Selenium Antagonizes Cadmium-Induced Inflammation and Oxidative Stress via Suppressing the Interplay between NLRP3 Inflammasome and HMGB1/NF-κB Pathway in Duck Hepatocytes
Abstract
:1. Introduction
2. Results
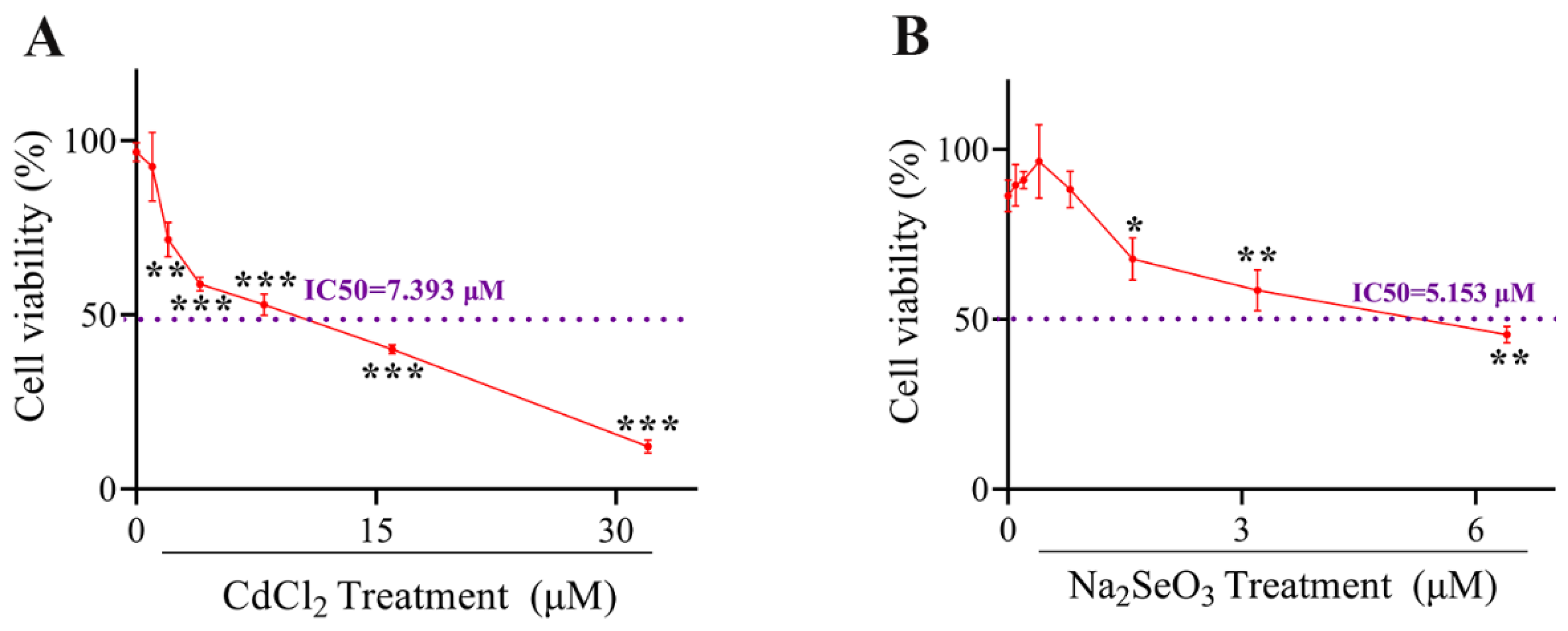
2.1. Selection of Cd and Se Dosage
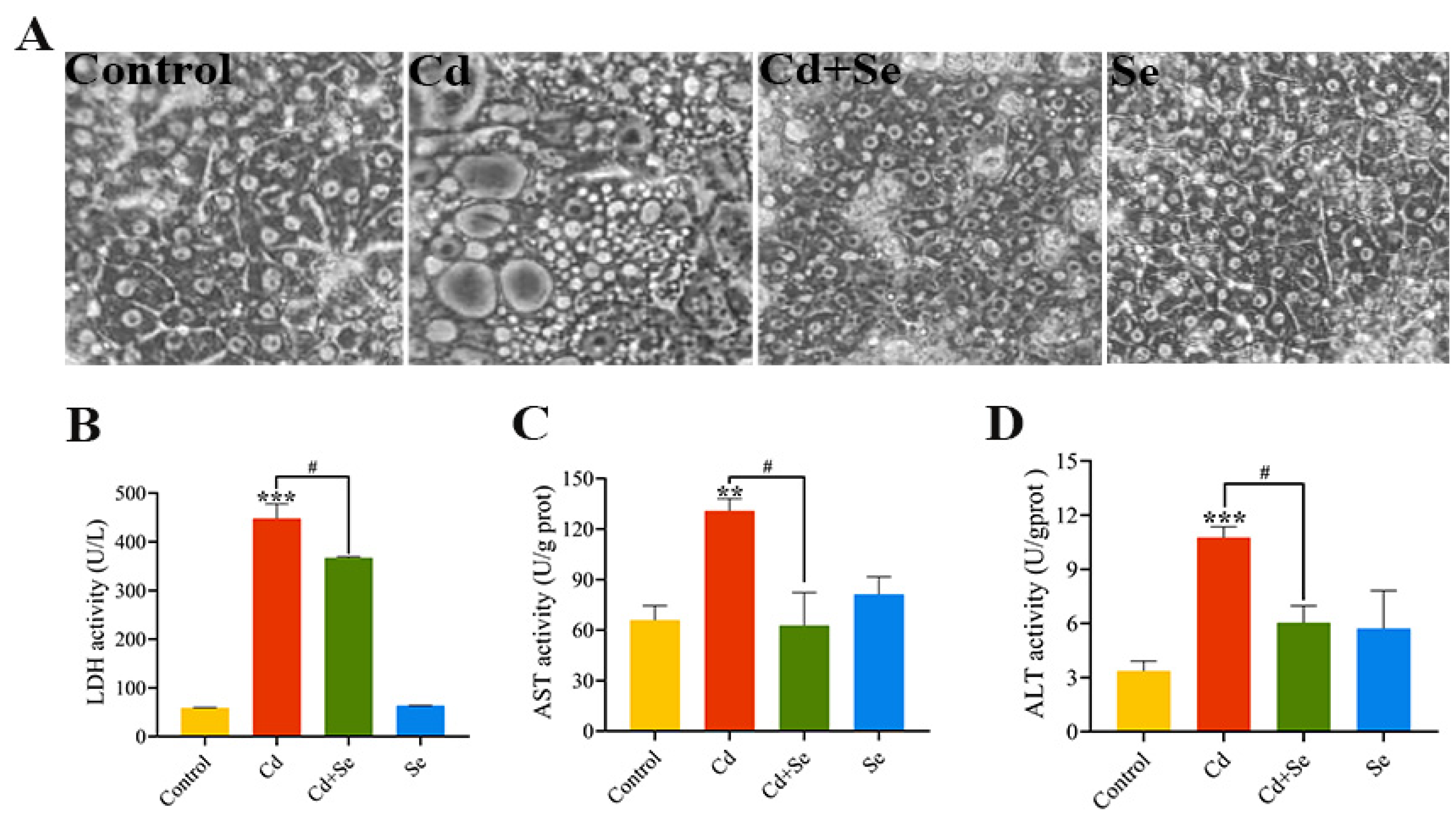
2.2. Se Alleviates Cd-Induced Hepatocytes Injury
2.3. Se Suppresses Cd-Stimulated Increases in ALT, AST and LDH Release from Hepatocytes
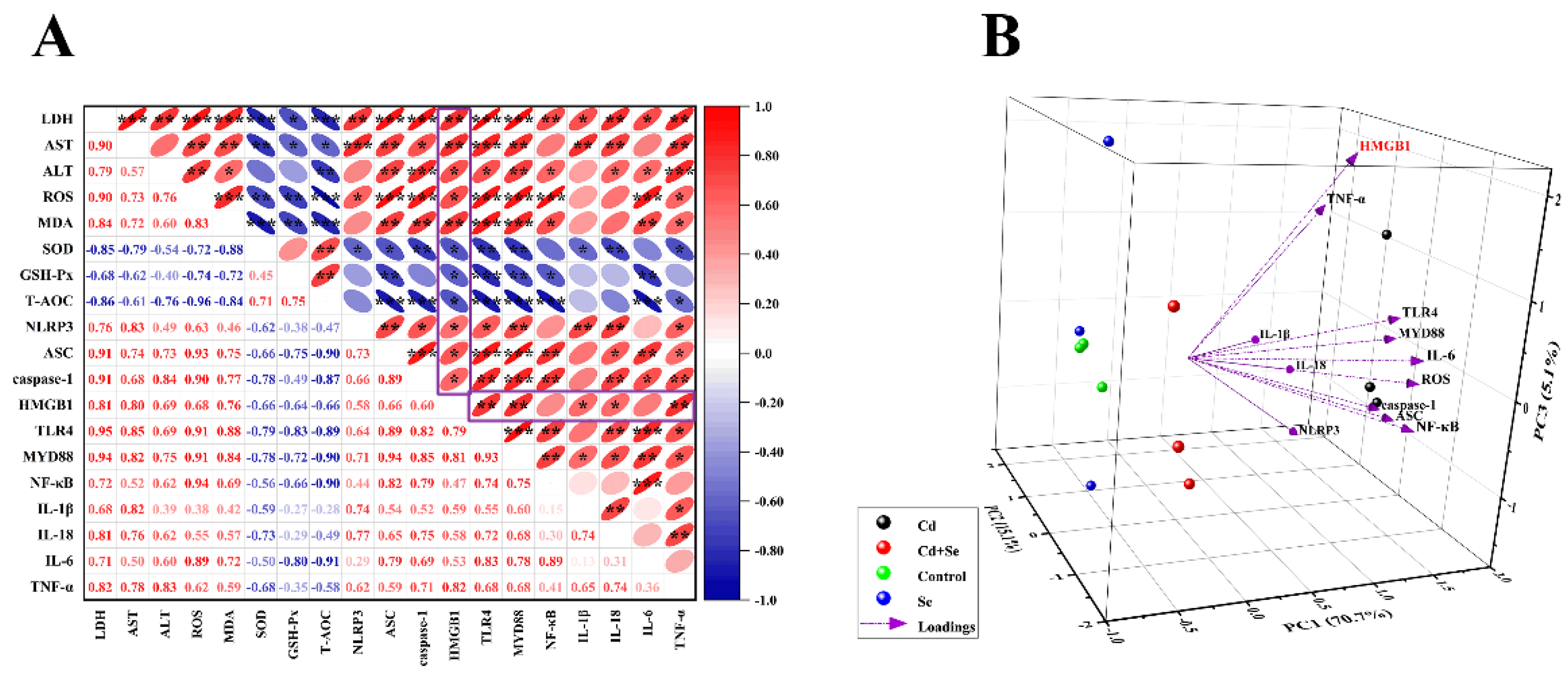
2.4. Se Relieves Cd-Induced Oxidative Stress and Inflammation of Hepatocyte
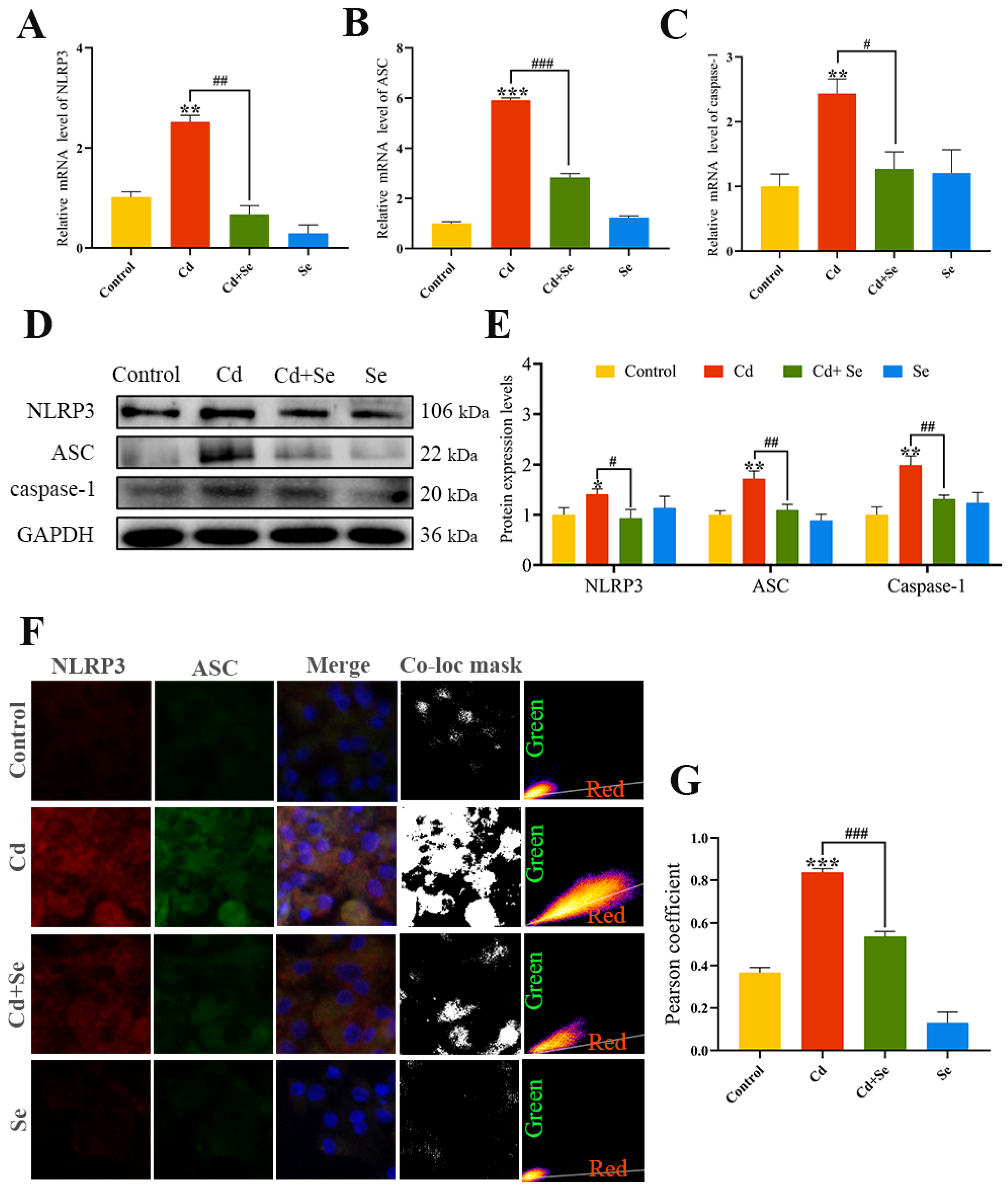
2.5. Se Represses Cd-Induced Activation of NLRP3 Inflammasomes
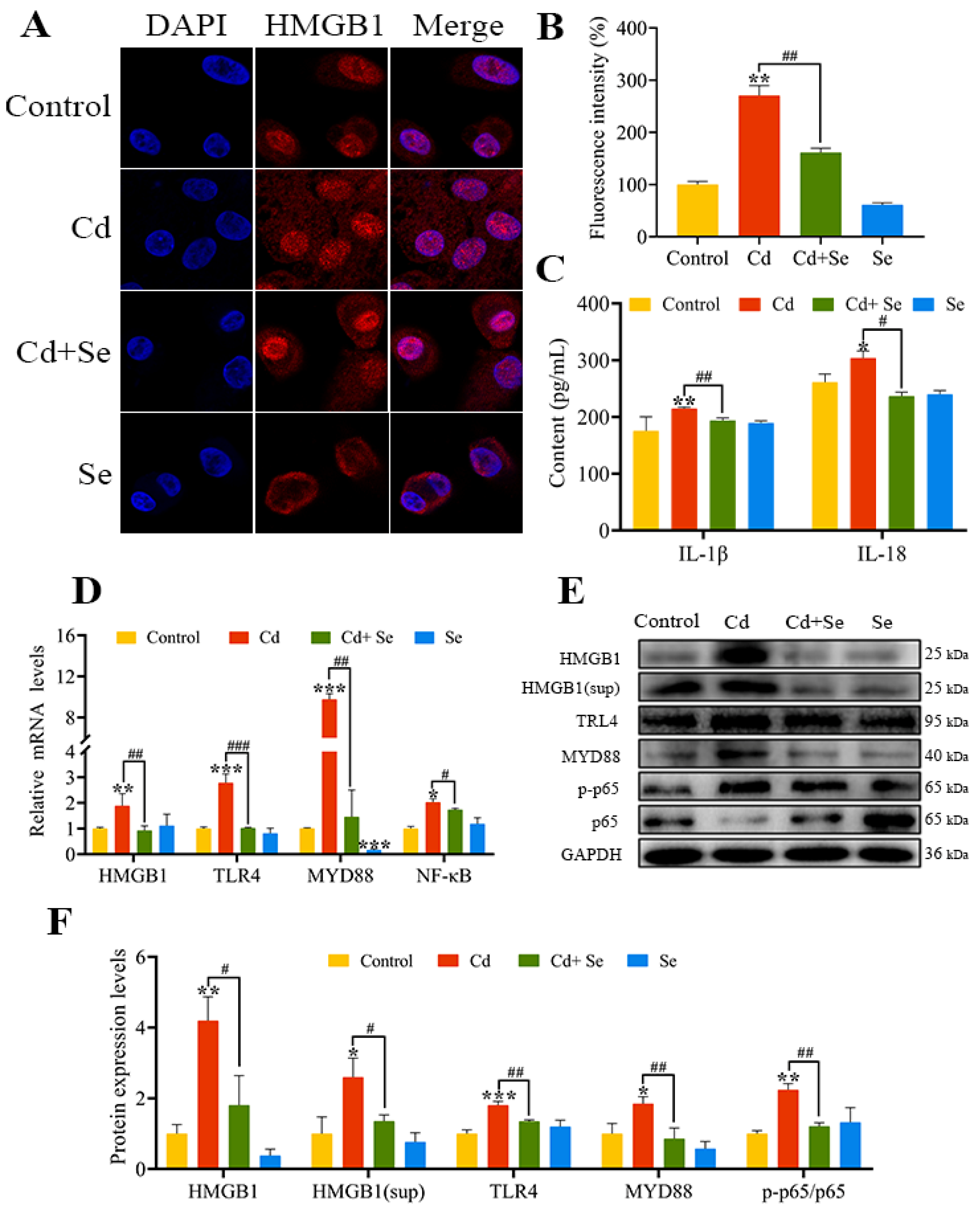
2.6. Se Curbs Cd-Caused Activation of HMGB1/NF-κB Signaling Pathway in Hepatocytes
2.7. HMGB1 Exerts a Vital Role in Se Restraining Cd-Induced Oxidative Stress and Inflammation of Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Cells Isolation and Cells Culture
4.2. Cells Viability Assay
4.3. Determination of LDH Release
4.4. Assay of Transaminase Index
4.5. Measurement of Antioxidant Function Index
4.6. Detection of Intracellular ROS
4.7. Immunofluorescence Staining
4.8. Colocalization Analysis of NLRP3 and ASC
4.9. Preparation of Culture Supernatant
4.10. Enzyme-Linked Immunosorbent Measure (ELISA)
4.11. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.12. Western Blotting Analyses
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Z.; Chen, S.; Han, X.; Zhang, J.; Qiao, G.; Jiang, Y.; Zhuo, R.; Qiu, W. A Single Amino Acid Change in Nramp6 from Sedum Alfredii Hance Affects Cadmium Accumulation. Int. J. Mol. Sci. 2020, 21, 3169. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yang, Y.; Deng, R.; Gong, X.; Zhou, W.; Chen, S.; Li, B.; Wang, G. Remediation of Cd-Contaminated Soil by Modified Nanoscale Zero-Valent Iron: Role of Plant Root Exudates and Inner Mechanisms. Int. J. Environ. Res. Public Health 2021, 18, 5887. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. In Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. J. Int. Assoc. Geochem. Cosmochem. 2019, 108, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sena, A.; Ebi, K. When Land Is Under Pressure Health Is Under Stress. Int. J. Environ. Res. Public Health 2020, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Eisa, R.A.; El-Shenawy, N.S. Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats. Biology 2022, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Yu, K.; Zeng, W.; Lian, G. Protective roles of Pyracantha fortuneana extract on acute renal toxicity induced by cadmium chloride in rats. Acta Cir. Bras. 2019, 34, e201900706. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Boursereau, R.; Abou-Samra, M.; Lecompte, S.; Noel, L.; Brichard, S.M. Downregulation of the NLRP3 inflammasome by adiponectin rescues Duchenne muscular dystrophy. BMC Biol. 2018, 16, 33. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Guo, W.; Hang, N.; Yang, Y.; Wu, X.; Shen, Y.; Cao, J.; Sun, Y.; Xu, Q. MALT1 inhibitors prevent the development of DSS-induced experimental colitis in mice via inhibiting NF-κB and NLRP3 inflammasome activation. Oncotarget 2016, 7, 30536–30549. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [Green Version]
- Ka, S.M.; Lin, J.C.; Lin, T.J.; Liu, F.C.; Chao, L.K.; Ho, C.L.; Yeh, L.T.; Sytwu, H.K.; Hua, K.F.; Chen, A. Citral alleviates an accelerated and severe lupus nephritis model by inhibiting the activation signal of NLRP3 inflammasome and enhancing Nrf2 activation. Arthritis Res. Ther. 2015, 17, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, J.K.; O’Neill, L.A. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biology. 2012, 47, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The inflammasome: An integrated view. Immunol. Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Cai, D.; Li, P.; Jin, J.; Jiang, X.; Li, Z.; Tian, L.; Chen, G.; Sun, J.; et al. Chronic oral exposure to cadmium causes liver inflammation by NLRP3 inflammasome activation in pubertal mice. Food Chem. Toxicol. 2021, 148, 111944. [Google Scholar] [CrossRef]
- Allam, N.G.; Ali, E.M.M.; Shabanna, S.; Abd-Elrahman, E. Protective Efficacy of Streptococcus Thermophilus Against Acute Cadmium Toxicity in Mice. Iran. J. Pharm. Res. 2018, 17, 695–707. [Google Scholar]
- Walle, L.V.; Kanneganti, T.D.; Lamkanfi, M. HMGB1 release by inflammasomes. Virulence 2011, 2, 162–165. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wu, S.; Chen, C.; Xie, B.; Fang, Z.; Hu, W.; Chen, J.; Fu, H.; He, H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2017, 14, 143. [Google Scholar] [CrossRef]
