Influence of Heat Treatment of Electrospun Carbon Nanofibers on Biological Response
Abstract
:1. Introduction
2. Results and Discussion
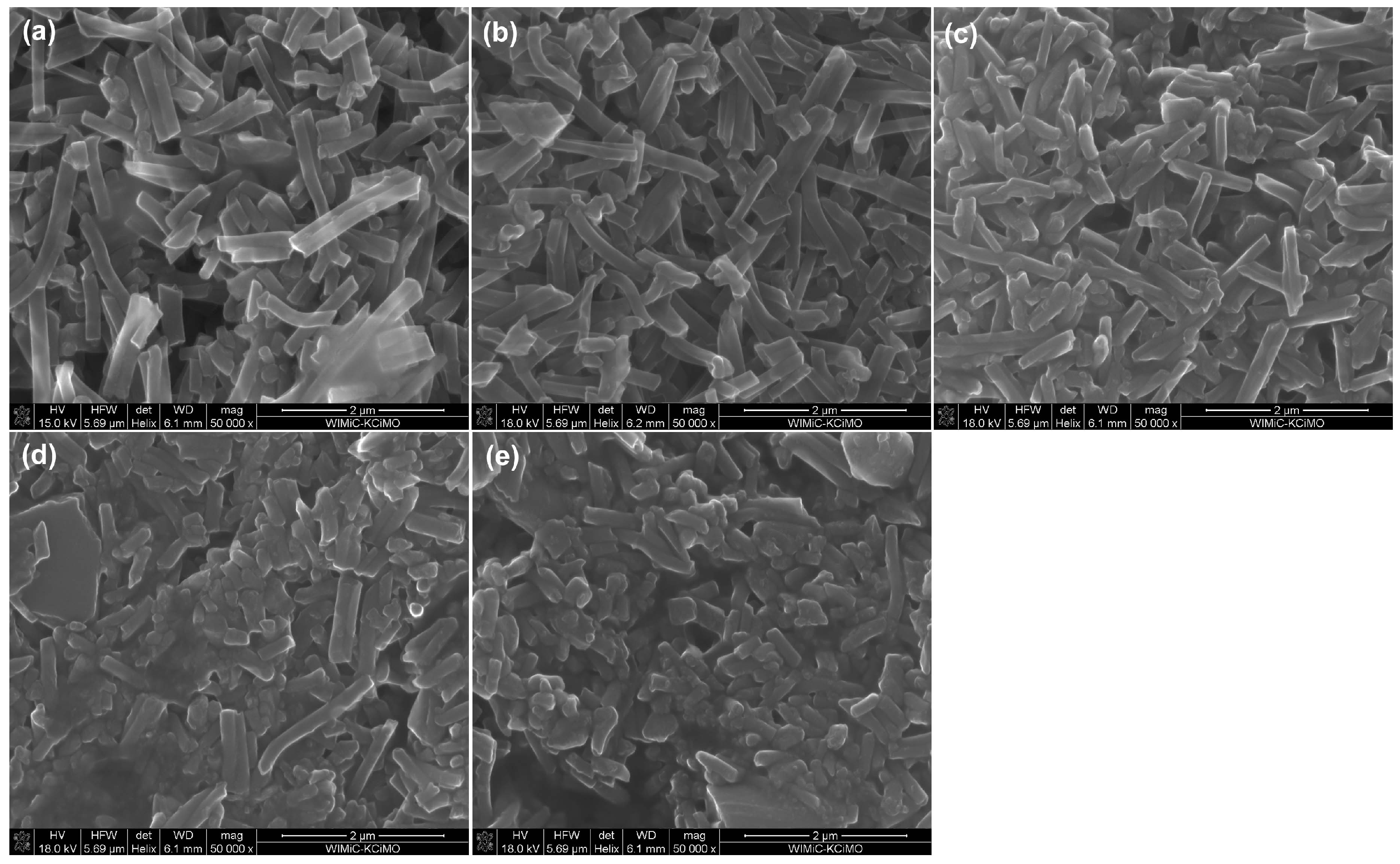
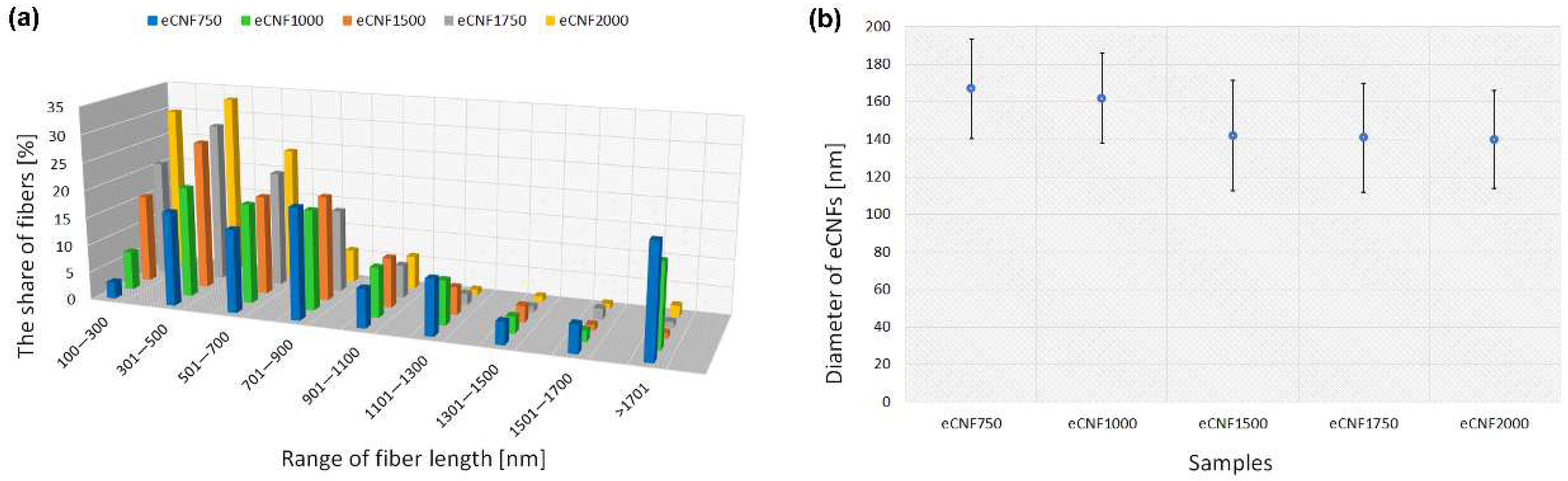
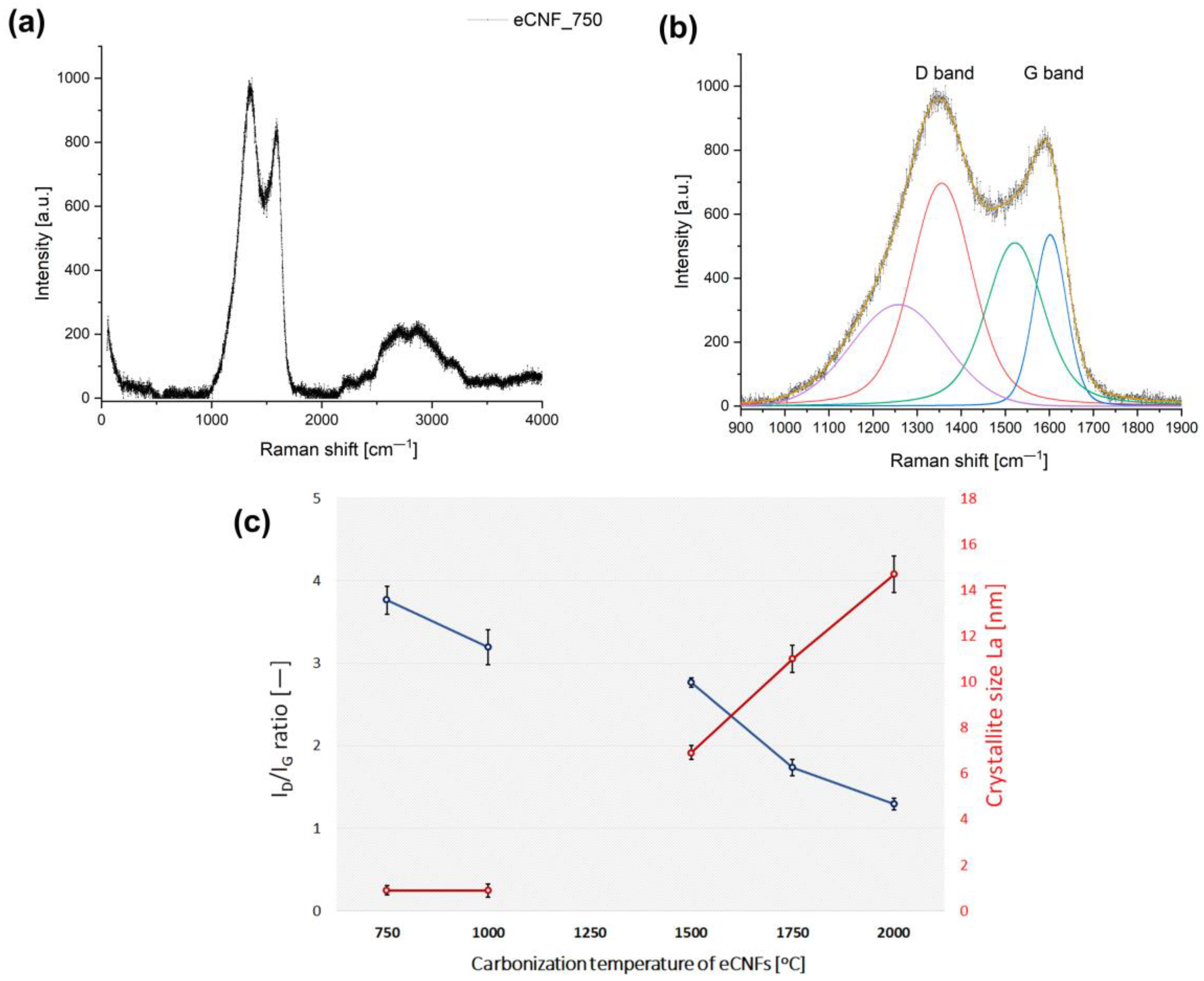
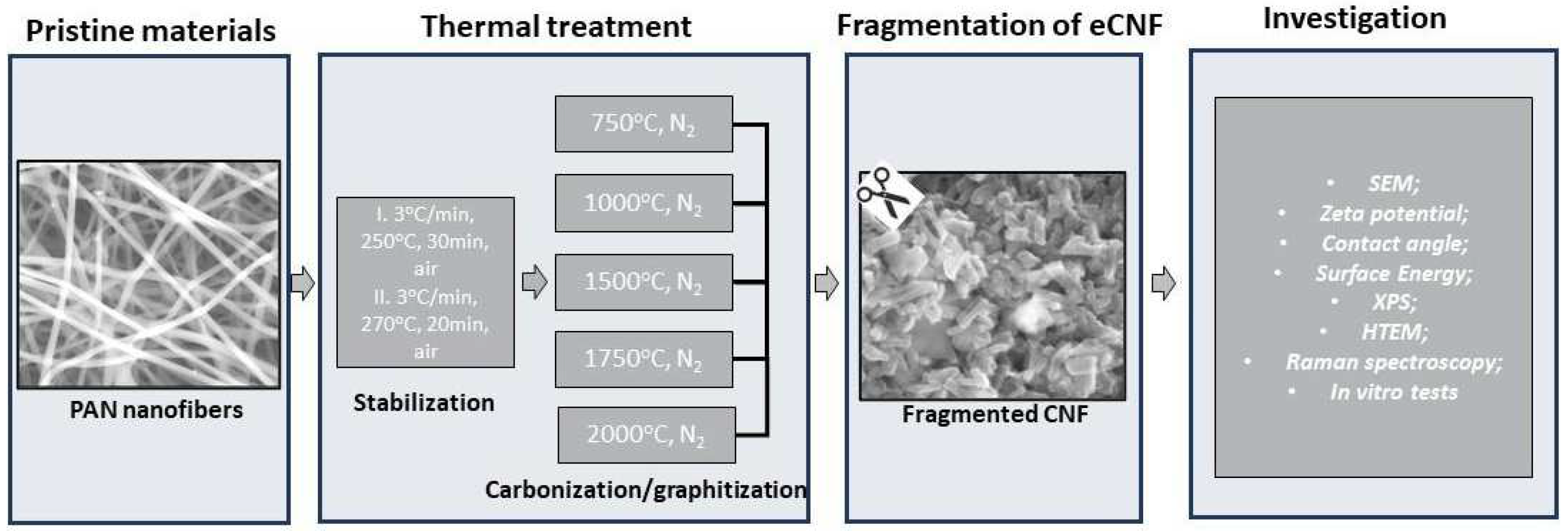
2.1. Physicochemical, Microstructural and Structural Characterization of eCNFs
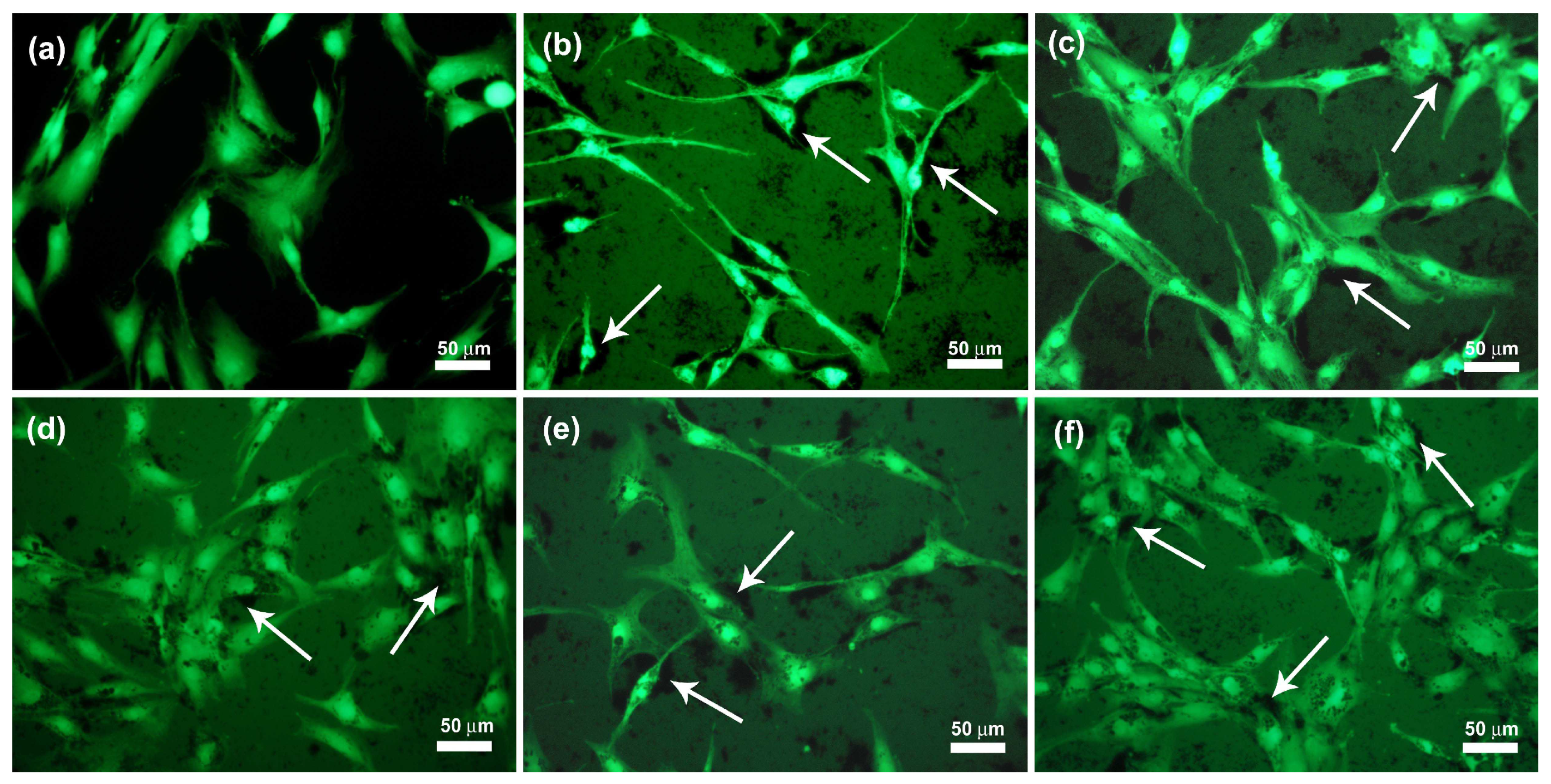
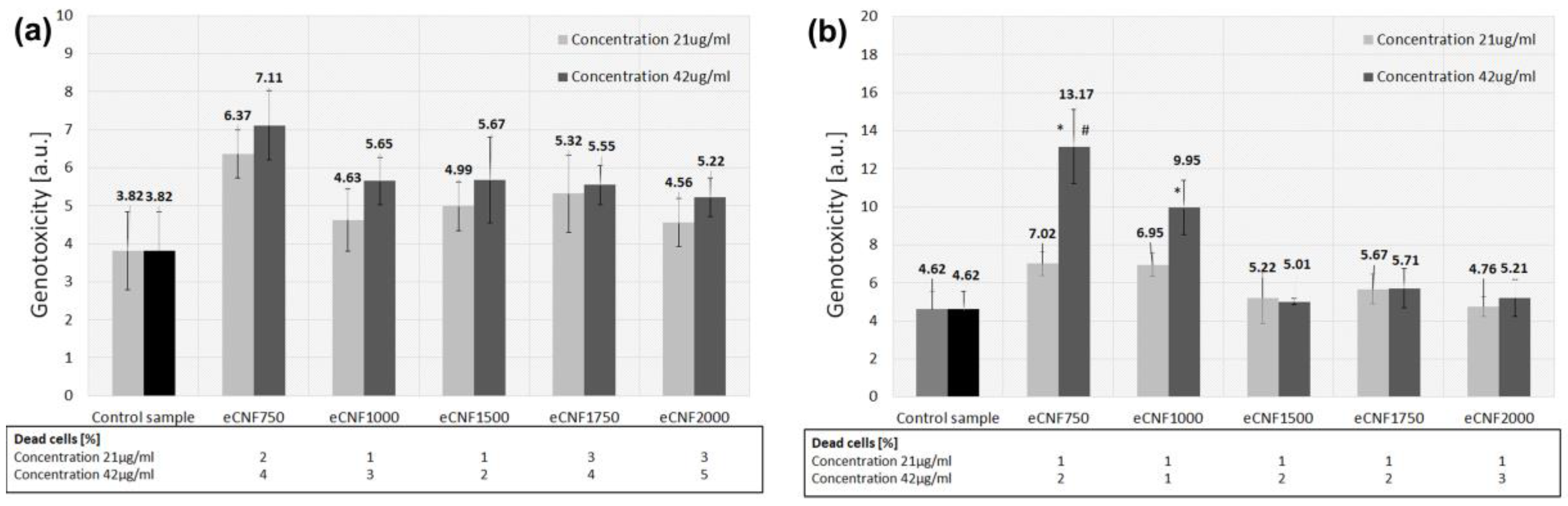
2.2. Biological Evaluation of eCNFs
3. Materials and Methods
3.1. Physicochemical Characterization of CNFs
3.2. Biological Investigation of CNF on Cell Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.-J. Precursors and Manufacturing of Carbon Fibers. In Carbon Fibers. Springer Series in Materials Science; Springer: Dordrecht, The Netherlands, 2018; pp. 31–67. [Google Scholar]
- Kumar, N.; Gangwar, A.K.; Devi, K.S. Carbon Fibers in Biomedical Applications. In Recent Developments in the Field of Carbon Fibers; InTech: London, UK, 2018; pp. 83–102. Available online: https://www.intechopen.com/chapters/61843 (accessed on 20 May 2022).
