Spheroid Formation and Peritoneal Metastasis in Ovarian Cancer: The Role of Stromal and Immune Components
Abstract
:1. Introduction
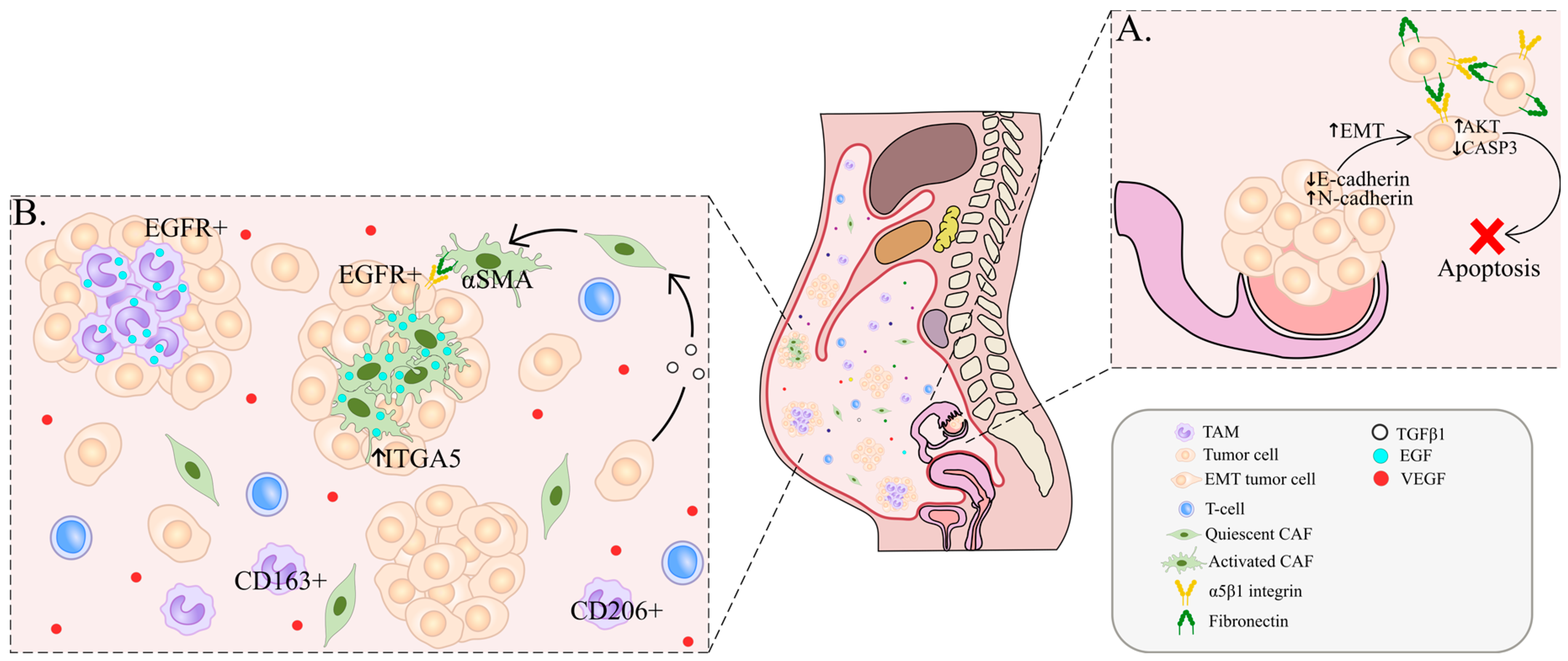
2. Cellular Composition of Ascites and Spheroid Formation
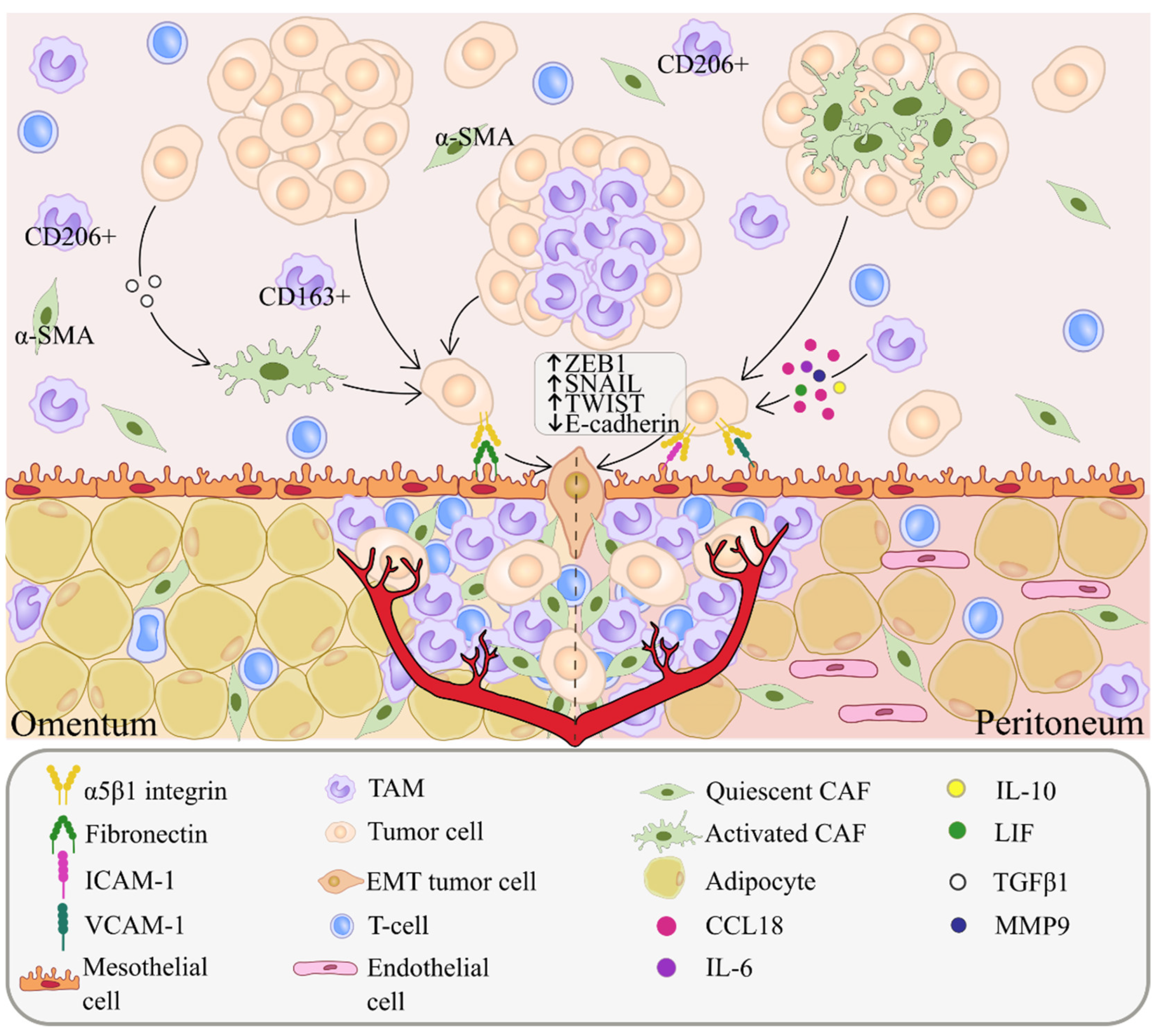
3. Peritoneal Metastasis
4. Cancer-Associated Fibroblasts (CAFs)
5. Tumor-Associated Macrophages (TAMs)
6. T Cells
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | Alpha-Smooth Muscle Actin |
| CAF | cancer-associated fibroblast |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| EMT | epithelial-mesenchymal transition |
| HGSOC | high grade serous ovarian carcinoma |
| MA | malignant ascites |
| MET | mesenchymal-epithelial transition |
| MS | milky spot |
| OC | ovarian cancer |
| OS | overall survival |
| PFS | progression-free survival |
| TAM | tumor-associated macrophage |
| TME | tumor microenvironment |
| VEGF | vascular endothelial growth factor |
References
- Kuroki, L.; Guntupalli, S.R. Treatment of Epithelial Ovarian Cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [Green Version]
- Radu, M.R.; Prădatu, A.; Duică, F.; Micu, R.; Creţoiu, S.M.; Suciu, N.; Creţoiu, D.; Varlas, V.N.; Rădoi, V.E. Ovarian Cancer: Biomarkers and Targeted Therapy. Biomedicines 2021, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Conrad, C.; Sorrin, A.J.; Ruhi, M.K.; Reader, J.C.; Huang, S.A.; Franco, W.; Scarcelli, G.; Polacheck, W.J.; Roque, D.M.; et al. Malignant Ascites in Ovarian Cancer: Cellular, Acellular, and Biophysical Determinants of Molecular Characteristics and Therapy Response. Cancers 2021, 13, 4318. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Bast, R.C.; Han, C.Y.; Lu, Z.; Lu, K.H. Next Steps in the Early Detection of Ovarian Cancer. Commun. Med. 2021, 1, 36. [Google Scholar] [CrossRef]
- Henderson, J.T.; Webber, E.M.; Sawaya, G.F. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial Ovarian Cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Yeung, T.-L.; Leung, C.S.; Yip, K.-P.; Au Yeung, C.L.; Wong, S.T.C.; Mok, S.C. Cellular and Molecular Processes in Ovarian Cancer Metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol. Physiol. 2015, 309, C444–C456. [Google Scholar] [CrossRef] [Green Version]
- Penet, M.F.; Krishnamachary, B.; Wildes, F.B.; Mironchik, Y.; Hung, C.F.; Wu, T.C.; Bhujwalla, Z.M. Ascites Volumes and the Ovarian Cancer Microenvironment. Front. Oncol. 2018, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Thomakos, N.; Diakosavvas, M.; Machairiotis, N.; Fasoulakis, Z.; Zarogoulidis, P.; Rodolakis, A. Rare Distant Metastatic Disease of Ovarian and Peritoneal Carcinomatosis: A Review of the Literature. Cancers 2019, 11, 1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.-L.; Xia, H.H.-X.; Zhu, S.-L. Ascitic Fluid Analysis in the Differential Diagnosis of Ascites: Focus on Cirrhotic Ascites. J. Clin. Transl. Hepatol. 2014, 2, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Li, Y.J.; Lan, C.Y.; Huang, Q.D.; Feng, Y.L.; Huang, Y.W.; Liu, J.H. Clinical Significance of Ascites in Epithelial Ovarian Cancer. Neoplasma 2013, 60, 546–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The Untapped Potential of Ascites in Ovarian Cancer Research and Treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.; Kazakova, E.; Gerashchenko, T.; Kzhyshkowska, J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers 2021, 13, 3253. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Guiral, D.; Hübner, M.; Alyami, M.; Bhatt, A.; Ceelen, W.; Glehen, O.; Lordick, F.; Ramsay, R.; Sgarbura, O.; Van Der Speeten, K.; et al. Primary and Metastatic Peritoneal Surface Malignancies. Nat. Rev. Dis. Prim. 2021, 7, 91. [Google Scholar] [CrossRef]