- Doz, E.; Noulin, N.; Boichot, E.; Guénon, I.; Fick, L.; le Bert, M.; Lagente, V.; Ryffel, B.; Schnyder, B.; Quesniaux, V.F.; et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J. Immunol. 2008, 180, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, R.; Galvez, B.G.; Pusterla, T.; de Marchis, F.; Cossu, G.; Marcu, K.B.; Bianchi, M.E. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J. Cell Biol. 2007, 179, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lu, B.; Fu, J.; Zhu, X.; Song, E.; Song, Y. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells. J. Hazard. Mater. 2021, 404, 124050. [Google Scholar] [CrossRef]
- Wang, C.; Nie, G.; Zhuang, Y.; Hu, R.; Wu, H.; Xing, C.; Li, G.; Hu, G.; Yang, F.; Zhang, C. Inhibition of autophagy enhances cadmium-induced apoptosis in duck renal tubular epithelial cells. Ecotoxicol. Environ. Saf. 2020, 205, 111188. [Google Scholar] [CrossRef] [PubMed]
- Noor, K.K.; Ijaz, M.U.; Ehsan, N.; Tahir, A.; Yeni, D.K.; Zihad, S.M.N.K.; Uddin, S.J.; Ashraf, A.; Simal-Gandara, J. Hepatoprotective role of vitexin against cadmium-induced liver damage in male rats: A biochemical, inflammatory, apoptotic and histopathological investigation. Biomed. Pharmacother. 2022, 150, 112934. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hu, R.; Huang, G.; Pu, W.; Chu, X.; Xing, C.; Zhang, C. Molybdenum and cadmium co-exposure induces endoplasmic reticulum stress-mediated apoptosis by Th1 polarization in Shaoxing duck (Anas platyrhyncha) spleens. Chemosphere 2022, 298, 134275. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Nie, G.; Luo, J.; Hu, R.; Li, G.; Hu, G.; Zhang, C. Molybdenum and Cadmium Co-induce Pyroptosis via Inhibiting Nrf2-Mediated Antioxidant Defense Response in the Brain of Ducks. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Wang, H.; Ward, M.F.; Sama, A.E. Targeting HMGB1 in the treatment of sepsis. Expert Opin. Ther. Targets 2014, 18, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kim, D.C.; Cho, E.S.; Ko, S.O.; Kwon, W.Y.; Suh, G.J.; Shin, H.K. Antioxidant and anti-inflammatory effects of selenium in oral buccal mucosa and small intestinal mucosa during intestinal ischemia-reperfusion injury. J. Inflamm. 