- Blazewicz, M. Carbon materials in the treatment of soft and hard tissue injuries. Eur. Cells Mater. 2001, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fraczek-Szczypta, A.; Rabiej, S.; Szparaga, G.; Pabjanczyk-Wlazlo, E.; Krol, P.; Brzezinska, M.; Blazewicz, S.; Bogun, M. The structure and properties of the carbon non-wovens modified with bioactive nanoceramics for medical applications. Mater. Sci. Eng. C 2015, 51, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Bencano, A.; Armas-Padrón, J.; Gili-Miner, M.; Lozano, M. Carbon fiber implants in osteochondral defects of the rabbit patella. Biomaterials 2000, 21, 2171–2176. [Google Scholar] [CrossRef]
- Robinson, D.; Efrat, M.; Mendes, D.G.; Halperin, N.; Nevo, Z. Implants composed of carbon fiber mesh and bone-marrow-derived, chondrocyte-enriched cultures for joint surface reconstruction. Bull. Hosp. Jt. Dis. 1993, 53, 75–82. [Google Scholar]
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [Green Version]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824. [Google Scholar] [CrossRef]
- Mäkisalo, S.E.; Visuri, T.; Viljanen, A.; Jokio, P. Reconstruction of the anterior cruciate ligament with carbon fibres: Unsatisfactory results after 8 years. Knee Surg. Sport. Traumatol. Arthrosc. 1996, 4, 132–136. [Google Scholar] [CrossRef]