- Feki, A.; Berardi, P.; Bellingan, G.; Major, A.; Krause, K.-H.; Petignat, P.; Zehra, R.; Pervaiz, S.; Irminger-Finger, I. Dissemination of Intraperitoneal Ovarian Cancer: Discussion of Mechanisms and Demonstration of Lymphatic Spreading in Ovarian Cancer Model. Crit. Rev. Oncol. Hematol. 2009, 72, 1–9. [Google Scholar] [CrossRef]
- Kipps, E.; Tan, D.S.P.; Kaye, S.B. Meeting the Challenge of Ascites in Ovarian Cancer: New Avenues for Therapy and Research. Nat. Rev. Cancer 2013, 13, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Sugarbaker, P.H. Prevention and Treatment of Peritoneal Metastases: A Comprehensive Review. Indian J. Surg. Oncol. 2019, 10, 3–23. [Google Scholar] [CrossRef]
- Nowak, M.; Klink, M. The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells 2020, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.F.; Grimm, A.J.; Chiang, C.L.-L.; Mookerjee, A.; Flies, D.; Jean, S.; McCann, G.A.; Michaux, J.; Pak, H.; Huber, F.; et al. Rapid Tumor Vaccine Using Toll-like Receptor-Activated Ovarian Cancer Ascites Monocytes. J. Immunother. Cancer 2020, 8, e000875. [Google Scholar] [CrossRef] [PubMed]
- Sheid, B. Angiogenic Effects of Macrophages Isolated from Ascitic Fluid Aspirated from Women with Advanced Ovarian Cancer. Cancer Lett. 1992, 62, 153–158. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.; Song, Y.S. Ascites Modulates Cancer Cell Behavior, Contributing to Tumor Heterogeneity in Ovarian Cancer. Cancer Sci. 2016, 107, 1173–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborn, G.; Stavraka, C.; Adams, R.; Sayasneh, A.; Ghosh, S.; Montes, A.; Lacy, K.E.; Kristeleit, R.; Spicer, J.; Josephs, D.H.; et al. Macrophages in Ovarian Cancer and Their Interactions with Monoclonal Antibody Therapies. Clin. Exp. Immunol. 2021, uxab020. [Google Scholar] [CrossRef]
- Steitz, A.M.; Steffes, A.; Finkernagel, F.; Unger, A.; Sommerfeld, L.; Jansen, J.M.; Wagner, U.; Graumann, J.; Müller, R.; Reinartz, S. Tumor-Associated Macrophages Promote Ovarian Cancer Cell Migration by Secreting Transforming Growth Factor Beta Induced (TGFBI) and Tenascin C. Cell Death Dis. 2020, 11, 249. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Kim, J.; Kim, B.; Kim, S.I.; Kim, M.A.; Kwon, S.; Song, Y.S. Evaluating Tumor Evolution via Genomic Profiling of Individual Tumor Spheroids in a Malignant Ascites. Sci. Rep. 2018, 8, 12724. [Google Scholar] [CrossRef]
- Zaman, G.; den Ouden, J.E.; Dylus, J.; van Doornmalen, A.M.; Buijsman, R.C.; Eijkelenboom, A.; Massuger, L.F.; van Altena, A.M. Abstract 2221: Chemotherapy Sensitivity of Tumor Cells from Ascites of Ovarian Cancer Patients: Relationship with Immune Status and Clinical Response. Cancer Res. 2019, 79, 2221. [Google Scholar] [CrossRef]
- Velletri, T.; Villa, C.E.; Cilli, D.; Barzaghi, B.; Lo Riso, P.; Lupia, M.; Luongo, R.; López-Tobón, A.; De Simone, M.; Bonnal, R.J.P.; et al. Single Cell-Derived Spheroids Capture the Self-Renewing Subpopulations of Metastatic Ovarian Cancer. Cell Death Differ. 2022, 29, 614–626. [Google Scholar] [CrossRef]
- Matte, I.; Garde-Granger, P.; Bessette, P.; Piché, A. Serum CA125 and Ascites Leptin Level Ratio Predicts Baseline Clinical Resistance to First-Line Platinum-Based Treatment and Poor Prognosis in Patients with High Grade Serous Ovarian Cancer. Am. J. Cancer Res. 2019, 9, 160–170. [Google Scholar]