2014, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.Y.; Alfulaij, N.; Berry, M.J.; Seale, L.A. From Selenium Absorption to Selenoprotein Degradation. Biol. Trace Elem. Res. 2019, 192, 26–37. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Chang, W.; Chen, R.; Xu, S.; Tao, D. Protective effects of selenium yeast against cadmium-induced necroptosis via inhibition of oxidative stress and MAPK pathway in chicken liver. Ecotoxicol. Environ. Saf. 2020, 206, 111329. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, W.; Zhang, Q.; Li, S. Inhibition of Lipopolysaccharide-Induced Inflammation of Chicken Liver Tissue by Selenomethionine via TLR4-NF-κB-NLRP3 Signaling Pathway. Biol. Trace Elem. Res. 2020, 195, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Aala, J.; Harchegani, A.B.; Monsef, H.A.; Mohsenifar, Z.; Ebrahimi, P.; Parvizi, M.R. N-Acetyl cysteine mitigates histopathological changes and inflammatory genes expressions in the liver of cadmium exposed rats. Environ. Anal. Health Toxicol. 2021, 36, e2021024-0. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, F.; Li, Y.; Cao, H.; Huang, A.; Zhuang, Y.; Zhang, C.; Hu, G.; Mao, Y.; Luo, J.; et al. The protection of selenium against cadmium-induced mitophagy via modulating nuclear xenobiotic receptors response and oxidative stress in the liver of rabbits. Environ. Pollut. 2021, 285, 117301. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ahmad, S.T.; Dayal, R.; Javid, K.; Umar, S.; Asiaf, A.; Nafees, S.; Bhat, J.U.; Wani, A.; Samim, M.; et al. Nephroprotective action of Peucedanum grande against cadmium chloride induced renal toxicity in Wistar rats. EXCLI J. 2012, 11, 444–452. [Google Scholar] [PubMed]
- Zhu, J.; Yu, L.; Shen, X.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice. Int. J. Mol. Sci. 2021, 22, 11045. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Z.; Hu, R.; Pi, S.; Wei, Z.; Wang, C.; Yang, F.; Xing, C.; Nie, G.; Hu, G. New insights into crosstalk between pyroptosis and autophagy co-induced by molybdenum and cadmium in duck renal tubular epithelial cells. J. Hazard. Mater. 2021, 416, 126138. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Sun, Y.; Xing, A.; Wu, Z.; Tian, Z.; Li, X.; Wang, Y. MicroRNA Omics Analysis of Camellia sinesis Pollen Tubes in Response to Low-Temperature and Nitric Oxide. Biomolecules 2021, 11, 930. [Google Scholar] [CrossRef]
