Plasma Sci. 2002, 30, 286–292. [Google Scholar] [CrossRef]
- Armstrong, C.; Matteson, D. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science 1985, 227, 65–67. [Google Scholar] [CrossRef]
- Bean, B.P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J. Gen. Physiol. 1985, 86, 1–30. [Google Scholar] [CrossRef]
- Curtis, B.M.; Catterall, W.A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry 1984, 23, 2113–2118. [Google Scholar] [CrossRef]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef]
- Bech-Hansen, N.T.; Naylor, M.J.; Maybaum, T.A.; Pearce, W.G.; Koop, B.; Fishman, G.A.; Mets, M.; Musarella, M.A.; Boycott, K.M. Loss-of-function mutations in a calcium-channel α 1-subunit gene in Xp11. 23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 264. [Google Scholar] [CrossRef]
- Mikami, A.; Imoto, K.; Tanabe, T.; Niidome, T.; Mori, Y.; Takeshima, H.; Narumiya, S.; Numa, S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature 1989, 340, 230. [Google Scholar] [CrossRef]
- Tanabe, T.; Takeshima, H.; Mikami, A.; Flockerzi, V.; Takahashi, H.; Kangawa, K.; Kojima, M.; Matsuo, H.; Hirose, T.; Numa, S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 1987, 328, 313. [Google Scholar] [CrossRef]
- Williams, M.E.; Feldman, D.H.; McCue, A.F.; Brenner, R.; Velicelebi, G.; Ellis, S.B.; Harpold, M.M. Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron 1992, 8, 71–84. [Google Scholar] [CrossRef]
- Randall, A.; Tsien, R.W. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J. Neurosci. 1995, 15, 2995–3012. [Google Scholar] [CrossRef]
- Bourinet, E.; Soong, T.W.; Sutton, K.; Slaymaker, S.; Mathews, E.; Monteil, A.; Zamponi, G.W.; Nargeot, J.; Snutch, T.P. Splicing of α 1A subunit gene generates phenotypic variants of P-and Q-type calcium channels. Nat. Neurosci. 1999, 2, 407. [Google Scholar] [CrossRef]
- Richards, K.S.; Swensen, A.M.; Lipscombe, D.; Bommert, K. Novel CaV2. 1 clone replicates many properties of Purkinje cell CaV2. 1 current. Eur. J. Neurosci. 2007, 26, 2950–2961. [Google Scholar] [CrossRef]
- Adams, M.E.; Myers, R.A.; Imperial, J.S.; Olivera, B.M. Toxityping rat brain calcium channels with. omega.-toxins from spider and cone snail venoms. Biochemistry 1993, 32, 12566–12570. [Google Scholar] [CrossRef]
- Dubel, S.J.; Starr, T.; Hell, J.; Ahlijanian, M.K.; Enyeart, J.J.; Catterall, W.A.; Snutch, T.P. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. Proc. Natl. Acad. Sci. USA 1992, 89, 5058–5062. [Google Scholar] [CrossRef] [Green Version]
- Soong, T.W.; Stea, A.; Hodson, C.D.; Dubel, S.J.; Vincent, S.R.; Snutch, T.P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science 1993, 260, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Stotz, S.C.; Spaetgens, R.L.; Dayanithi, G.; Lemos, J.; Nargeot, J.; Zamponi, G.W. Interaction of SNX482 with domains III and IV inhibits activation gating of α1E (CaV2. 3) calcium channels. Biophys. J. 2001, 81, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Newcomb, R.; Szoke, B.; Palma, A.; Wang, G.; Chen, X.; Hopkins, W.; Cong, R.; Miller, J.; Urge, L.; Tarczy-Hornoch, K.; et al. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry 1998, 37, 15353–15362. [Google Scholar] [CrossRef]
- Cribbs, L.L.; Lee, J.H.; Yang, J.; Satin, J.; Zhang, Y.; Daud, A.; Barclay, J.; Williamson, M.P.; Fox, M.; Rees, M.; et al. Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res. 1998, 83, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Daud, A.N.; Cribbs, L.L.; Lacerda, A.E.; Pereverzev, A.; Klöckner, U.; Schneider, T.; Perez-Reyes, E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J. Neurosci. 1999, 19, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Perez-Reyes, E.; Cribbs, L.L.; Daud, A.; Lacerda, A.E.; Barclay, J.; Williamson, M.P.; Fox, M.; Rees, M.; Lee, J.H. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 1998, 391, 896. [Google Scholar] [CrossRef]
- Perez-Reyes, E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev. 2003, 83, 117–161. [Google Scholar] [CrossRef] [Green Version]
- Catterall, W.A. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron 2010, 67, 915–928. [Google Scholar] [CrossRef] [Green Version]
- Ellinor, P.T.; Yang, J.; Sather, W.A.; Zhang, J.F.; Tsien, R.W. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron 1995, 15, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; El-Din, T.M.G.; Payandeh, J.; Martinez, G.Q.; Heard, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 2014, 505, 56. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Elllnor, P.T.; Sather, W.A.; Zhang, J.F.; Tsien, R.W. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature 1993, 366, 158. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Zamponi, G.W.; Stea, A.; Soong, T.W.; Lewis, B.A.; Jones, L.P.; Yue, D.T.; Snutch, T.P. The α1E calcium channel exhibits permeation properties similar to low-voltage-activated calcium channels. J. Neurosci. 1996, 16, 4983–4993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansman, J.B.; Hess, P.; Tsien, R.W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 1986, 88, 321–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.h.; Li, M.h.; Zhang, Y.; He, L.l.; Yamada, Y.; Fitzmaurice, A.; Shen, Y.; Zhang, H.; Tong, L.; Yang, J. Structural basis of the α 1-β subunit interaction of voltage-gated Ca2+ channels. Nature 2004, 429, 675. [Google Scholar] [CrossRef] [PubMed]