- Mo, L.; Pospichalova, V.; Huang, Z.; Murphy, S.K.; Payne, S.; Wang, F.; Kennedy, M.; Cianciolo, G.J.; Bryja, V.; Pizzo, S.V.; et al. Ascites Increases Expression/Function of Multidrug Resistance Proteins in Ovarian Cancer Cells. PLoS ONE 2015, 10, e0131579. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; DeGray, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. In Vitro Microtumors Provide a Physiologically Predictive Tool for Breast Cancer Therapeutic Screening. PLoS ONE 2015, 10, e0123312. [Google Scholar] [CrossRef]
- Piché, A. Malignant Peritoneal Effusion Acting as a Tumor Environment in Ovarian Cancer Progression: Impact and Significance. World J. Clin. Oncol. 2018, 9, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Masoumi Moghaddam, S.; Amini, A.; Morris, D.L.; Pourgholami, M.H. Significance of Vascular Endothelial Growth Factor in Growth and Peritoneal Dissemination of Ovarian Cancer. Cancer Metastasis Rev. 2012, 31, 143–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halkia, E.; Spiliotis, J.; Sugarbaker, P. Diagnosis and Management of Peritoneal Metastases from Ovarian Cancer. Gastroenterol. Res. Pract. 2012, 2012, 541842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; Karaminejadranjbar, M.; Hu, Z.; et al. An Evolving Story of the Metastatic Voyage of Ovarian Cancer Cells: Cellular and Molecular Orchestration of the Adipose-Rich Metastatic Microenvironment. Oncogene 2019, 38, 2885–2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or Permissive for Metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ge, X.; Zhao, Y.; Wang, D.; Ling, L.; Zheng, S.; Ding, K.; Wang, J.; Sun, L. Molecular Alterations in Metastatic Ovarian Cancer from Gastrointestinal Cancer. Front. Oncol. 2020, 10, 605349. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Li, Y. Milky Spots: Omental Functional Units and Hotbeds for Peritoneal Cancer Metastasis. Tumor Biol. 2016, 37, 5715–5726. [Google Scholar] [CrossRef] [Green Version]
- Sacchi, G.; Di Paolo, N.; Venezia, F.; Rossi, A.; Nicolai, G.A.; Garosi, G. Possible Role of Milky Spots in Mesothelial Transplantation. Int. J. Artif. Organs 2007, 30, 520–526. [Google Scholar] [CrossRef]
- Collins, D.; Hogan, A.M.; O’Shea, D.; Winter, D.C. The Omentum: Anatomical, Metabolic, and Surgical Aspects. J. Gastrointest. Surg. 2009, 13, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Krishnan, V.; Schoof, M.; Rodriguez, I.; Theriault, B.; Chekmareva, M.; Rinker-Schaeffer, C. Milky Spots Promote Ovarian Cancer Metastatic Colonization of Peritoneal Adipose in Experimental Models. Am. J. Pathol. 2013, 183, 576–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, V.; Stadick, N.; Clark, R.; Bainer, R.; Veneris, J.T.; Khan, S.; Drew, A.; Rinker-Schaeffer, C. Using MKK4’s Metastasis Suppressor Function to Identify and Dissect Cancer Cell-Microenvironment Interactions during Metastatic Colonization. Cancer Metastasis Rev. 2012, 31, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Funk, H.M.; Thiolloy, S.; Lotan, T.L.; Hickson, J.; Prins, G.S.; Drew, A.F.; Rinker-Schaeffer, C.W. In Vitro Metastatic Colonization of Human Ovarian Cancer Cells to the Omentum. Clin. Exp. Metastasis 2010, 27, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Krist, L.F.; Kerremans, M.; Broekhuis-Fluitsma, D.M.; Eestermans, I.L.; Meyer, S.; Beelen, R.H. Milky Spots in the Greater Omentum Are Predominant Sites of Local Tumour Cell Proliferation and Accumulation in the Peritoneal Cavity. Cancer Immunol. Immunother. 1998, 47, 205–212. [Google Scholar] [CrossRef]
- Adeshakin, F.O.; Adeshakin, A.O.; Afolabi, L.O.; Yan, D.; Zhang, G.; Wan, X. Mechanisms for Modulating Anoikis Resistance in Cancer and the Relevance of Metabolic Reprogramming. Front. Oncol. 2021, 11, 626577. [Google Scholar] [CrossRef]