- Chen, F.; Hou, L.; Zhu, L.; Yang, C.; Zhu, F.; Qiu, H.; Qin, S. Effects of selenide chitosan sulfate on glutathione system in hepatocytes and specific pathogen-free chickens. Poult. Sci. 2020, 99, 3979–3986. [Google Scholar] [CrossRef]
- Yan, X.S.; Yang, Z.J.; Jia, J.X.; Song, W.; Fang, X.; Cai, Z.P.; Huo, D.S.; Wang, H. Protective mechanism of testosterone on cognitive impairment in a rat model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 649–657. [Google Scholar]
- Cao, P.; Nie, G.; Luo, J.; Hu, R.; Li, G.; Hu, G.; Zhang, C. Cadmium and molybdenum co-induce pyroptosis and apoptosis via the PTEN/PI3K/AKT axis in the livers of Shaoxing ducks (Anas platyrhynchos). Food Funct. 2022, 13, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, X.; Liu, G. The Antagonistic Effect of Selenium on Lead Toxicity Is Related to the Ion Profile in Chicken Liver. Biol. Trace Elem. Res. 2016, 169, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Min, X.; Zhang, S.; Song, C.; Xiong, K. Effect of Heavy Metal Contamination in the Environment on Antioxidant Function in Wumeng Semi-fine Wool Sheep in Southwest China. Biol. Trace Elem. Res. 2020, 198, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Cho, Y.K.; Kang, M.S.; Yoo, T.W.; Park, J.H.; Kim, H.J.; Park, D.I.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; et al. The association between increased alanine aminotransferase activity and metabolic factors in nonalcoholic fatty liver disease. Metab. Clin. Exp. 2006, 55, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Singh, S.; Zhou, P.; Yang, S.; Wang, Y.; Zhu, Z.; Zhang, J.; Chen, A.; Billiar, T.; et al. ADAR1 Prevents Liver Injury from Inflammation and Suppresses Interferon Production in Hepatocytes. Am. J. Pathol. 2015, 185, 3224–3237. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, L.; Khare, S.; Misharin, A.V.; Patel, R.; Ratsimandresy, R.A.; Wallin, M.C.; Perlman, H.; Greaves, D.R.; Hoffman, H.M.; Dorfleutner, A.; et al. The PYRIN Domain-only Protein POP1 Inhibits Inflammasome Assembly and Ameliorates Inflammatory Disease. Immunity 2015, 43, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Jia, J.A.; Li, H.; Wan, N.; Zhang, S.; Ma, X. Lymphocyte-monocyte-neutrophil index: A predictor of severity of coronavirus disease 2019 patients produced by sparse principal component analysis. Virol. J. 2021, 18, 115. [Google Scholar] [CrossRef]