- Van Petegem, F.; Clark, K.A.; Chatelain, F.C.; Minor, D.L., Jr. Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature 2004, 429, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallon, J.L.; Halling, D.B.; Hamilton, S.L.; Quiocho, F.A. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Cav1. 2 calcium channel. Structure 2005, 13, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Fallon, J.L.; Baker, M.R.; Xiong, L.; Loy, R.E.; Yang, G.; Dirksen, R.T.; Hamilton, S.L.; Quiocho, F.A. Crystal structure of dimeric cardiac L-type calcium channel regulatory domains bridged by Ca2+· calmodulins. Proc. Natl. Acad. Sci. USA 2009, 106, 5135–5140. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.Y.; Rumpf, C.H.; Fujiwara, Y.; Cooley, E.S.; Van Petegem, F.; Minor, D.L., Jr. Structures of CaV2 Ca2+/CaM-IQ domain complexes reveal binding modes that underlie calcium-dependent inactivation and facilitation. Structure 2008, 16, 1455–1467. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.Y.; Rumpf, C.H.; Van Petegem, F.; Arant, R.J.; Findeisen, F.; Cooley, E.S.; Isacoff, E.Y.; Minor, D.L. Multiple C-terminal tail Ca2+/CaMs regulate CaV1. 2 function but do not mediate channel dimerization. EMBO J. 2010, 29, 3924–3938. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Vogel, H. Structural basis for the regulation of L-type voltage-gated calcium channels: Interactions between the N-terminal cytoplasmic domain and Ca2+-calmodulin. Front. Mol. Neurosci. 2012, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.; Vander Kooi, C.; Leahy, D.; Yue, D. Structure of the CaV2 IQ domain in complex with Ca2+/calmodulin. In Proceedings of the Annual Meeting of the Physiological Society of Japan, Virtual Event, 3 April 2008; p. 072. [Google Scholar]
- Van Petegem, F.; Chatelain, F.C.; Minor Jr, D.L. Insights into voltage-gated calcium channel regulation from the structure of the Ca V 1.2 IQ domain–Ca2+/calmodulin complex. Nat. Struct. Mol. Biol. 2005, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Serysheva, I.; Ludtke, S.; Baker, M.; Chiu, W.; Hamilton, S. Structure of the voltage-gated L-type Ca2+ channel by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 2002, 99, 10370–10375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.P.; Davies, A.; Butcher, A.J.; Dolphin, A.C.; Kitmitto, A. Three-dimensional Structure of CaV3. 1 comparison with the cardiac l-type voltage-gated calcium channel monomer architecture. J. Biol. Chem. 2009, 284, 22310–22321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.P.; Davies, A.; Nieto-Rostro, M.; Dolphin, A.C.; Kitmitto, A. Labelling of the 3D structure of the cardiac L-type voltage-gated calcium channel. Channels 2009, 3, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, M.; Eberhart, A.; Glossmann, H.; Striessnig, J.; Grigorieff, N. Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. J. Mol. Biol. 2003, 332, 171–182. [Google Scholar] [CrossRef]
- Long, S.B.; Campbell, E.B.; MacKinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 2005, 309, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Huber, I.; Wappl, E.; Herzog, A.; Mitterdorfer, J.; Glossmann, H.; Langer, T.; Striessnig, J. Conserved Ca2+-antagonist-binding properties and putative folding structure of a recombinant high-affinity dihydropyridine-binding domain. Biochem. J. 2000, 347, 829–836. [Google Scholar] [CrossRef]
- Lipkind, G.M.; Fozzard, H.A. Molecular modeling of interactions of dihydropyridines and phenylalkylamines with the inner pore of the L-type Ca2+ channel. Mol. Pharmacol. 2003, 63, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Zamponi, G.W.; Stotz, S.C.; Staples, R.J.; Andro, T.M.; Nelson, J.K.; Hulubei, V.; Blumenfeld, A.; Natale, N.R. Unique structure- activity relationship for 4-isoxazolyl-1, 4-dihydropyridines. J. Med. Chem. 2003, 46, 87–96. [Google Scholar] [CrossRef]
- Delemotte, L.; Tarek, M.; Klein, M.L.; Amaral, C.; Treptow, W. Intermediate states of the Kv1. 2 voltage sensor from atomistic molecular dynamics simulations. Proc. Natl. Acad. Sci. USA 2011, 108, 6109–6114. [Google Scholar] [CrossRef] [Green Version]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Elsevier 2014, 82, 24–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, W.R.; Merritt, J.H.; Comeaux, J.A.; Kuhnel, C.T.; Moreland, D.F.; Teltschik, D.G.; Lucas, J.H.; Murphy, M.R. Strength-duration curve for an electrically excitable tissue extended down to near 1 nanosecond. IEEE Trans. Plasma Sci. 2004, 32, 1587–1599. [Google Scholar] [CrossRef]
