A Multifunctional and Fast-Response Lysosome-Targetable Fluorescent Probe for Monitoring pH and Isoxaflutole
Abstract
:1. Introduction
2. Results and Discussion
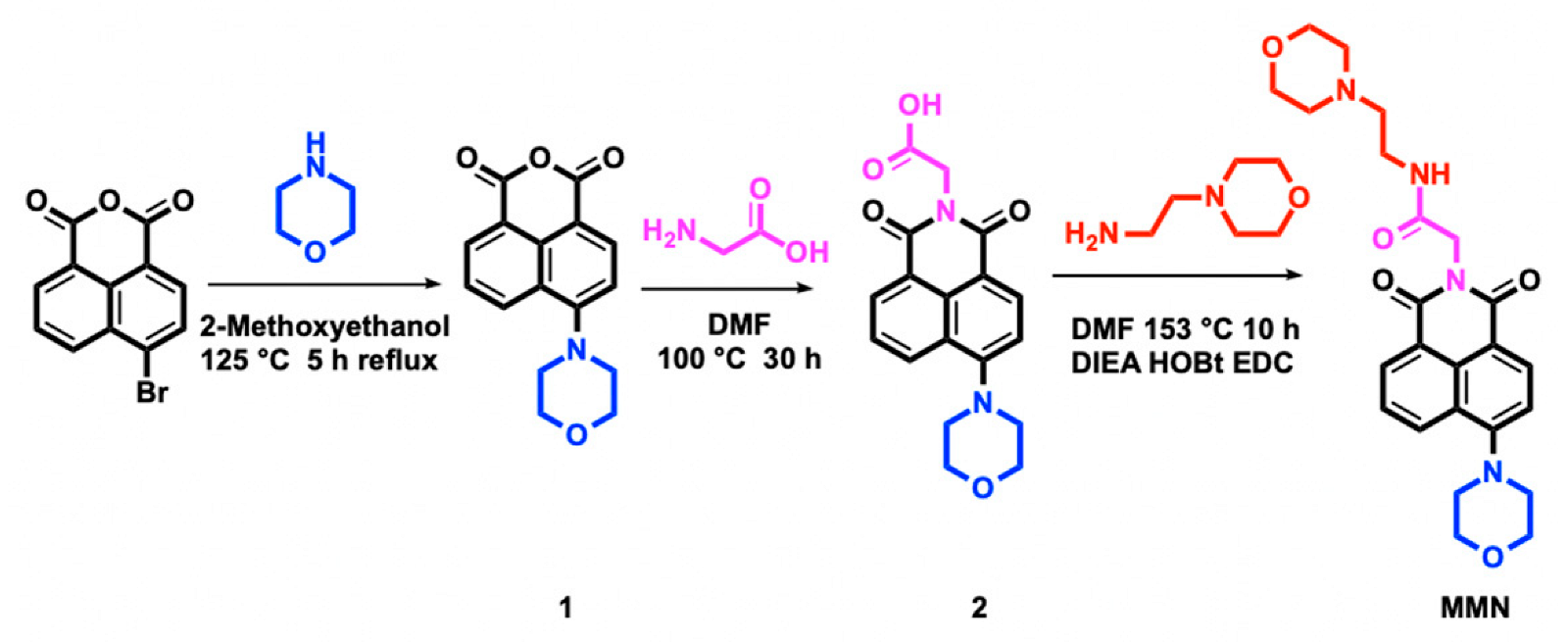
2.1. Design and Synthesis of MMN
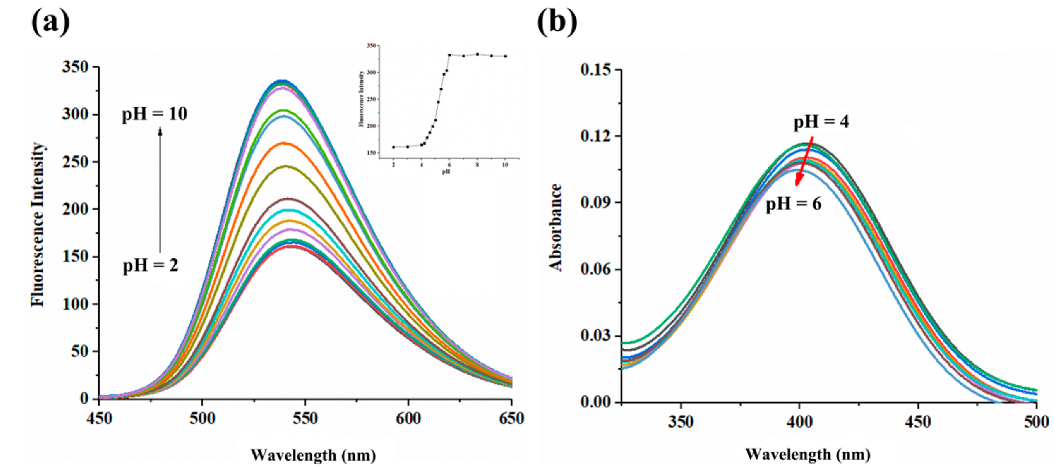
2.2. pH-Dependent Sensing Performance
2.3. pKa Value, Reversibility and Photostability
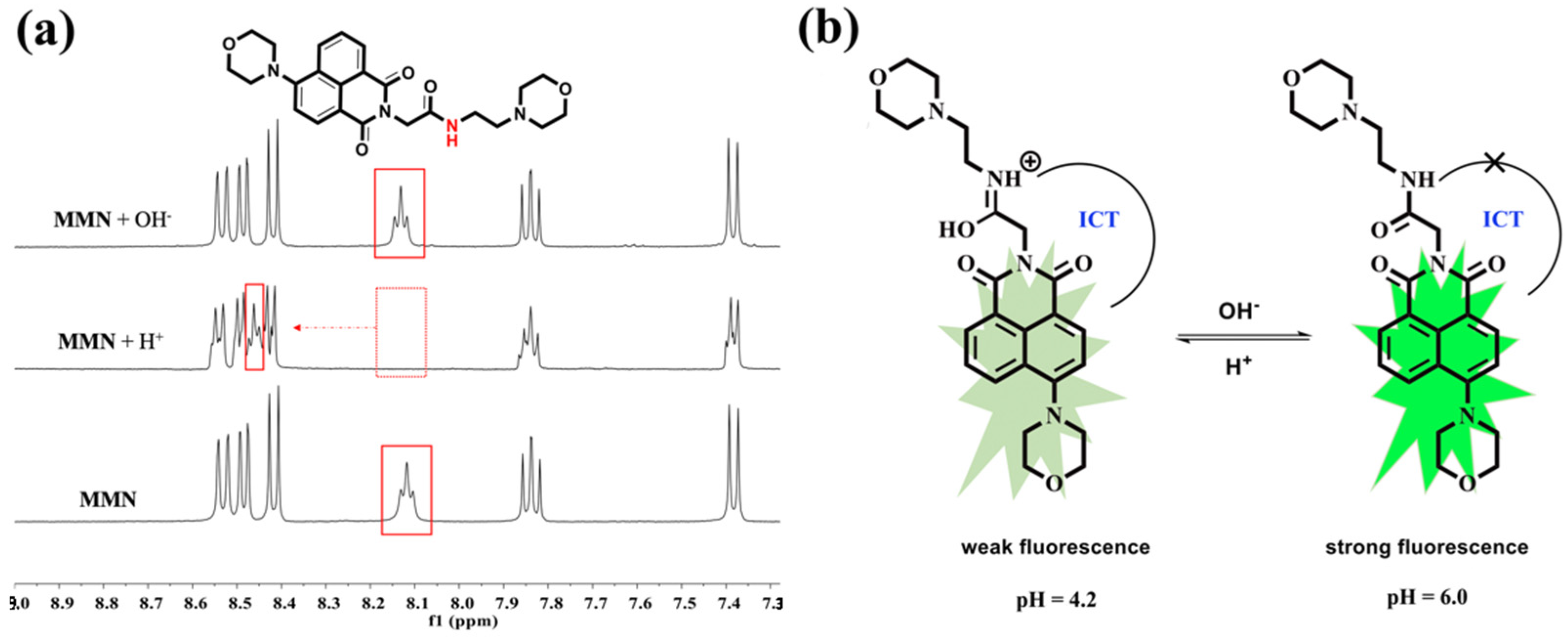
2.4. Recognition Mechanism of MMN to H+
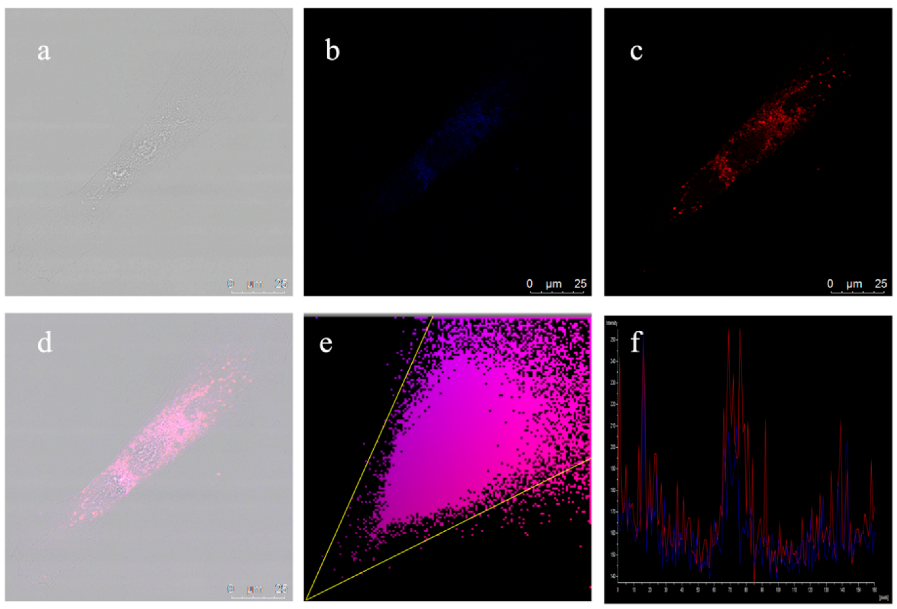
2.5. Colocalization Imaging Experiment and Test Stri
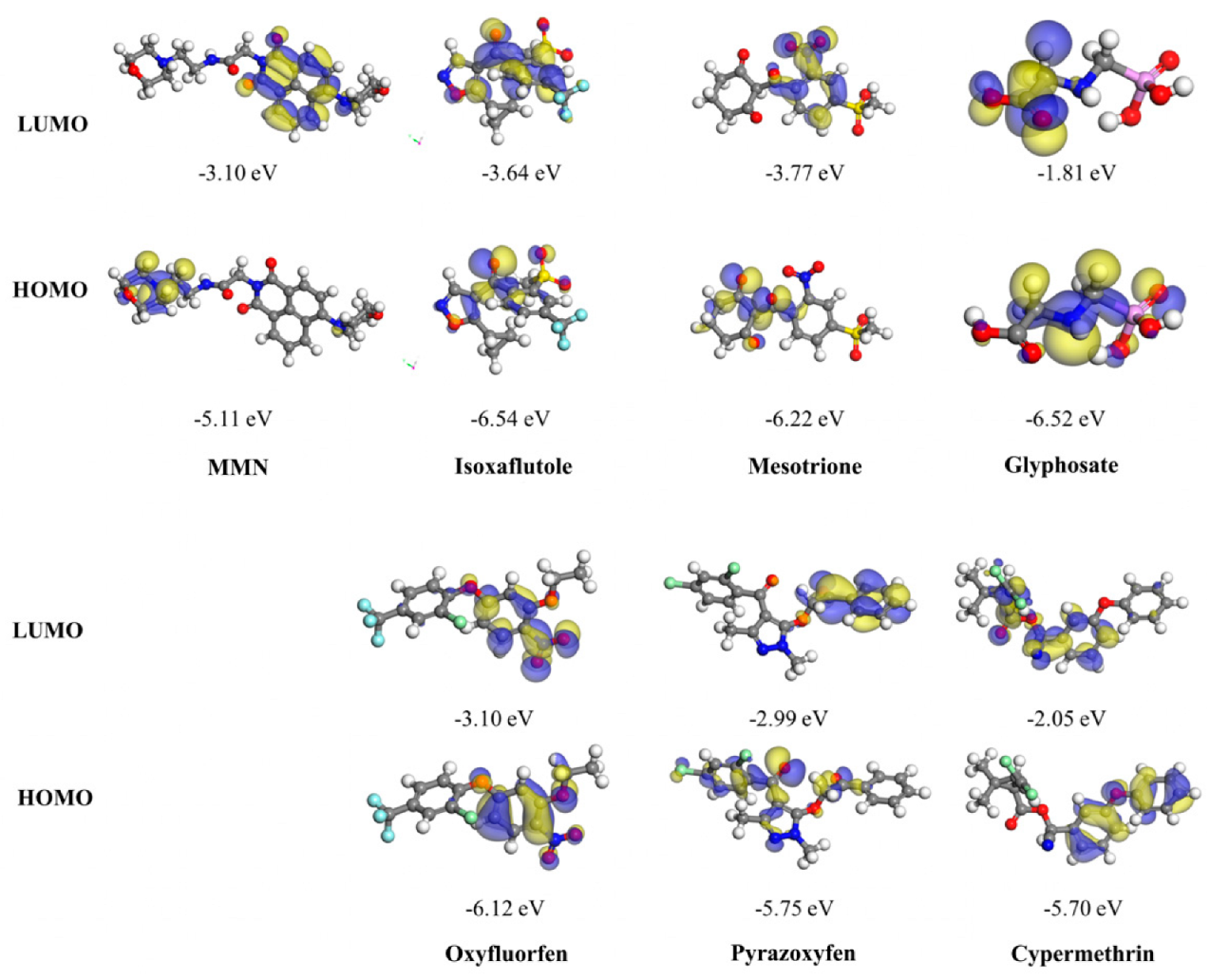
2.6. Detection of Isoxaflutole
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis
3.2.1. Synthesis of 4-Morpholine-1,8-naphthalic Anhydride (1)
3.2.2. Synthesis of N-Carboxymethyl-4-morpholine-1,8-naphthalimide (2)
3.2.3. Synthesis of N-(2-Morpholinoethyl)acetamide-4-morpholine-1,8-naphthimide (MMN)
3.3. General Method for the Spectra Experiments of MMN
3.4. Cell Incubation and Fluorescence Imaging
3.5. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chao, J.B.; Wang, H.J.; Zhang, Y.B.; Li, Z.Q.; Liu, Y.H.; Huo, F.J.; Yin, C.X.; Shi, Y.W.; Wang, J.J. A single pH fluorescent probe for bio-sensing and imaging of extreme acidity and extreme alkalinity. Anal. Chim. Acta 2017, 975, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Grabe, M.; Oster, G. Regulation of organelle acidity. J. Gen. Physiol. 2001, 117, 329–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosio, F.; Wiktor, J.; Pasquarello, A. pH-dependent catalytic reaction pathway for water splitting at the BiVO4–water interface from the band alignment. ACS Energy Lett. 2018, 3, 829–834. [Google Scholar] [CrossRef]