- Pesáková, V.; Klézl, Z.; Balík, K.; Adam, M. Biomechanical and biological properties of the implant material carbon-carbon composite covered with pyrolytic carbon. J. Mater. Sci. Mater. Med. 2000, 11, 793–798. [Google Scholar] [CrossRef]
- Czajkowska, B.; Błażewicz, M. Phagocytosis of chemically modified carbon materials. Biomaterials 1997, 18, 69–74. [Google Scholar] [CrossRef]
- Debnath, U.; Fairclough, J.; Williams, R. Long-term local effects of carbon fibre in the knee. Knee 2004, 11, 259–264. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Stodolak-Zych, E.; Benko, A.; Szatkowski, P.; Długoń, E.; Nocuń, M.; Paluszkiewicz, C.; Błażewicz, M. Spectroscopic studies of the influence of CNTs on the thermal conversion of PAN fibrous membranes to carbon nanofibers. J. Mol. Struct. 2016, 1126, 94–102. [Google Scholar] [CrossRef]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The Use of Electrospun Organic and Carbon Nanofibers in Bone Regeneration. Nanomaterials 2020, 10, 562. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, J.; Qiao, S.; Zhang, W. Carbon-Based Nanomaterials for Bone and Cartilage Regeneration: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4718–4735. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Yougbare, S.; Chang, T.-K.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Kuo, T.-R. Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Čapek, J.; Roušar, T. Detection of Oxidative Stress Induced by Nanomaterials in Cells—The Roles of Reactive Oxygen Species and Glutathione. Molecules 2021, 26, 4710. [Google Scholar] [CrossRef]
- Kohl, Y.; Rundén-Pran, E.; Mariussen, E.; Hesler, M.; El Yamani, N.; Longhin, E.M.; Dusinska, M. Genotoxicity of Nanomaterials: Advanced In Vitro Models and High Throughput Methods for Human Hazard Assessment—A Review. Nanomaterials 2020, 10, 1911. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and In Vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef]
- Ling, C.; An, H.; Li, L.; Wang, J.; Lu, T.; Wang, H.; Hu, Y.; Song, G.; Liu, S. Genotoxicity Evaluation of Titanium Dioxide Nanoparticles In Vitro: A Systematic Review of the Literature and Meta-analysis. Biol. Trace Elem. Res. 2021, 199, 2057–2076. [Google Scholar] [CrossRef]