- Van Baal, J.O.A.M.; van Noorden, C.J.F.; Nieuwland, R.; Van de Vijver, K.K.; Sturk, A.; van Driel, W.J.; Kenter, G.G.; Lok, C.A.R. Development of Peritoneal Carcinomatosis in Epithelial Ovarian Cancer: A Review. J. Histochem. Cytochem. 2017, 66, 67–83. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the Role of Sialylation in Cancer Progression and Metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef]
- Yabushita, H.; Shimazu, M.; Noguchi, M.; Kishida, T.; Narumiya, H.; Sawaguchi, K.; Noguchi, M. Vascular Endothelial Growth Factor Activating Matrix Metalloproteinase in Ascitic Fluid during Peritoneal Dissemination of Ovarian Cancer. Oncol. Rep. 2003, 10, 89–95. [Google Scholar] [CrossRef]
- Wieser, V.; Marth, C. Resistance to Chemotherapy and Anti-Angiogenic Therapy in Ovarian Cancer. Memo–Mag. Eur. Med. Oncol. 2019, 12, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Loges, S.; Schmidt, T.; Carmeliet, P. Mechanisms of Resistance to Anti-Angiogenic Therapy and Development of Third-Generation Anti-Angiogenic Drug Candidates. Genes Cancer 2010, 1, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Ferriss, J.S.; Java, J.J.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Walker, J.L.; Homesley, H.D.; Fowler, J.; Greer, B.E.; Boente, M.P.; et al. Ascites Predicts Treatment Benefit of Bevacizumab in Front-Line Therapy of Advanced Epithelial Ovarian, Fallopian Tube and Peritoneal Cancers: An NRG Oncology/GOG Study. Gynecol. Oncol. 2015, 139, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachana, S.-P.; Pilalis, E.; Gavalas, N.G.; Tzannis, K.; Papadodima, O.; Liontos, M.; Rodolakis, A.; Vlachos, G.; Thomakos, N.; Haidopoulos, D.; et al. The Development of an Angiogenic Protein “Signature” in Ovarian Cancer Ascites as a Tool for Biologic and Prognostic Profiling. PLoS ONE 2016, 11, e0156403. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Huang, B.; Huang, Z.; Cai, J.; Gong, L.; Zhang, Y.; Jiang, J.; Dong, W.; Wang, Z. Tumor Cell-fibroblast Heterotypic Aggregates in Malignant Ascites of Patients with Ovarian Cancer. Int. J. Mol. Med. 2019, 44, 2245–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Li, W.-J.; Weng, J.-J.; Chen, Z.-J.; Wen, Y.-Y.; Deng, T.; Le, H.-B.; Zhang, Y.-K.; Zhang, B.-J. Cancer-Associated Fibroblasts, Matrix Metalloproteinase-9 and Lymphatic Vessel Density Are Associated with Progression from Adenocarcinoma in Situ to Invasive Adenocarcinoma of the Lung. Oncol. Lett. 2020, 20, 130. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zheng, S.; Zhang, T.; Wu, J.; Sun, Y.; Zhang, J.; Liu, G. Role of Exosomes in the Immune Microenvironment of Ovarian Cancer (Review). Oncol. Lett. 2021, 21, 377. [Google Scholar] [CrossRef]
- Xie, J.; Qi, X.; Wang, Y.; Yin, X.; Xu, W.; Han, S.; Cai, Y.; Han, W. Cancer-associated Fibroblasts Secrete Hypoxia-induced Serglycin to Promote Head and Neck Squamous Cell Carcinoma Tumor Cell Growth in Vitro and in Vivo by Activating the Wnt/β-Catenin Pathway. Cell. Oncol. 2021, 44, 661–671. [Google Scholar] [CrossRef]
- Fullár, A.; Dudás, J.; Oláh, L.; Hollósi, P.; Papp, Z.; Sobel, G.; Karászi, K.; Paku, S.; Baghy, K.; Kovalszky, I. Remodeling of Extracellular Matrix by Normal and Tumor-Associated Fibroblasts Promotes Cervical Cancer Progression. BMC Cancer 2015, 15, 256. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, B.; Webb, D.J. Cancer-Associated Fibroblasts Modulate Growth Factor Signaling and Extracellular Matrix Remodeling to Regulate Tumor Metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Smid, M.; Iaria, J.; Salvatori, D.C.F.; van Dam, H.; Zhu, H.J.; Martens, J.W.M.; Ten Dijke, P. Cancer-Associated Fibroblast-Derived Gremlin 1 Promotes Breast Cancer Progression. Breast Cancer Res. 2019, 21, 109. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Kobayashi, H.; Mizutani, Y.; Hara, A.; Iida, T.; Miyai, Y.; Asai, N.; Enomoto, A. Roles of the Mesenchymal Stromal/Stem Cell Marker Meflin/Islr in Cancer Fibrosis. Front. Cell Dev. Biol. 2021, 9, 749924. [Google Scholar] [CrossRef] [PubMed]