- Craviso, G.L.; Choe, S.; Chatterjee, P.; Chatterjee, I.; Vernier, P.T. Nanosecond electric pulses: A novel stimulus for triggering Ca2+ influx into chromaffin cells via voltage-gated Ca2+ channels. Cell. Mol. Neurobiol. 2010, 30, 1259–1265. [Google Scholar] [CrossRef]
- Burke, R.C.; Bardet, S.M.; Carr, L.; Romanenko, S.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R.P. Nanosecond pulsed electric fields depolarize transmembrane potential via voltage-gated K+, Ca2+ and TRPM8 channels in U87 glioblastoma cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Semenov, I.; Casciola, M.; Xiao, S. Neuronal excitation and permeabilization by 200-ns pulsed electric field: An optical membrane potential study with FluoVolt dye. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1273–1281. [Google Scholar] [CrossRef]
- Bagalkot, T.R.; Leblanc, N.; Craviso, G.L. Stimulation or Cancellation of Ca2+ Influx by Bipolar Nanosecond Pulsed Electric Fields in Adrenal Chromaffin Cells Can Be Achieved by Tuning Pulse Waveform. Sci. Rep. 2019, 9, 11545. [Google Scholar] [CrossRef] [Green Version]
- Bagalkot, T.R.; Terhune, R.C.; Leblanc, N.; Craviso, G.L. Different membrane pathways mediate Ca2+ influx in adrenal chromaffin cells exposed to 150–400 ns electric pulses. BioMed Res. Int. 2018, 2018, 9046891. [Google Scholar] [CrossRef] [Green Version]
- Hristov, K.; Mangalanathan, U.; Casciola, M.; Pakhomova, O.N.; Pakhomov, A.G. Expression of voltage-gated calcium channels augments cell susceptibility to membrane disruption by nanosecond pulsed electric field. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2175–2183. [Google Scholar] [CrossRef]
- Esser, A.T.; Smith, K.C.; Gowrishankar, T.; Vasilkoski, Z.; Weaver, J.C. Mechanisms for the intracellular manipulation of organelles by conventional electroporation. Biophys. J. 2010, 98, 2506–2514. [Google Scholar] [CrossRef] [Green Version]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane electroporation and electropermeabilization: Mechanisms and models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef]
- Teissie, J.; Tsong, T.Y. Evidence of voltage-induced channel opening in Na/K ATPase of human erythrocyte membrane. J. Membr. Biol. 1980, 55, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tsong, T.Y. Electroporation of cell membranes. In Electroporation and Electrofusion in Cell Biology; Spring: Berlin/Heidelberg, Germany, 1989; pp. 149–163. [Google Scholar]
- Marracino, P.; Bernardi, M.; Liberti, M.; Del Signore, F.; Trapani, E.; Garate, J.A.; Burnham, C.J.; Apollonio, F.; English, N.J. Transprotein-electropore characterization: A molecular dynamics investigation on human AQP4. ACS Omega 2018, 3, 15361–15369. [Google Scholar] [CrossRef] [PubMed]
- Rems, L.; Kasimova, M.A.; Testa, I.; Delemotte, L. Pulsed electric fields can create pores in the voltage sensors of voltage-gated ion channels. Biophys. J. 2020, 119, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C. Electroporation: A general phenomenon for manipulating cells and tissues. J. Cell. Biochem. 1993, 51, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Vernier, P.T. Pore lifetimes in cell electroporation: Complex dark pores? arXiv 2017, arXiv:1708.07478. [Google Scholar]
- Weaver, J.C.; Barnett, A. Progress toward a theoretical model for electroporation mechanism: Membrane electrical behavior and molecular transport. In Guide to Electroporation and Electrofusion; Spring: Berlin/Heidelberg, Germany, 1992; pp. 91–117. [Google Scholar]
- Nesin, V.; Bowman, A.M.; Xiao, S.; Pakhomov, A.G. Cell permeabilization and inhibition of voltage-gated Ca2+ and Na+ channel currents by nanosecond pulsed electric field. Bioelectromagnetics 2012, 33, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Nesin, V.; Pakhomov, A.G. Inhibition of voltage-gated Na+ current by nanosecond pulsed electric field (nsPEF) is not mediated by Na+ influx or Ca2+ signaling. Bioelectromagnetics 2012, 33, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Craviso, G.L.; Vernier, P.T.; Chatterjee, I.; Leblanc, N. Nanosecond electric pulses differentially affect inward and outward currents in patch clamped adrenal chromaffin cells. PLoS ONE 2017, 12, e0181002. [Google Scholar] [CrossRef]
- Chen, W.; Han, Y.; Chen, Y.; Astumian, D. Electric field-induced functional reductions in the K+ channels mainly resulted from supramembrane potential-mediated electroconformational changes. Biophys. J. 1998, 75, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Levine, Z.A.; Vernier, P.T. Life cycle of an electropore: Field-dependent and field-independent steps in pore creation and annihilation. J. Membr. Biol. 2010, 236, 27–36. [Google Scholar] [CrossRef]