- Huk, A.; Izak-Nau, E.; Reidy, B.; Boyles, M.; Duschl, A.; Lynch, I.; Dušinska, M. Is the toxic potential of nanosilver dependent on its size? Part. Fibre Toxicol. 2014, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Jorio, A.; Souza Filho, A.G.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 5355–5377. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Zambrzycki, M.; Łoś, S.; Fraczek-Szczypta, A. Structure and electrical transport properties of carbon nanofibres/carbon nanotubes 3D hierarchical nanocomposites: Impact of the concentration of acetylacetonate catalyst. Ceram. Int. 2021, 47, 4020–4033. [Google Scholar] [CrossRef]
- Cagliostro, D.E. Carbon Fiber Production at Low Temperatures from Polyacryonitrile. Text. Res. J. 1980, 50, 632–638. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon Fibers: Precursor Systems, Processing, Structure, and Properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Tang, M.; Rice, G.G.; Fellers, J.F.; Lin, J.S. X-ray scattering studies of graphite fibers. J. Appl. Phys. 1986, 60, 803–810. [Google Scholar] [CrossRef]
- Johnson, W. Optical anisotropy of carbon fibres. Nature 1979, 279, 142–145. [Google Scholar] [CrossRef]
- Knibbs, R.H. The use of polarized light microscopy in examining the structure of carbon fibres*. J. Microsc. 1971, 94, 273–281. [Google Scholar] [CrossRef]
- Paris, O.; Loidl, D.; Peterlik, H. Texture of PAN- and pitch-based carbon fibers. Carbon 2002, 40, 551–555. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Y.; He, L.; Guo, Q.; Ye, H. Microstructure difference between core and skin of T700 carbon fibers in heat-treated carbon/carbon composites. Carbon 2011, 49, 2883–2892. [Google Scholar] [CrossRef]
- Qin, X.; Lu, Y.; Xiao, H.; Wen, Y.; Yu, T. A comparison of the effect of graphitization on microstructures and properties of polyacrylonitrile and mesophase pitch-based carbon fibers. Carbon 2012, 50, 4459–4469. [Google Scholar] [CrossRef]
- Karacan, I.; Erzurumluoğlu, L. The effect of carbonization temperature on the structure and properties of carbon fibers prepared from poly(m-phenylene isophthalamide) precursor. Fibers Polym. 2015, 16, 1629–1645. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1993, 70, A25. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Xue, Y.; Zuo, Z.; Li, Y.; Liu, H.; Li, Y. Graphdiyne-Supported NiCo2S4 Nanowires: A Highly Active and Stable 3D Bifunctional Electrode Material. Small 2017, 13, 1700936. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W. NIST X-ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 20 May 2022).