- Attieh, Y.; Clark, A.G.; Grass, C.; Richon, S.; Pocard, M.; Mariani, P.; Elkhatib, N.; Betz, T.; Gurchenkov, B.; Vignjevic, D.M. Cancer-Associated Fibroblasts Lead Tumor Invasion through Integrin-Β3-Dependent Fibronectin Assembly. J. Cell Biol. 2017, 216, 3509–3520. [Google Scholar] [CrossRef] [Green Version]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, C.H.; Sandoval, P.; Muñoz-Hernández, P.; Pascual-Antón, L.; López-Cabrera, M.; Jiménez-Heffernan, J.A. Mesothelial-to-Mesenchymal Transition Contributes to the Generation of Carcinoma-Associated Fibroblasts in Locally Advanced Primary Colorectal Carcinomas. Cancers 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matte, I.; Legault, C.M.; Garde-Granger, P.; Laplante, C.; Bessette, P.; Rancourt, C.; Piché, A. Mesothelial Cells Interact with Tumor Cells for the Formation of Ovarian Cancer Multicellular Spheroids in Peritoneal Effusions. Clin. Exp. Metastasis 2016, 33, 839–852. [Google Scholar] [CrossRef]
- Capellero, S.; Erriquez, J.; Battistini, C.; Porporato, R.; Scotto, G.; Borella, F.; Di Renzo, M.F.; Valabrega, G.; Olivero, M. Ovarian Cancer Cells in Ascites Form Aggregates That Display a Hybrid Epithelial-Mesenchymal Phenotype and Allows Survival and Proliferation of Metastasizing Cells. Int. J. Mol. Sci. 2022, 23, 833. [Google Scholar] [CrossRef]
- Wintzell, M.; Hjerpe, E.; Åvall Lundqvist, E.; Shoshan, M. Protein Markers of Cancer-Associated Fibroblasts and Tumor-Initiating Cells Reveal Subpopulations in Freshly Isolated Ovarian Cancer Ascites. BMC Cancer 2012, 12, 359. [Google Scholar] [CrossRef] [Green Version]
- Izar, B.; Tirosh, I.; Stover, E.H.; Wakiro, I.; Cuoco, M.S.; Alter, I.; Rodman, C.; Leeson, R.; Su, M.-J.; Shah, P.; et al. A Single-Cell Landscape of High-Grade Serous Ovarian Cancer. Nat. Med. 2020, 26, 1271–1279. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-Tumor Spheroids Promote Early Peritoneal Metastasis of Ovarian Cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef] [Green Version]
- Winter, S.J.; Miller, H.A.; Steinbach-Rankins, J.M. Multicellular Ovarian Cancer Model for Evaluation of Nanovector Delivery in Ascites and Metastatic Environments. Pharmaceutics 2021, 13, 1891. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, F.H.C.; Tedrow, J.; Chang, W.; Zhang, C.; Mitra, A.K. Modulation of Immune Infiltration of Ovarian Cancer Tumor Microenvironment by Specific Subpopulations of Fibroblasts. Cancers 2020, 12, 3184. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, H. Fibroblasts: Dangerous Travel Companions. J. Exp. Med. 2019, 216, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Tang, H.; Xu, L.; Wang, X.; Yang, C.; Ruan, S.; Guo, J.; Hu, S.; Wang, Z. Fibroblasts in Omentum Activated by Tumor Cells Promote Ovarian Cancer Growth, Adhesion and Invasiveness. Carcinogenesis 2012, 33, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact Spheroid Formation by Ovarian Cancer Cells Is Associated with Contractile Behavior and an Invasive Phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A Mechanically Active Heterotypic E-Cadherin/N-Cadherin Adhesion Enables Fibroblasts to Drive Cancer Cell Invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef]