- Bennett, W.D.; Sapay, N.; Tieleman, D.P. Atomistic simulations of pore formation and closure in lipid bilayers. Biophys. J. 2014, 106, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Fernández, A.R.; Campos, L.; Villanelo, F.; Gutiérrez-Maldonado, S.E.; Perez-Acle, T. Exploring the Conformational Changes Induced by Nanosecond Pulsed Electric Fields on the Voltage Sensing Domain of a Ca2+ Channel. Membranes 2021, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, V.; Marracino, P.; Apollonio, F.; Liberti, M.; Amadei, A.; d’Inzeo, G. Molecular Dynamics Simulations of a Nanosecond E-Field Pulse Acting on Single DNA Strand. Available online: https://www.researchgate.net/profile/Andrea-Amadei-3/publication/228895176_Molecular_Dynamics_Simulations_of_a_Nanosecond_E-Field_Pulse_acting_on_Single_DNA_Strand/links/0912f509a6eb6b3492000000/Molecular-Dynamics-Simulations-of-a-Nanosecond-E-Field-Pulse-acting-on-Single-DNA-Strand.pdf (accessed on 27 April 2022).
- Li, H.; Liu, S.; Yang, X.; Du, Y.; Luo, J.; Tan, J.; Sun, Y. Cellular Processes Involved in Jurkat Cells Exposed to Nanosecond Pulsed Electric Field. Int. J. Mol. Sci. 2019, 20, 5847. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, J.; Chen, M.T.; Vernier, P.T.; Gundersen, M.A.; Valderrábano, M. Cardiac myocyte excitation by ultrashort high-field pulses. Biophys. J. 2009, 96, 1640–1648. [Google Scholar] [CrossRef] [Green Version]
- Azarov, J.E.; Semenov, I.; Casciola, M.; Pakhomov, A.G. Excitation of murine cardiac myocytes by nanosecond pulsed electric field. J. Cardiovasc. Electrophysiol. 2019, 30, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Xiao, S.; Novickij, V.; Casciola, M.; Semenov, I.; Mangalanathan, U.; Kim, V.; Zemlin, C.; Sozer, E.; Muratori, C.; et al. Excitation and electroporation by MHz bursts of nanosecond stimuli. Biochem. Biophys. Res. Commun. 2019, 518, 759–764. [Google Scholar] [CrossRef]
- Semenov, I.; Grigoryev, S.; Neuber, J.U.; Zemlin, C.W.; Pakhomova, O.N.; Casciola, M.; Pakhomov, A.G. Excitation and injury of adult ventricular cardiomyocytes by nano-to millisecond electric shocks. Sci. Rep. 2018, 8, 8233. [Google Scholar] [CrossRef] [Green Version]
- Romanenko, S.; Arnaud-Cormos, D.; Leveque, P.; O’Connor, R.P. Ultrashort pulsed electric fields induce action potentials in neurons when applied at axon bundles. In Proceedings of the 2016 9th International Kharkiv Symposium on Physics and Engineering of Microwaves, Millimeter and Submillimeter Waves (MSMW), Kharkiv, Ukraine, 20–24 June 2016; pp. 1–5. [Google Scholar]
- Casciola, M.; Xiao, S.; Pakhomov, A.G. Damage-free peripheral nerve stimulation by 12-ns pulsed electric field. Sci. Rep. 2017, 7, 10453. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, P.; Tucci, V.; Zeni, O.; Romeo, S. Analysis of ionic channel currents under nsPEFs-stimulation by a circuital model of an excitable cell. In Proceedings of the 2020 IEEE 20th Mediterranean Electrotechnical Conference (MELECON), Palermo, Italy, 16–18 June 2020; pp. 411–414. [Google Scholar]
- Roth, C.C.; Tolstykh, G.P.; Payne, J.A.; Kuipers, M.A.; Thompson, G.L.; DeSilva, M.N.; Ibey, B.L. Nanosecond pulsed electric field thresholds for nanopore formation in neural cells. J. Biomed. Opt. 2013, 18, 035005. [Google Scholar] [CrossRef] [Green Version]
- Vadlamani, R.A.; Nie, Y.; Detwiler, D.A.; Dhanabal, A.; Kraft, A.M.; Kuang, S.; Gavin, T.P.; Garner, A.L. Nanosecond pulsed electric field induced proliferation and differentiation of osteoblasts and myoblasts. J. R. Soc. Interface 2019, 16, 20190079. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Ge, Z.; Zhang, J. Nanosecond pulsed electric fields (nsPEFs) regulate phenotypes of chondrocytes through Wnt/β-catenin signaling pathway. Sci. Rep. 2014, 4, 5836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morotomi-Yano, K.; Uemura, Y.; Katsuki, S.; Akiyama, H.; Yano, K. Activation of the JNK pathway by nanosecond pulsed electric fields. Biochem. Biophys. Res. Commun. 2011, 408, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Muratori, C.; Pakhomov, A.G.; Gianulis, E.; Meads, J.; Casciola, M.; Mollica, P.A.; Pakhomova, O.N. Activation of the phospholipid scramblase TMEM16F by nanosecond pulsed electric fields (nsPEF) facilitates its diverse cytophysiological effects. J. Biol. Chem. 2017, 292, 19381–19391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beebe, S.J.; Blackmore, P.F.; White, J.; Joshi, R.P.; Schoenbach, K.H. Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol. Meas. 2004, 25, 1077. [Google Scholar] [CrossRef]