- Smolka, W.; Ptas, M.; Panek, A.; Krok-Borkowicz, M.; Zambrzycki, M.; Gubernat, M.; Markowski, J.; Fraczek-Szczypta, A. Surface Modification of Carbon Nanofibers to Improve Their Biocompatibility in Contact with Osteoblast and Chondrocytes Cell Lines. Materials 2021, 14, 6370. [Google Scholar] [CrossRef]
- Fowkes, F.M.; Harkins, W.D. The State of Monolayers Adsorbed at the Interface Solid—Aqueous Solution. J. Am. Chem. Soc. 1940, 62, 3377–3386. [Google Scholar] [CrossRef]
- Taherian, F.; Marcon, V.; van der Vegt, N.F.A.; Leroy, F. What Is the Contact Angle of Water on Graphene? Langmuir 2013, 29, 1457–1465. [Google Scholar] [CrossRef]
- Raj, R.; Maroo, S.C.; Wang, E.N. Wettability of Graphene. Nano Lett. 2013, 13, 1509–1515. [Google Scholar] [CrossRef]
- Belyaeva, L.A.; Schneider, G.F. Wettability of graphene. Surf. Sci. Rep. 2020, 75, 100482. [Google Scholar] [CrossRef]
- Shih, C.-J.; Wang, Q.H.; Lin, S.; Park, K.-C.; Jin, Z.; Strano, M.S.; Blankschtein, D. Breakdown in the Wetting Transparency of Graphene. Phys. Rev. Lett. 2012, 109, 176101. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Pacheco Benito, S.; Lefferts, L. Wettability of carbon nanofiber layers on nickel foils. J. Colloid Interface Sci. 2011, 364, 530–538. [Google Scholar] [CrossRef]
- ASTM Standard D 4182-4187; Zeta Potential of Colloids in Water and Waste Water. American Society for Testing and Materials: West Conshohocken, PA, USA; p. 1985.
- Castro, M.R.S.; Al-Dahoudi, N.; Oliveira, P.W.; Schmidt, H.K. Multi-walled carbon nanotube-based transparent conductive layers deposited on polycarbonate substrate. J. Nanoparticle Res. 2009, 11, 801–806. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A.; Dlugon, E.; Weselucha-Birczynska, A.; Nocun, M.; Blazewicz, M. Multi walled carbon nanotubes deposited on metal substrate using EPD technique. A spectroscopic study. J. Mol. Struct. 2013, 1040, 238–245. [Google Scholar] [CrossRef]
- Sokolowski, K.; Blazewicz, S.; Nocun, M.; Fraczek-Szczypta, A. Carbon micro- and nanofibrous materials with high adsorption capacity for water desalination. Desalination 2021, 503, 114936. [Google Scholar] [CrossRef]
- Metwally, S.; Ferraris, S.; Spriano, S.; Krysiak, Z.J.; Kaniuk, Ł.; Marzec, M.M.; Kim, S.K.; Szewczyk, P.K.; Gruszczyński, A.; Wytrwal-Sarna, M.; et al. Surface potential and roughness controlled cell adhesion and collagen formation in electrospun PCL fibers for bone regeneration. Mater. Des. 2020, 194, 108915. [Google Scholar] [CrossRef]
- George, J.H.; Shaffer, M.S.; Stevens, M.M. Investigating the cellular response to nanofibrous materials by use of a multi-walled carbon nanotube model. J. Exp. Nanosci. 2006, 1, 1–12. [Google Scholar] [CrossRef]
- Xie, J.; Shen, H.; Yuan, G.; Lin, K.; Su, J. The effects of alignment and diameter of electrospun fibers on the cellular behaviors and osteogenesis of BMSCs. Mater. Sci. Eng. C 2021, 120, 111787. [Google Scholar] [CrossRef]
- Panek, A.; Błażewicz, M.; Frączek-Szczypta, A.; Adamczyk, J.; Wiltowska-Zuber, J.; Paluszkiewicz, C. Applications of Comet Assay for the Evaluation of Genotoxicity and DNA Repair Efficiency in Nanomaterials Research. Acta Phys. Pol. A 2018, 133, 280–282. [Google Scholar] [CrossRef]
- Sramkova, M.; Kozics, K.; Masanova, V.; Uhnakova, I.; Razga, F.; Nemethova, V.; Mazancova, P.; Kapka-Skrzypczak, L.; Kruszewski, M.; Novotova, M.; et al. Kidney nanotoxicity studied in human renal proximal tubule epithelial cell line TH1. Mutat. Res. Toxicol. Environ. Mutagen. 2019, 845, 403017. [Google Scholar] [CrossRef]