- Xu, S.; Yang, Y.; Dong, L.; Qiu, W.; Yang, L.; Wang, X.; Liu, L. Construction and Characteristics of an E-Cadherin-Related Three-Dimensional Suspension Growth Model of Ovarian Cancer. Sci. Rep. 2014, 4, 5646. [Google Scholar] [CrossRef] [Green Version]
- Mikuła-Pietrasik, J.; Uruski, P.; Tykarski, A.; Książek, K. The Peritoneal “Soil” for a Cancerous “Seed”: A Comprehensive Review of the Pathogenesis of Intraperitoneal Cancer Metastases. Cell. Mol. Life Sci. 2018, 75, 509–525. [Google Scholar] [CrossRef]
- Feng, W.; Dean, D.C.; Hornicek, F.J.; Shi, H.; Duan, Z. Exosomes Promote Pre-Metastatic Niche Formation in Ovarian Cancer. Mol. Cancer 2019, 18, 124. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, C.; Chang, X.; Qi, Y.; Zhu, Z.; Yang, X. Fibrosis of Mesothelial Cell-Induced Peritoneal Implantation of Ovarian Cancer Cells. Cancer Manag. Res. 2018, 10, 6641–6647. [Google Scholar] [CrossRef] [Green Version]
- Uruski, P.; Mikuła-Pietrasik, J.; Pakuła, M.; Budkiewicz, S.; Drzewiecki, M.; Gaiday, A.N.; Wierzowiecka, M.; Naumowicz, E.; Moszyński, R.; Tykarski, A.; et al. Malignant Ascites Promote Adhesion of Ovarian Cancer Cells to Peritoneal Mesothelium and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 4222. [Google Scholar] [CrossRef]
- Gupta, V.; Yull, F.; Khabele, D. Bipolar Tumor-Associated Macrophages in Ovarian Cancer as Targets for Therapy. Cancers 2018, 10, 366. [Google Scholar] [CrossRef] [Green Version]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, K.; Komohara, Y.; Tashiro, H.; Ohtake, H.; Nakagawa, T.; Katabuchi, H.; Takeya, M. Involvement of M2-Polarized Macrophages in the Ascites from Advanced Epithelial Ovarian Carcinoma in Tumor Progression via Stat3 Activation. Cancer Sci. 2010, 101, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-Associated Macrophages Drive Spheroid Formation during Early Transcoelomic Metastasis of Ovarian Cancer. J. Clin. Investig. 2016, 126, 4157–4173. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Hu, Y.; Long, T.; Lu, X.; Tuo, Y.; Li, Y.; Ke, Z. Tumor-Associated Macrophages Induced Spheroid Formation by CCL18-ZEB1-M-CSF Feedback Loop to Promote Transcoelomic Metastasis of Ovarian Cancer. J. Immunother. Cancer 2021, 9, e003973. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Shen, J.; Yu, S.; Fei, J.; Zhu, X.; Zhao, J.; Zhai, L.; Sadhukhan, A.; Zhou, J. Tumor-Associated Macrophages (TAMs): A Critical Activator in Ovarian Cancer Metastasis. Oncol. Targets. Ther. 2019, 12, 8687–8699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibault, B.; Castells, M.; Delord, J.-P.; Couderc, B. Ovarian Cancer Microenvironment: Implications for Cancer Dissemination and Chemoresistance Acquisition. Cancer Metastasis Rev. 2014, 33, 17–39. [Google Scholar] [CrossRef]
- Moughon, D.L.; He, H.; Schokrpur, S.; Jiang, Z.K.; Yaqoob, M.; David, J.; Lin, C.; Iruela-Arispe, M.L.; Dorigo, O.; Wu, L. Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer. Cancer Res. 2015, 75, 4742–4752. [Google Scholar] [CrossRef] [Green Version]
- Yin, M.; Zhou, H.J.; Zhang, J.; Lin, C.; Li, H.; Li, X.; Li, Y.; Zhang, H.; Breckenridge, D.G.; Ji, W.; et al. ASK1-Dependent Endothelial Cell Activation Is Critical in Ovarian Cancer Growth and Metastasis. JCI Insight 2017, 2, e91828. [Google Scholar] [CrossRef] [Green Version]
- Duluc, D.; Delneste, Y.; Tan, F.; Moles, M.-P.; Grimaud, L.; Lenoir, J.; Preisser, L.; Anegon, I.; Catala, L.; Ifrah, N.; et al. Tumor-Associated Leukemia Inhibitory Factor and IL-6 Skew Monocyte Differentiation into Tumor-Associated Macrophage-like Cells. Blood 2007, 110, 4319–4330. [Google Scholar] [CrossRef]