- Parks, S.K.; Chiche, J.; Pouyssegur, J. pH control mechanisms of tumor survival and growth. J. Cell. Physiol. 2011, 226, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Ren, D. Lysosomal physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Luka, G.S.; Nowak, E.; Kawchuk, J.; Hoorfar, M.; Najjaran, H. Portable device for the detection of colorimetric assays. R. Soc. Open Sci. 2017, 4, 171025. [Google Scholar] [CrossRef] [Green Version]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, J.; Fang, K. New technology for the detection of pH. J. Biochem. Bioph. Meth. 2005, 63, 1–9. [Google Scholar]
- Ma, Q.; Zhuo, W.; Zhai, Z.; Gong, G.; Zhang, T.; Xiao, H.; Zhou, Z.; Liu, Y. A new fluorescent probe for neutral to alkaline pH and imaging application in live cells. Spectrochim. Acta A 2021, 261, 120031. [Google Scholar] [CrossRef]
- Kang, T.; Gao, S.; Zhao, L.X.; Zhai, Y.; Ye, F.; Fu, Y. Design, synthesis and SAR of novel 1,3-disubstituted imidazolidine or hexahydropyrimidine derivatives as herbicide safeners. J. Agric. Food Chem. 2021, 69, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pallett, K.E.; Cramp, S.M.; Little, J.P.; Veerasekaran, P.; Crudace, A.J.; Slater, A.E. Isoxaflutole: The background to its discovery and the basis of its herbicidal properties. Pest Manage. Sci. 2001, 57, 133–142. [Google Scholar] [CrossRef]
- Stephenson, D.O.; Bond, J.A. Evaluation of thiencarbazone-methyl– and isoxaflutole-based herbicide programs in corn. Weed Technol. 2012, 26, 37–42. [Google Scholar] [CrossRef]
- Simmons, J.T.; Kells, J.J. Variation and inheritance of isoxaflutole tolerance in corn (zea mays). Weed Technol. 2003, 17, 177–180. [Google Scholar] [CrossRef]
- Nelson, E.A.; Penner, D. Leaching of isoxaflutole and the herbicide safeners R-29148 and furilazole. Weed Technol. 2007, 21, 106–109. [Google Scholar] [CrossRef]
- Alletto, L.; Benoit, P.; Bergheaud, V.; Coquet, Y. Variability of retention process of isoxaflutole and its diketonitrile metabolite in soil under conventional and conservation tillage. Pest Manag. Sci. 2012, 68, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Rouchaud, J.; Nues, O.; Eelen, H.; Bulcke, R. Soil metabolism of isoxaflutole in corn. Arch. Environ. Contam. Toxicol. 2002, 42, 280–285. [Google Scholar] [CrossRef]