- Guo, S.; Jackson, D.L.; Burcus, N.I.; Chen, Y.J.; Xiao, S.; Heller, R. Gene electrotransfer enhanced by nanosecond pulsed electric fields. Mol. Ther. Methods Clin. Dev. 2014, 1, 14043. [Google Scholar] [CrossRef]
- Estlack, L.E.; Roth, C.C.; Thompson, G.L.; Lambert, W.A.; Ibey, B.L. Nanosecond pulsed electric fields modulate the expression of Fas/CD95 death receptor pathway regulators in U937 and Jurkat Cells. Apoptosis 2014, 19, 1755–1768. [Google Scholar] [CrossRef]
- Zhang, R.; Aji, T.; Shao, Y.; Jiang, T.; Yang, L.; Lv, W.; Chen, Y.; Chen, X.; Wen, H. Nanosecond pulsed electric field (nsPEF) disrupts the structure and metabolism of human Echinococcus granulosus protoscolex in vitro with a dose effect. Parasitol. Res. 2017, 116, 1345–1351. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Aji, T.; Shao, Y.; Chen, Y.; Wen, H. Novel interventional management of hepatic hydatid cyst with nanosecond pulses on experimental mouse model. Sci. Rep. 2017, 7, 4491. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Wen, H. Experimental nanopulse ablation of multiple membrane parasite on ex vivo hydatid cyst. BioMed Res. Int. 2018, 2018, 8497283. [Google Scholar] [CrossRef] [Green Version]
- Hargrave, B.; Li, F. Nanosecond pulse electric field activation of platelet-rich plasma reduces myocardial infarct size and improves left ventricular mechanical function in the rabbit heart. J. Extra-Corpor. Technol. 2012, 44, 198. [Google Scholar]
- Xiao, S.; Kiyan, T.; Blackmore, P.; Schoenbach, K. Pulsed Power for Wound Healing. In Proceedings of the 2008 IEEE International Power Modulators and High-Voltage Conference, Las Vegas, NV, USA, 27–31 May 2008; pp. 69–72. [Google Scholar]
- Hargrave, B.; Li, F. Nanosecond Pulse Electric Field Activated-Platelet Rich Plasma Enhances the Return of Blood Flow to Large and Ischemic Wounds in a Rabbit Model. Physiol. Rep. 2015, 3, e12461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccitelli, R.; Berridge, J.C.; Mallon, Z.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanoelectroablation of murine tumors triggers a CD8-dependent inhibition of secondary tumor growth. PLoS ONE 2015, 10, e0134364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Sain, N.M.; Harlow, K.T.; Chen, Y.J.; Shires, P.K.; Heller, R.; Beebe, S.J. A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur. J. Cancer 2014, 50, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jing, Y.; Burcus, N.I.; Lassiter, B.P.; Tanaz, R.; Heller, R.; Beebe, S.J. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int. J. Cancer 2018, 142, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Skeate, J.G.; Da Silva, D.M.; Chavez-Juan, E.; Anand, S.; Nuccitelli, R.; Kast, W.M. Nano-Pulse Stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLoS ONE 2018, 13, e0191311. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, R.; Tran, K.; Lui, K.; Huynh, J.; Athos, B.; Kreis, M.; Nuccitelli, P.; De Fabo, E.C. Non-thermal nanoelectroablation of UV-induced murine melanomas stimulates an immune response. Pigment Cell Melanoma Res. 2012, 25, 618–629. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, R.; McDaniel, A.; Anand, S.; Cha, J.; Mallon, Z.; Berridge, J.C.; Uecker, D. Nano-Pulse Stimulation is a physical modality that can trigger immunogenic tumor cell death. J. Immunother. Cancer 2017, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, R.; Wood, R.; Kreis, M.; Athos, B.; Huynh, J.; Lui, K.; Nuccitelli, P.; Epstein, E.H., Jr. First-in-human trial of nanoelectroablation therapy for basal cell carcinoma: Proof of method. Exp. Dermatol. 2014, 23, 135–137. [Google Scholar] [CrossRef]
- Garon, E.B.; Sawcer, D.; Vernier, P.T.; Tang, T.; Sun, Y.; Marcu, L.; Gundersen, M.A.; Koeffler, H.P. In vitro and in vivo evaluation and a case report of intense nanosecond pulsed electric field as a local therapy for human malignancies. Int. J. Cancer 2007, 121, 675–682. [Google Scholar] [CrossRef]
- Mir, L.M.; Orlowski, S.; Belehradek, J., Jr.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer Clin. Oncol. 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Serša, G.; Čemažar, M.; Miklavčič, D. Antitumor effectiveness of electrochemotherapy with cis-diamminedichloroplatinum (II) in mice. Cancer Res. 1995, 55, 3450–3455. [Google Scholar] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Salido, G.M.; Rosado, J.A. Apoptosis: Involvement of Oxidative Stress and Intracellular Ca2+ Homeostasis; Universty of Extremadure: Badajoz, Spain, 2009; pp. 229–235. [Google Scholar]
- Stacey, M.; Stickley, J.; Fox, P.; O’Donnell, C.; Schoenbach, K.; Beebe, S.; Buescher, S. Increased cell killing and DNA damage in cells exposed to ultra-short pulsed electric fields. In Proceedings of the Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 20–24 October 2002; pp. 79–82. [Google Scholar]