- Reinartz, S.; Finkernagel, F.; Adhikary, T.; Rohnalter, V.; Schumann, T.; Schober, Y.; Nockher, W.A.; Nist, A.; Stiewe, T.; Jansen, J.M.; et al. A Transcriptome-Based Global Map of Signaling Pathways in the Ovarian Cancer Microenvironment Associated with Clinical Outcome. Genome Biol. 2016, 17, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutyser, E.; Struyf, S.; Proost, P.; Opdenakker, G.; Laureys, G.; Verhasselt, B.; Peperstraete, L.; Van de Putte, I.; Saccani, A.; Allavena, P.; et al. Identification of Biologically Active Chemokine Isoforms from Ascitic Fluid and Elevated Levels of CCL18/Pulmonary and Activation-Regulated Chemokine in Ovarian Carcinoma. J. Biol. Chem. 2002, 277, 24584–24593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbecki, J.; Olbromski, M.; Dzięgiel, P. CCL18 in the Progression of Cancer. Int. J. Mol. Sci. 2020, 21, 7955. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Matte, I.; Laplante, C.; Garde-Granger, P.; Carignan, A.; Bessette, P.; Rancourt, C.; Piché, A. CCL18 from Ascites Promotes Ovarian Cancer Cell Migration through Proline-Rich Tyrosine Kinase 2 Signaling. Mol. Cancer 2016, 15, 58. [Google Scholar] [CrossRef] [Green Version]
- Macciò, A.; Gramignano, G.; Cherchi, M.C.; Tanca, L.; Melis, L.; Madeddu, C. Role of M1-Polarized Tumor-Associated Macrophages in the Prognosis of Advanced Ovarian Cancer Patients. Sci. Rep. 2020, 10, 6096. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, T.; Wortmann, A.; Finkernagel, F.; Lieber, S.; Nist, A.; Stiewe, T.; Wagner, U.; Müller-Brüsselbach, S.; Reinartz, S.; Müller, R. Interferon Signaling in Ascites-Associated Macrophages Is Linked to a Favorable Clinical Outcome in a Subgroup of Ovarian Carcinoma Patients. BMC Genom. 2017, 18, 243. [Google Scholar] [CrossRef] [Green Version]
- Worzfeld, T.; Finkernagel, F.; Reinartz, S.; Konzer, A.; Adhikary, T.; Nist, A.; Stiewe, T.; Wagner, U.; Looso, M.; Graumann, J.; et al. Proteotranscriptomics Reveal Signaling Networks in the Ovarian Cancer Microenvironment. Mol. Cell. Proteom. 2018, 17, 270–289. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the Immune System in Cancer: From Tumor Initiation to Metastatic Progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kühnel, F.; Woller, N. CD4 and CD8 T Lymphocyte Interplay in Controlling Tumor Growth. Cell. Mol. Life Sci. 2018, 75, 689–713. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.; Yew, P.-Y.; Hasegawa, K.; Ikeda, Y.; Fujiwara, K.; Fleming, G.F.; Nakamura, Y.; Park, J.-H. Characterization of T Cell Repertoire of Blood, Tumor, and Ascites in Ovarian Cancer Patients Using next Generation Sequencing. Oncoimmunology 2015, 4, e1030561. [Google Scholar] [CrossRef] [Green Version]
- Wefers, C.; Duiveman-de Boer, T.; Yigit, R.; Zusterzeel, P.L.M.; van Altena, A.M.; Massuger, L.F.A.G.; De Vries, I.J.M. Survival of Ovarian Cancer Patients Is Independent of the Presence of DC and T Cell Subsets in Ascites. Front. Immunol. 2019, 9, 3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, J.; Chavarria, M.; Lopez, G.E.; Felder, M.A.; Kapur, A.; Romo Chavez, A.; Karst, N.; Barroilhet, L.; Patankar, M.S.; Stanic, A.K. Identification of Unique Clusters of T, Dendritic, and Innate Lymphoid Cells in the Peritoneal Fluid of Ovarian Cancer Patients. Am. J. Reprod. Immunol. 2020, 84, e13284. [Google Scholar] [CrossRef] [PubMed]
- GiuntolI, R.L.; Webb, T.J.; Zoso, A.; Rogers, O.; Diaz-montes, T.P.; Bristow, R.E.; Oelke, M. Ovarian Cancer-Associated Ascites Demonstrates Altered Immune Environment: Implications for Antitumor Immunity. Anticancer Res. 2009, 29, 2875–2884. [Google Scholar] [PubMed]