- Lin, C.H.; Lerch, R.N.; Thuiman, E.M.; Garrett, H.E.; George, M.F. Determination of isoxaflutole (balance) and its metabolites in water using solid phase extraction followed by high-performance liquid chromatography with ultraviolet or mass spectrometry. J. Agric. Food Chem. 2002, 50, 5816–5824. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lerch, R.N.; Garrett, H.E.; Li, Y.X.; George, M.F. Improved HPLC-MS/MS method for determination of isoxaflutole (balance) and its metabolites in soils and forage plants. J. Agric. Food Chem. 2007, 55, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xiong, C.; Pan, J.; Su, D.; Zeng, L. Highly photostable, lysosometargeted BODIPYs with green to near-infrared emission for lysosome imaging in living cells. Dyes Pigm. 2018, 155, 30–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Chen, D.; Wu, D.; Chen, Z.; Zhang, J.; Chen, X.; Liu, S.; Yin, J. A colorimetric and ratiometric fluorescent probe for mercury (II) in lysosome. Sens. Actuators B Chem. 2016, 224, 907–914. [Google Scholar] [CrossRef]
- Ye, F.; Wu, N.; Li, P.; Liu, Y.L.; Li, S.J.; Fu, Y. A lysosome-targetable fluorescent probe for imaging trivalent cations Fe3+, Al3+ and Cr3+ in living cells. Spectrochim. Acta A 2019, 222, 117242. [Google Scholar] [CrossRef]
- Zhou, H.; Feng, R.; Liang, Q.; Su, X.; Deng, L.; Yang, L.; Ma, L.J. A sensitive pH fluorescent probe based on triethylenetetramine bearing double dansyl groups in aqueous solutions and its application in cells. Spectrochim. Acta A 2020, 229, 117881. [Google Scholar] [CrossRef]
- Fu, Y.; Pang, X.X.; Wang, Z.Q.; Chai, Q.; Ye, F. A highly sensitive and selective fluorescent probe for determination of Cu (II) and application in live cell imaging. Spectrochim. Acta A 2019, 208, 198–205. [Google Scholar] [CrossRef]