- Yang, F.; Liao, J.; Pei, R.; Yu, W.; Han, Q.; Li, Y.; Guo, J.; Hu, L.; Pan, J.; Tang, Z. Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere 2018, 204, 36–43. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, T.; Nie, G.; Hu, R.; Pi, S.; Wei, Z.; Wang, C.; Li, G.; Hu, G. In vivo assessment of molybdenum and cadmium co-induce nephrotoxicity via causing calcium homeostasis disorder and autophagy in ducks (Anas platyrhyncha). Ecotoxicol. Environ. Saf. 2021, 230, 113099. [Google Scholar] [CrossRef]
- Sharif, H.; Wang, L.; Wang, W.L.; Magupalli, V.G.; Andreeva, L.; Qiao, Q.; Hauenstein, A.V.; Wu, Z.; Núñez, G.; Mao, Y.; et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 2019, 570, 338–343. [Google Scholar] [CrossRef]
- Gritsenko, A.; Green, J.P.; Brough, D.; Lopez-Castejon, G. Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev. 2020, 55, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Hornung, V. The NLRP3 Inflammasome Renders Cell Death Pro-inflammatory. J. Mol. Biol. 2018, 430, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cai, W.; Du, R.; Li, H.; Wang, B.; Zhou, Y.; Shen, D.; Shen, H.; Lan, Y.; Chen, L.; et al. Sevoflurane Alleviates Myocardial Ischemia Reperfusion Injury by Inhibiting P2X7-NLRP3 Mediated Pyroptosis. Front. Mol. Biosci. 2021, 8, 768594. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, C.; Yao, Y.; Li, H.; Yao, Y.; Zhu, Y.; Cui, Y.; Yuan, Y.; Sha, J. Mebendazole-Induced Blood-Testis Barrier Injury in Mice Testes by Disrupting Microtubules in Addition to Triggering Programmed Cell Death. Int. J. Mol. Sci. 2022, 23, 4220. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Allen, I.C.; Bergstralh, D.T.; Brickey, W.J.; Huang, M.T.; Taxman, D.J.; Duncan, J.A.; Ting, J.P. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 2009, 183, 2008–2015. [Google Scholar] [CrossRef]
- Tsung, A.; Klune, J.R.; Zhang, X.; Jeyabalan, G.; Cao, Z.; Peng, X.; Stolz, D.B.; Geller, D.A.; Rosengart, M.R.; Billiar, T.R. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 2007, 204, 2913–2923. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, M.; Saqib, U.; Liu, D.; Faisal, S.M.; Sugathan, S.; Bishnoi, S.; Baig, M.S. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 2016, 40, 79–89. [Google Scholar] [CrossRef]