- Lieber, S.; Reinartz, S.; Raifer, H.; Finkernagel, F.; Dreyer, T.; Bronger, H.; Jansen, J.M.; Wagner, U.; Worzfeld, T.; Müller, R.; et al. Prognosis of Ovarian Cancer Is Associated with Effector Memory CD8+ T Cell Accumulation in Ascites, CXCL9 Levels and Activation-Triggered Signal Transduction in T Cells. Oncoimmunology 2018, 7, e1424672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific Recruitment of Regulatory T Cells in Ovarian Carcinoma Fosters Immune Privilege and Predicts Reduced Survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Idorn, M.; Olsen, M.; Halldórsdóttir, H.R.; Skadborg, S.K.; Pedersen, M.; Høgdall, C.; Høgdall, E.; Met, Ö.; Thor Straten, P. Improved Migration of Tumor Ascites Lymphocytes to Ovarian Cancer Microenvironment by CXCR2 Transduction. Oncoimmunology 2017, 7, e1412029. [Google Scholar] [CrossRef]
- Dutsch-Wicherek, M.M.; Szubert, S.; Dziobek, K.; Wisniewski, M.; Lukaszewska, E.; Wicherek, L.; Jozwicki, W.; Rokita, W.; Koper, K. Analysis of the Treg Cell Population in the Peripheral Blood of Ovarian Cancer Patients in Relation to the Long-Term Outcomes. Ginekol. Pol. 2019, 90, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Landskron, J.; Helland, Ø.; Torgersen, K.M.; Aandahl, E.M.; Gjertsen, B.T.; Bjørge, L.; Taskén, K. Activated Regulatory and Memory T-Cells Accumulate in Malignant Ascites from Ovarian Carcinoma Patients. Cancer Immunol. Immunother. 2015, 64, 337–347. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Interleukin 6 Present in Inflammatory Ascites from Advanced Epithelial Ovarian Cancer Patients Promotes Tumor Necrosis Factor Receptor 2-Expressing Regulatory T Cells. Front. Immunol. 2017, 8, 1482. [Google Scholar] [CrossRef]
- Wefers, C.; Duiveman-de Boer, T.; Zusterzeel, P.L.M.; Massuger, L.F.A.G.; Fuchs, D.; Torensma, R.; Wheelock, C.E.; de Vries, I.J.M. Different Lipid Regulation in Ovarian Cancer: Inhibition of the Immune System. Int. J. Mol. Sci. 2018, 19, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, E.; Nielsen, J.S.; Wick, D.A.; Ng, A.V.; Johnson, L.D.S.; Nesslinger, N.J.; McMurtrie, E.; Webb, J.R.; Nelson, B.H. Polyfunctional T-Cell Responses Are Disrupted by the Ovarian Cancer Ascites Environment and Only Partially Restored by Clinically Relevant Cytokines. PLoS ONE 2010, 5, e15625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Sandoval, T.A.; Chae, C.-S.; Chopra, S.; Tan, C.; Rutkowski, M.R.; Raundhal, M.; Chaurio, R.A.; Payne, K.K.; Konrad, C.; et al. IRE1α-XBP1 Controls T Cell Function in Ovarian Cancer by Regulating Mitochondrial Activity. Nature 2018, 562, 423–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakina, M.; Kazakova, A.; Villert, A.; Kolomiets, L.; Larionova, I. Spheroid Formation and Peritoneal Metastasis in Ovarian Cancer: The Role of Stromal and Immune Components. Int. J. Mol. Sci. 2022, 23, 6215. https://doi.org/10.3390/ijms23116215
Rakina M, Kazakova A, Villert A, Kolomiets L, Larionova I. Spheroid Formation and Peritoneal Metastasis in Ovarian Cancer: The Role of Stromal and Immune Components. International Journal of Molecular Sciences. 2022; 23(11):6215. https://doi.org/10.3390/ijms23116215
Chicago/Turabian StyleRakina, Militsa, Anna Kazakova, Alisa Villert, Larisa Kolomiets, and Irina Larionova. 2022. "Spheroid Formation and Peritoneal Metastasis in Ovarian Cancer: The Role of Stromal and Immune Components" International Journal of Molecular Sciences 23, no. 11: 6215. https://doi.org/10.3390/ijms23116215