- Ye, F.; Liang, X.M.; Xu, K.X.; Pang, X.X.; Chai, Q.; Fu, Y. A novel dithiourea-appended naphthalimide “on-off” fluorescent probe for detecting Hg2+ and Ag+ and its application in cell imaging. Talanta 2019, 200, 494–502. [Google Scholar] [CrossRef]
- Dong, B.; Song, X.; Kong, X.; Wang, C.; Zhang, N.; Lin, W. A tumor-targeting and lysosome-specific two-photon fluorescent probe for imaging pH changes in living cells. J. Mater. Chem. B 2017, 5, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Guo, P.; Chai, L.; Shi, Q.; Xu, B.H.; Yuan, J.P.; Wang, X.G.; Shi, X.F.; Zhang, W.Q. Fluorescent and colorimetric detection of pH by a rhodamine-based probe. Sens. Actuators B Chem. 2014, 194, 498–502. [Google Scholar] [CrossRef]
- Dong, Y.; Xiao, H.; Xing, L.; Wu, C.; Zhou, J.; Zhou, Z.; Liu, Y.; Zhuo, S.; Li, P. Two-photon fluorescence visualization of lysosomal pH changes during mitophagy and cell apoptosis. Talanta 2020, 209, 120549. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Yaneva, I.S.; Surleva, A.R.; Asiri, A.M.; Bojinov, V.B. Synthesis, sensor activity and logic behavior of a highly water–soluble naphthalimide derivative. Sens. Actuators B Chem. 2013, 184, 54–63. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, G.; Hu, J. Ratiometric fluorescent mapping of pH and glutathione dictates intracellular transport pathways of micellar nanoparticles. Biomacromolecules 2020, 21, 3436–3446. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.L.; Liu, C.G.; Fu, Y.; Ye, F. A built-in self-calibrating luminescence sensor based on RhB@Zr-MOF for detection of cations, nitro explosives and pesticides. RSC Adv. 2020, 10, 19149–19156. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.L.; Liu, C.G.; Fu, Y.; Ye, F. A cationic metal-organic framework for dye adsorption and separation based on column-chromatography. J. Mol. Liq. 2020, 300, 112311. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.L.; Ji, X.X.; Liu, C.G.; Fu, Y.; Ye, F. A novel luminescent sensor based on Tb@UiO-66 for highly detecting Sm3+ and teflubenzuron. J. Taiwan Inst. Chem. Eng. 2021, 126, 173–181. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Yue, M.; Yang, L.; Sun, F.; Xu, G.; Fu, Y.; Ye, F. A switch-on fluorescent probe for detection of mesotrione based on the straight forward cleavage of carbon-nitrogen double bond of Schiff base. Chem. Eng. J. 2022, 430, 132758. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.L.; Liu, C.G.; Ye, F.; Fu, Y. Two luminescent dye@MOFs systems as dual-emitting platforms for efficient pesticides detection. J. Hazard. Mater. 2020, 381, 120966. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Yang, L.; Li, S.; Wang, Y.; Wang, L.; Li, P.; Ye, F.; Fu, Y. New fluorescent probes for the sensitive determination of glyphosate in food and environmental samples. J. Agric. Food Chem. 2021, 69, 12661–12673. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, S.; Yang, L.; Liu, Y.L.; Li, P.; Ye, F.; Fu, Y. Cobalt (II) complex as a fluorescent sensing platform for the selective and sensitive detection of triketone HPPD inhibitors. J. Hazard. Mater. 2021, 404, 124015. [Google Scholar] [CrossRef]
- Dai, H.; Xu, H. Selective and sensitive fluorescent chemosensors for Cu2+ ion based upon bis-1,8-naphthalimide dyads. Chin. J. Chem. 2012, 30, 267–272. [Google Scholar] [CrossRef]
- Liang, S.; Yu, H.; Xiang, J.; Yang, W.; Chen, X.; Liu, Y.; Gao, C.; Yan, G. New naphthalimide modified polyethylenimine nanoparticles as fluorescent probe for DNA detection. Spectrochim. Acta A 2012, 97, 359–365. [Google Scholar] [CrossRef]