- Benedetti, G.; Bonaventura, P.; Lavocat, F.; Miossec, P. IL-17A and TNF-α Increase the Expression of the Antiapoptotic Adhesion Molecule Amigo-2 in Arthritis Synoviocytes. Front. Immunol. 2016, 7, 254. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Pan, L.; Lin, F.; Dai, H.; Fu, R. Monoclonal antibody against Toll-like receptor 4 attenuates ventilator-induced lung injury in rats by inhibiting MyD88- and NF-κB-dependent signaling. Int. J. Mol. Med. 2017, 39, 693–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Luo, L.; Bian, X.; Liu, R.H.; Zhao, S.; Chen, Y.; Sun, K.; Jiang, J.; Liu, Z.; Zeng, L. Pu-erh Tea Restored Circadian Rhythm Disruption by Regulating Tryptophan Metabolism. J. Agric. Food Chem. 2022, 70, 5610–5623. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Cao, Y.; Wang, J.; Zhao, X.; Jiao, J.; Li, J.; Zhang, K.; Yin, G. Inhibition of miR-155 Attenuates CD14(+) Monocyte-Mediated Inflammatory Response and Oxidative Stress in Psoriasis Through TLR4/MyD88/NF-κB Signaling Pathway. Clin. Cosmet. Investig. Dermatol. 2022, 15, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Kang, J.; He, Z.; Liu, Q.; Wu, J.; Li, D.; Wang, X.; Tao, Z.; Guan, X.; et al. Role of ROS-Induced NLRP3 Inflammasome Activation in the Formation of Calcium Oxalate Nephrolithiasis. Front. Immunol. 2022, 13, 818625. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhou, W.; Rui, Y.; Liu, L.; Chen, B.; Su, X. MicroRNA-574–5p Attenuates Acute Respiratory Distress Syndrome by Targeting HMGB1. Am. J. Respir. Cell Mol. Biol. 2021, 64, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Dickson, A.J. Hormonal regulation of glycogen metabolism in hepatocyte suspensions isolated from chicken embryos. Comp. Biochem. Physiol. B 1982, 71, 689–693. [Google Scholar] [CrossRef]
- Wei, Z.; Nie, G.; Yang, F.; Pi, S.; Wang, C.; Cao, H.; Guo, X.; Liu, P.; Li, G.; Hu, G.; et al. Inhibition of ROS/NLRP3/Caspase-1 mediated pyroptosis attenuates cadmium-induced apoptosis in duck renal tubular epithelial cells. Environ. Pollut 2020, 273, 115919. [Google Scholar] [CrossRef]