- Chen, X.; Zhuang, J.; Kolb, J.F.; Schoenbach, K.H.; Beebe, S.J. Long term survival of mice with hepatocellular carcinoma after pulse power ablation with nanosecond pulsed electric fields. Technol. Cancer Res. Treat. 2012, 11, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccitelli, R.; Huynh, J.; Lui, K.; Wood, R.; Kreis, M.; Athos, B.; Nuccitelli, P. Nanoelectroablation of human pancreatic carcinoma in a murine xenograft model without recurrence. Int. J. Cancer 2013, 132, 1933–1939. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [Green Version]
- Stacey, M.; Stickley, J.; Fox, P.; Statler, V.; Schoenbach, K.; Beebe, S.; Buescher, S. Differential effects in cells exposed to ultra-short, high intensity electric fields: Cell survival, DNA damage, and cell cycle analysis. Mutat. Res. Toxicol. Environ. Mutagen. 2003, 542, 65–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, F.; Liu, Z.; Guo, J.; Zhang, J.; Fang, J. Nanosecond pulsed electric fields promoting the proliferation of porcine iliac endothelial cells: An in vitro study. PLoS ONE 2018, 13, e0196688. [Google Scholar]
- Dong, F.; Liu, Z.; Zhang, J.; Fang, J.; Guo, J.; Zhang, Y. Nspefs Promoting the Proliferation of Piec Cells: An in Vitro Study. In Proceedings of the 2017 IEEE International Conference on Plasma Science (ICOPS), Atlantic City, NJ, USA, 21–25 May 2017; p. 1. [Google Scholar]
- Guo, J.; Ma, R.; Su, B.; Li, Y.; Zhang, J.; Fang, J. Raising the avermectins production in Streptomyces avermitilis by utilizing nanosecond pulsed electric fields (nsPEFs). Sci. Rep. 2016, 6, 25949. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, F.; Gusbeth, C.; Frey, W.; Maisch, J.; Nick, P. Nanosecond pulsed electrical fields enhance product recovery in plant cell fermentation. Protoplasma 2020, 257, 1585–1594. [Google Scholar] [CrossRef]
- Prorot, A.; Arnaud-Cormos, D.; Lévêque, P.; Leprat, P. Bacterial stress induced by nanosecond pulsed electric fields (nsPEF): Potential applications for food industry and environment. In Proceedings of the IV International Conference on Environmental, Industrial and Applied Microbiolog, Malaga, Spain, 14–16 September 2011. [Google Scholar]
- Haberkorn, I.; Buchmann, L.; Häusermann, I.; Mathys, A. Nanosecond pulsed electric field processing of microalgae based biorefineries governs growth promotion or selective inactivation based on underlying microbial ecosystems. Bioresour. Technol. 2020, 319, 124173. [Google Scholar] [CrossRef]
- Su, B.; Guo, J.; Nian, W.; Feng, H.; Wang, K.; Zhang, J.; Fang, J. Early growth effects of nanosecond pulsed electric field (nsPEFs) exposure on Haloxylon ammodendron. Plasma Process. Polym. 2015, 12, 372–379. [Google Scholar] [CrossRef]
- Eing, C.J.; Bonnet, S.; Pacher, M.; Puchta, H.; Frey, W. Effects of nanosecond pulsed electric field exposure on Arabidopsis thaliana. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1322–1328. [Google Scholar] [CrossRef] [Green Version]
- Songnuan, W.; Kirawanich, P. Early growth effects on Arabidopsis thaliana by seed exposure of nanosecond pulsed electric field. J. Electrost. 2012, 70, 445–450. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Panacek, A.; Prucek, R.; Hrbac, J.; Vecerova, R.; Zboril, R. Comparative study of antimicrobial activity of AgBr and Ag nanoparticles (NPs). PLoS ONE 2015, 10, e0119202. [Google Scholar] [CrossRef] [Green Version]
- Vadlapudi, V.; Kaladhar, D.; Behara, M.; Sujatha, B.; Naidu, G.K. Synthesis of green metallic nanoparticles (NPs) and applications. Orient. J. Chem 2013, 29, 1589–1595. [Google Scholar] [CrossRef] [Green Version]
- Boedeker, K.L.; Cooper, V.N.; McNitt-Gray, M.F. Application of the noise power spectrum in modern diagnostic MDCT: Part I. Measurement of noise power spectra and noise equivalent quanta. Phys. Med. Biol. 2007, 52, 4027. [Google Scholar] [CrossRef]
- Dobbins, J.T., III; Samei, E.; Ranger, N.T.; Chen, Y. Intercomparison of methods for image quality characterization. II. Noise power spectrum a. Med Phys. 2006, 33, 1466–1475. [Google Scholar] [CrossRef] [Green Version]
- Grisaffe, D.B. Questions about the ultimate question: Conceptual considerations in evaluating Reichheld’s net promoter score (NPS). J. Consum. Satisf. Dissatisfaction Complain. Behav. 2007, 20, 36. [Google Scholar]
- Baehre, S.; O’Dwyer, M.; O’Malley, L.; Lee, N. The use of Net Promoter Score (NPS) to predict sales growth: Insights from an empirical investigation. J. Acad. Mark. Sci. 2022, 50, 67–84. [Google Scholar] [CrossRef]
- Melikov, K.C.; Frolov, V.A.; Shcherbakov, A.; Samsonov, A.V.; Chizmadzhev, Y.A.; Chernomordik, L.V. Voltage-induced nonconductive pre-pores and metastable single pores in unmodified planar lipid bilayer. Biophys. J. 2001, 80, 1829–1836. [Google Scholar] [CrossRef] [Green Version]