- Johansen, M.D.; Kremer, L.; Kumar, V. Variedly connected 1,8-naphthalimide-7-chloroquinoline conjugates: Synthesis, anti-mycobacterial and cytotoxic evaluation. Bioorg. Chem. 2019, 92, 103241. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, Z.; Fan, X.; Yao, Y.; Chen, Z.; Zhang, W.; Qian, J. Synthesis of 1,8-naphthalimide-based fluorescent nano-probes and their application in pH detection. Chin. Chem. Lett. 2018, 29, 1500–1502. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Wang, H.; Chen, J.L.; Zhao, P.; Chen, G.R.; He, X.P. Intracellular pH sensing and targeted imaging of lysosome by a galactosyl naphthalimide-piperazine probe. Dyes Pigm. 2016, 133, 372–379. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Chem. Phys. 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]




| Type | Working Media | Sensitive Range (pH) | Sensing Mechanism | Application | References |
|---|---|---|---|---|---|
| Off–on | MeCN/H2O (9/1) | 4.2–6.0 | ICT | Paper strip/Targetable Imaging/pesticide detection | MMN |
| On–off | PBS buffer | 5.0–7.2 | PET | Cell imaging | [28] |
| Off–on–off | H2O | 2.0–10 | PET | - | [29] |
| Colorimetric probe | DMSO | 4.5–8.0 | FRET | Cell imaging | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Liu, Y.; Yue, M.; Li, P.; Liu, Y.; Ye, F.; Fu, Y. A Multifunctional and Fast-Response Lysosome-Targetable Fluorescent Probe for Monitoring pH and Isoxaflutole. Int. J. Mol. Sci. 2022, 23, 6256. https://doi.org/10.3390/ijms23116256
Yang L, Liu Y, Yue M, Li P, Liu Y, Ye F, Fu Y. A Multifunctional and Fast-Response Lysosome-Targetable Fluorescent Probe for Monitoring pH and Isoxaflutole. International Journal of Molecular Sciences. 2022; 23(11):6256. https://doi.org/10.3390/ijms23116256
Chicago/Turabian StyleYang, Liu, Yan Liu, Mingli Yue, Ping Li, Yulong Liu, Fei Ye, and Ying Fu. 2022. "A Multifunctional and Fast-Response Lysosome-Targetable Fluorescent Probe for Monitoring pH and Isoxaflutole" International Journal of Molecular Sciences 23, no. 11: 6256. https://doi.org/10.3390/ijms23116256