| Gene Name | Accession Number | Primer Sequences (5′ to 3′) |
|---|---|---|
| HMGB1 | XM_027469875.2 | Forward:AGTGTGAGGAGGCTGCGTAT Reverse: TAGACCTTTGGGGCCGTGTG |
| TLR4 | NM_001310413.1 | Forward:CACCAGTTTCACTTCCCCTTGT Reverse: GCTTTGCTAGGGATGACTCCAA |
| MYD88 | NM_001310832.1 | Forward:GCTTATAGAAAGGAGGTGTCGG Reverse: TGAAAGTCGCATTCGTCGCT |
| TNF-α | XM_005027491.5 | Forward: GCATTTCGTTTTCCTTTTCAACT Reverse: ACCGTCTGAACTGTAACGGG |
| IL-1β | XM_038166869.1 | Forward: TGGGCATCAAGGGCTACA Reverse: TCGGGTTGGTTGGTGATG |
| caspase-1 | XM_038165654.1 | Forward: CACTGCAAGGCACTGATTGG Reverse: CCAGGAGACGGTATCTCCAC |
| NLRP3 | XM_005029958.4 | Forward:CCAGCCTGAAGATCGGAGACCT Reverse: AGGAGCCACCCTAGAGGAGAGT |
| β-actin | NM_001310421.1 | Forward:CAGCACGATGAAAATCAAGATCA Reverse: CAAGGGTGTGGGTGTTGGTAA |
| IL-6 | XM_027450925.2 | Forward:TGGCTTCGACGAGGAGAAATG Reverse: CGTCGTTGCCAGATGCTTTG |
| ASC | XM_013201308. 1 | Forward: CAGCATTCTGGATCGGCTCT Reverse: ATTTTCTCCTGCCTGATGCTT |
| IL-18 | XM_027444356.2 | Forward: CCTGAAATCCCCTCCCGCTA Reverse: AGCTCATCTTCACCCTCGGT |
| NF-κB | XM_005017679.4 | Forward:ACAACGTCCTTCATTTAGCAA Reverse: TCTGATAAAGGTCGTTCCTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Yang, F.; Lin, Y.; Shan, J.; Cao, H.; Zhang, C.; Zhuang, Y.; Xing, C.; Hu, G. Selenium Antagonizes Cadmium-Induced Inflammation and Oxidative Stress via Suppressing the Interplay between NLRP3 Inflammasome and HMGB1/NF-κB Pathway in Duck Hepatocytes. Int. J. Mol. Sci. 2022, 23, 6252. https://doi.org/10.3390/ijms23116252
Cao Z, Yang F, Lin Y, Shan J, Cao H, Zhang C, Zhuang Y, Xing C, Hu G. Selenium Antagonizes Cadmium-Induced Inflammation and Oxidative Stress via Suppressing the Interplay between NLRP3 Inflammasome and HMGB1/NF-κB Pathway in Duck Hepatocytes. International Journal of Molecular Sciences. 2022; 23(11):6252. https://doi.org/10.3390/ijms23116252
Chicago/Turabian StyleCao, Zhanyou, Fan Yang, Yiqun Lin, Jiyi Shan, Huabin Cao, Caiying Zhang, Yu Zhuang, Chenghong Xing, and Guoliang Hu. 2022. "Selenium Antagonizes Cadmium-Induced Inflammation and Oxidative Stress via Suppressing the Interplay between NLRP3 Inflammasome and HMGB1/NF-κB Pathway in Duck Hepatocytes" International Journal of Molecular Sciences 23, no. 11: 6252. https://doi.org/10.3390/ijms23116252