- Vernier, P.T.; Sun, Y.; Gundersen, M.A. Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. BMC Cell Biol. 2006, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilkoski, Z.; Esser, A.T.; Gowrishankar, T.; Weaver, J.C. Membrane electroporation: The absolute rate equation and nanosecond time scale pore creation. Phys. Rev. E 2006, 74, 021904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakhomov, A.G.; Miklavcic, D.; Markov, M.S. Advanced Electroporation Techniques in Biology and Medicine; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Pakhomov, A.G.; Pakhomova, O.N. Nanopores: A distinct transmembrane passageway in electroporated cells. In Advanced Electroporation Techniques in Biology in Medicine; CRC Press: Boca Raton, FL, USA, 2010; pp. 178–194. [Google Scholar]
- Bowman, A.M.; Nesin, O.M.; Pakhomova, O.N.; Pakhomov, A.G. Analysis of plasma membrane integrity by fluorescent detection of Tl+ uptake. J. Membr. Biol. 2010, 236, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibey, B.L.; Pakhomov, A.G.; Gregory, B.W.; Khorokhorina, V.A.; Roth, C.C.; Rassokhin, M.A.; Bernhard, J.A.; Wilmink, G.J.; Pakhomova, O.N. Selective cytotoxicity of intense nanosecond-duration electric pulses in mammalian cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010, 1800, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- LITJTER, J.; JD, L. Stability of lipid bilayers and red blood cell membranes. Phys. Lett. A 1975, 53, 193–194. [Google Scholar] [CrossRef]
- Brochard-Wyart, F.; de Gennes, P.G.; Sandre, O. Transient pores in stretched vesicles: Role of leak-out. Phys. A Stat. Mech. Appl. 2000, 278, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Leontiadou, H.; Mark, A.E.; Marrink, S.J. Molecular dynamics simulations of hydrophilic pores in lipid bilayers. Biophys. J. 2004, 86, 2156–2164. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.Y.; Bouret, Y.; Kindt, J.T. Molecular dynamics simulations of the lipid bilayer edge. Biophys. J. 2004, 87, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Gianulis, E.C.; Labib, C.; Saulis, G.; Novickij, V.; Pakhomova, O.N.; Pakhomov, A.G. Selective susceptibility to nanosecond pulsed electric field (nsPEF) across different human cell types. Cell. Mol. Life Sci. 2017, 74, 1741–1754. [Google Scholar] [CrossRef]
- Li, Y.C.; Park, M.J.; Ye, S.K.; Kim, C.W.; Kim, Y.N. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am. J. Pathol. 2006, 168, 1107–1118. [Google Scholar] [CrossRef] [Green Version]
- Merchant, T.E.; Kasimos, J.N.; Vroom, T.; de Bree, E.; Iwata, J.L.; de Graaf, P.W.; Glonek, T. Malignant breast tumor phospholipid profiles using 31P magnetic resonance. Cancer Lett. 2002, 176, 159–167. [Google Scholar] [CrossRef]
- Sterin, M.; Cohen, J.S.; Ringel, I. Hormone sensitivity is reflected in the phospholipid profiles of breast cancer cell lines. Breast Cancer Res. Treat. 2004, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dória, M.L.; Cotrim, Z.; Macedo, B.; Simões, C.; Domingues, P.; Helguero, L.; Domingues, M.R. Lipidomic approach to identify patterns in phospholipid profiles and define class differences in mammary epithelial and breast cancer cells. Breast Cancer Res. Treat. 2012, 133, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Dória, M.L.; Cotrim, C.Z.; Simões, C.; Macedo, B.; Domingues, P.; Domingues, M.R.; Helguero, L.A. Lipidomic analysis of phospholipids from human mammary epithelial and breast cancer cell lines. J. Cell. Physiol. 2013, 228, 457–468. [Google Scholar] [CrossRef]
- Patra, S.K. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2008, 1785, 182–206. [Google Scholar] [CrossRef]
- Niemelä, P.S.; Ollila, S.; Hyvönen, M.T.; Karttunen, M.; Vattulainen, I. Assessing the nature of lipid raft membranes. PLoS Comput. Biol. 2007, 3, e34. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Staubach, S.; Hanisch, F.G. Lipid rafts: Signaling and sorting platforms of cells and their roles in cancer. Expert Rev. Proteom. 2011, 8, 263–277. [Google Scholar] [CrossRef]
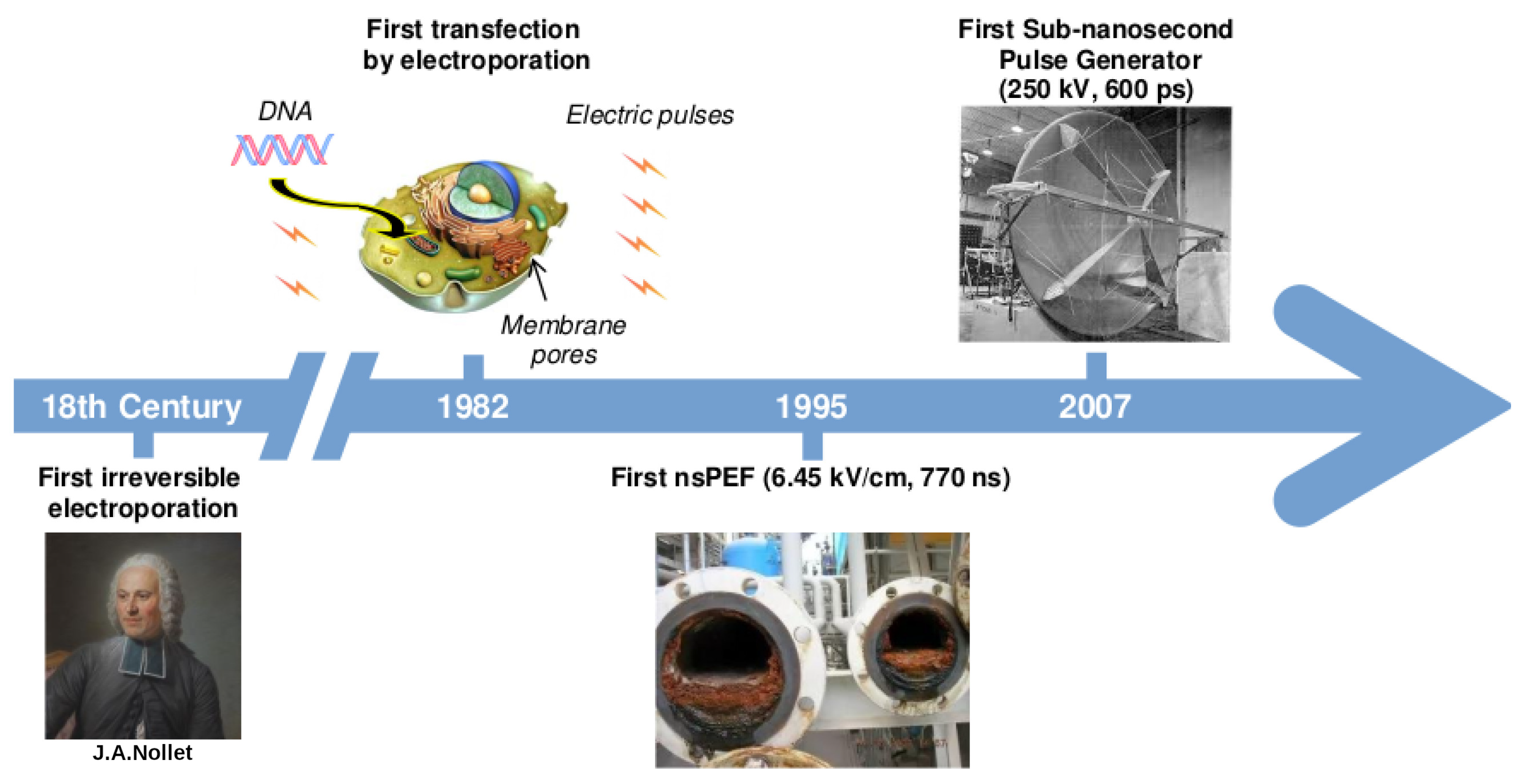
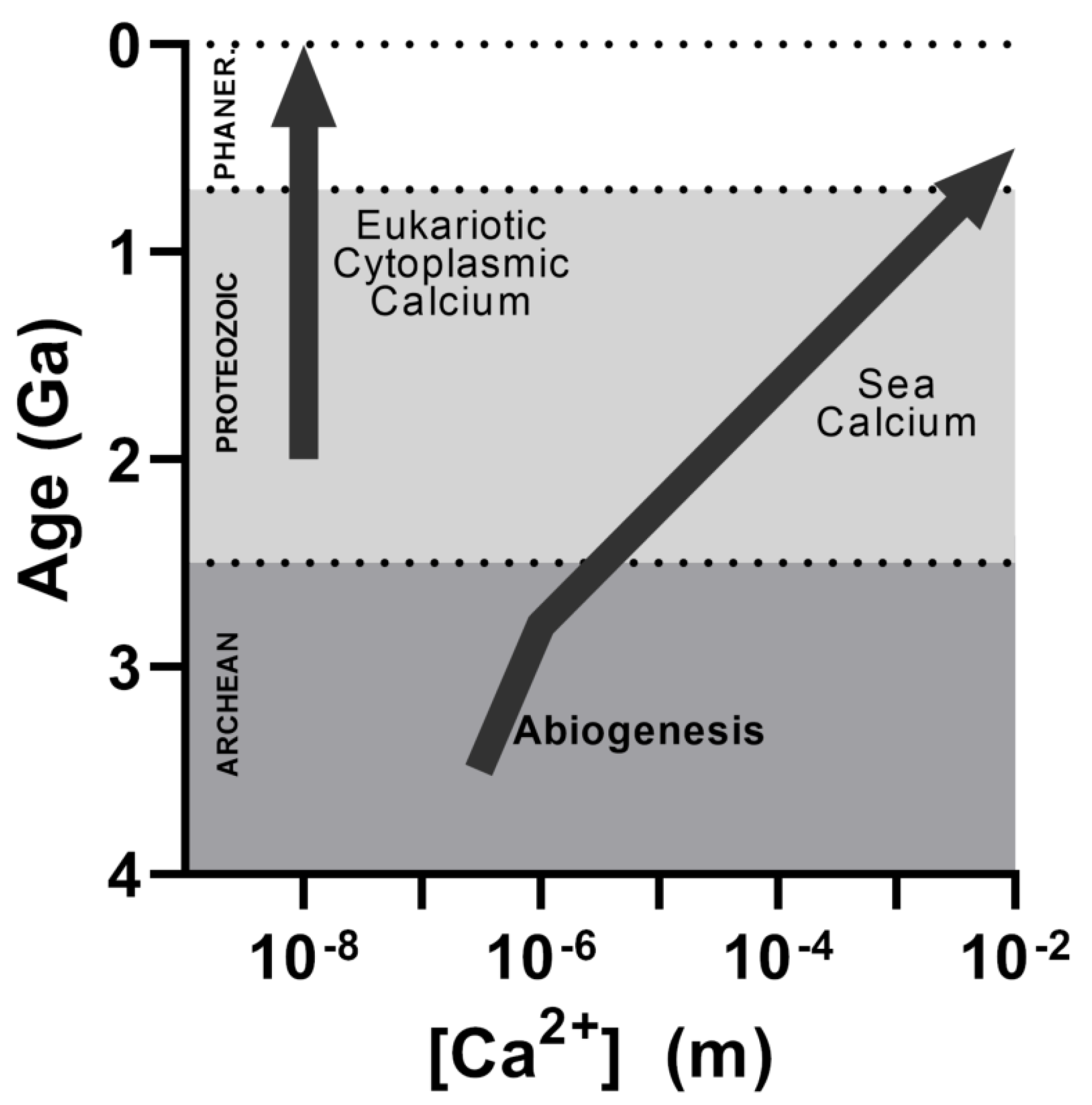


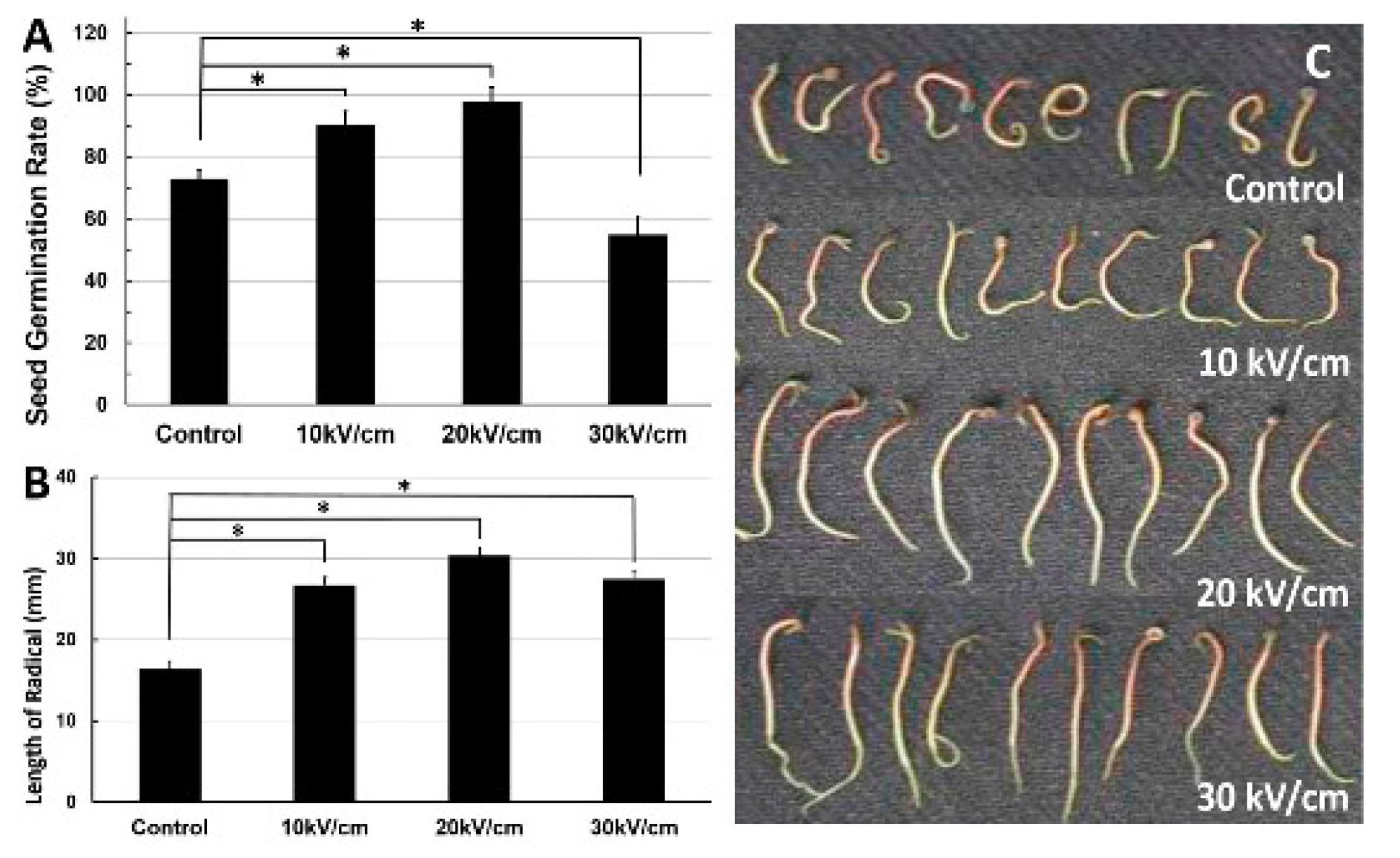
| nsPEF (kV/cm) | Pulse Time (ns) | Cell Line Used | Observed Effect | Year and Citation |
|---|---|---|---|---|
| 13.5 | 50 | HL-60 leukemia cells | nsPEF affects the nucleus but not the plasma membrane | 1997 [47] |
| 60 | 60 | Theoretical cell model including representations of several organelles | nsPEF goes through cell membrane, extensively penetrating organelles | 2006 [48] |
| ∼150 | 10–100 | Sp2, mouse murine myeloma cells | The cytoplasmic membrane is capable of withstanding nsPEF application, suggesting that permeabilization of organelles is the main effect | 2001 [49] |
| 53 | 60 | Human neutrophil and eosinophil cells | nsPEF induces poration of eosinophils’ intracellular granules. This occurs without permanent disruption of the cytoplasmic membrane. Of note, neutrophils show no changes | 2001 [50] |
| 4–15 | 60 | HL-60 leukemia cells | nsPEF induces a rise in cytoplasmic Ca concentration without incorporation of external propidium iodide and using a Ca-free media, suggesting no cell membrane poration, but poration of internal membranes | 2004 [51] |
| 20–80 | 4 | Chromaffin cells | nsPEF induces a rise in cytoplasmic Ca concentration not affected by the depletion of intracellular calcium storage with either caffeine or thapsigargin, being completely prevented by the presence of EGTA (a Ca chelator) in the extracellular medium | 2008 [52] |
| 22–24 | 60 | GH3 murine pituitary, PC-12 murine adrenal, and Jurkat cells (immortalized human T-lymphocytes) | nsPEF induces a long-lasting effect (∼100 s) on cytoplasmic membrane permeabilization that can be monitored by patch-clamp | 2007 [53] |
| 2.4–4.8 | 600 | GH3 and CHO-K1 Chinese hamster ovary cells | nsPEF induces an incremental increase in cell conductance, attributed to the formation of ion-channel-like nanopores in the cytoplasmic membrane with a maximum width of 1 nm in both studied cell lines. The size was proposed because the membrane remained mostly impermeable to propidium iodine | 2009 [3] |
| nsPEF (kV/cm) | Pulse Time (ns) | Cell Line or Tissue Used | Observed Effect of nsPEF | Year and Citation |
|---|---|---|---|---|
| 3.1 | 150–400 | Bovine chromaffin cells | Similar results to [186]. Bagalkot et al. in 2019 incorporated a symmetrical bipolar pulse (a second identical pulse but with opposite polarity) that attenuated Ca entry across possible nanopores while preserving Ca influx through VGCCs [189]. | 2018 [189,190] |
| 190 | 0.5 | GH3, CHO-K1, and NG108 cells (murine neuroblastoma–rat glioma hybrid) | This sub-nanosecond electric pulse activated VGCCs on GH3 and NG108 cells (which express multiple types of VGCCs) and CHO-K1 cells (no VGCC expression). Trains of up to 100 pulses did not change the cytoplasmic Ca concentration (followed by Fura-2 imaging) in CHO-K1 cells, while in GH3 and NG108, a single pulse significantly increased it. Trains of 100 pulses increased cytoplasmic Ca concentration to 379 ± 33 nM in GH3 and 719 ± 315 nM in NG108. To corroborate that Ca is passing through a VGCC and not nanopores, they used verapamil (L-type VGCC blocker) and –conotoxin (wide-spectrum N, P, and Q type VGCC blocker). They observed 80-100% inhibition of Ca uptake with both VGCC blockers. | 2015 [34] |
| 2.3 | 300 | HEK293 cells | In cells with and without assembled Ca1.3 L-type VGCC, the nsPEF pulse caused a lasting (>80 s) increase in membrane conductance for all cells. Although the elicited membrane potential did not depolarize enough for VGCC activation, the increase in conductance in cells that expressed VGCC was about two-fold greater than in cells which did not. This result suggests an important role of VGCC in the increase in cytoplasmic Ca concentration induced by nsPEF. | 2018 [191] |
| 1.6–1.9 | 200 | E18 rat hippocampal neurons | Using fast optical membrane potential imaging, it was shown that a single nsPEF pulse was able to trigger a single action potential 4–6 ms after the nsPEF pulse in 40% of neurons. The addition of tetrodotoxin (selective sodium channel blocker) to cell media abolished the induced nsPEF action potential, demonstrating that nsPEF managed to activate VGNCs. | 2017 [188] |
| 3.3–8.8 | 12 | Xenopus laevis peripheral nerve | Using thousands of nsPEF pulses, nerve excitation was achieved without electroporation for the first time. The nerves did not register cumulative damage, as refractory properties were not affected. The authors claimed that their data proved that VGNC are activated by nsPEFs and also manifested that nsPEFs are a promising tool for biomedical applications. | 2010 [186] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Fernández, A.R.; Campos, L.; Gutierrez-Maldonado, S.E.; Núñez, G.; Villanelo, F.; Perez-Acle, T. Nanosecond Pulsed Electric Field (nsPEF): Opening the Biotechnological Pandora’s Box. Int. J. Mol. Sci. 2022, 23, 6158. https://doi.org/10.3390/ijms23116158
Ruiz-Fernández AR, Campos L, Gutierrez-Maldonado SE, Núñez G, Villanelo F, Perez-Acle T. Nanosecond Pulsed Electric Field (nsPEF): Opening the Biotechnological Pandora’s Box. International Journal of Molecular Sciences. 2022; 23(11):6158. https://doi.org/10.3390/ijms23116158
Chicago/Turabian StyleRuiz-Fernández, Alvaro R., Leonardo Campos, Sebastian E. Gutierrez-Maldonado, Gonzalo Núñez, Felipe Villanelo, and Tomas Perez-Acle. 2022. "Nanosecond Pulsed Electric Field (nsPEF): Opening the Biotechnological Pandora’s Box" International Journal of Molecular Sciences 23, no. 11: 6158. https://doi.org/10.3390/ijms23116158