- Danielsen, P.H.; Knudsen, K.B.; Štrancar, J.; Umek, P.; Koklič, T.; Garvas, M.; Vanhala, E.; Savukoski, S.; Ding, Y.; Madsen, A.M.; et al. Effects of physicochemical properties of TiO2 nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice. Toxicol. Appl. Pharmacol. 2020, 386, 114830. [Google Scholar] [CrossRef]
- Fraser, K.; Kodali, V.; Yanamala, N.; Birch, M.E.; Cena, L.; Casuccio, G.; Bunker, K.; Lersch, T.L.; Evans, D.E.; Stefaniak, A.; et al. Physicochemical characterization and genotoxicity of the broad class of carbon nanotubes and nanofibers used or produced in U.S. facilities. Part. Fibre Toxicol. 2020, 17, 62. [Google Scholar] [CrossRef]
- Kisin, E.R.; Murray, A.R.; Sargent, L.; Lowry, D.; Chirila, M.; Siegrist, K.J.; Schwegler-Berry, D.; Leonard, S.; Castranova, V.; Fadeel, B.; et al. Genotoxicity of carbon nanofibers: Are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol. Appl. Pharmacol. 2011, 252, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Chen, F.; Shi, X.; Yucesoy, B.; Mossman, B.; Vallyathan, V. Diseases caused by silica: Mechanisms of injury and disease development. Int. Immunopharmacol. 2002, 2, 173–182. [Google Scholar] [CrossRef]
- Manning, C.B.; Vallyathan, V.; Mossman, B.T. Diseases caused by asbestos: Mechanisms of injury and disease development. Int. Immunopharmacol. 2002, 2, 191–200. [Google Scholar] [CrossRef]
- Panek, A.; Frączek-Szczypta, A.; Długoń, E.; Nocuń, M.; Paluszkiewicz, C.; Błażewicz, M. Genotoxicity Study of Carbon Nanoforms using a Comet Assay. Acta Phys. Pol. A 2018, 133, 306–308. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Duarte Silva, I.; Caria, M.H.; Laires, A.; Chaveca, T.; Glatt, H.R.; Rueff, J. Genotoxicity assessment of aromatic amines and amides in genetically engineered V79 cells. Mutat. Res. Toxicol. 1994, 341, 93–100. [Google Scholar] [CrossRef]
- DeMarini, D.M.; Carreón-Valencia, T.; Gwinn, W.M.; Hopf, N.B.; Sandy, M.S.; Bahadori, T.; Calaf, G.M.; Chen, G.; de Conti, A.; Fritschi, L.; et al. Carcinogenicity of some aromatic amines and related compounds. Lancet Oncol. 2020, 21, 1017–1018. [Google Scholar] [CrossRef]
- Bhatia, S.P.; Politano, V.T.; Api, A.M. Evaluation of genotoxicity of nitrile fragrance ingredients using in vitro and in vivo assays. Food Chem. Toxicol. 2013, 59, 784–792. [Google Scholar] [CrossRef]
- Shamovsky, I.; Ripa, L.; Börjesson, L.; Mee, C.; Nordén, B.; Hansen, P.; Hasselgren, C.; O’Donovan, M.; Sjö, P. Explanation for Main Features of Structure–Genotoxicity Relationships of Aromatic Amines by Theoretical Studies of Their Activation Pathways in CYP1A2. J. Am. Chem. Soc. 2011, 133, 16168–16185. [Google Scholar] [CrossRef]
- Hirano, T.; Higashi, K.; Sakai, A.; Tsurudome, Y.; Ootsuyama, Y.; Kido, R.; Kasai, H. Analyses of Oxidative DNA Damage and Its Repair Activity in the Livers of 3′-Methyl-4-dimethylaminoazobenzene-treated Rodents. Jpn. J. Cancer Res. 2000, 91, 681–685. [Google Scholar] [CrossRef]
- Siraki, A.G.; Chan, T.S.; Galati, G.; Teng, S.; O’Brien, P.J. N-Oxidation of Aromatic Amines by Intracellular Oxidases. Drug Metab. Rev. 2002, 34, 549–564. [Google Scholar] [CrossRef]
- Végh, J. The Shirley background revised. J. Electron Spectros. Relat. Phenom. 2006, 151, 159–164. [Google Scholar] [CrossRef]
- Meier, R.J. On art and science in curve-fitting vibrational spectra. Vib. Spectrosc. 2005, 39, 266–269. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]

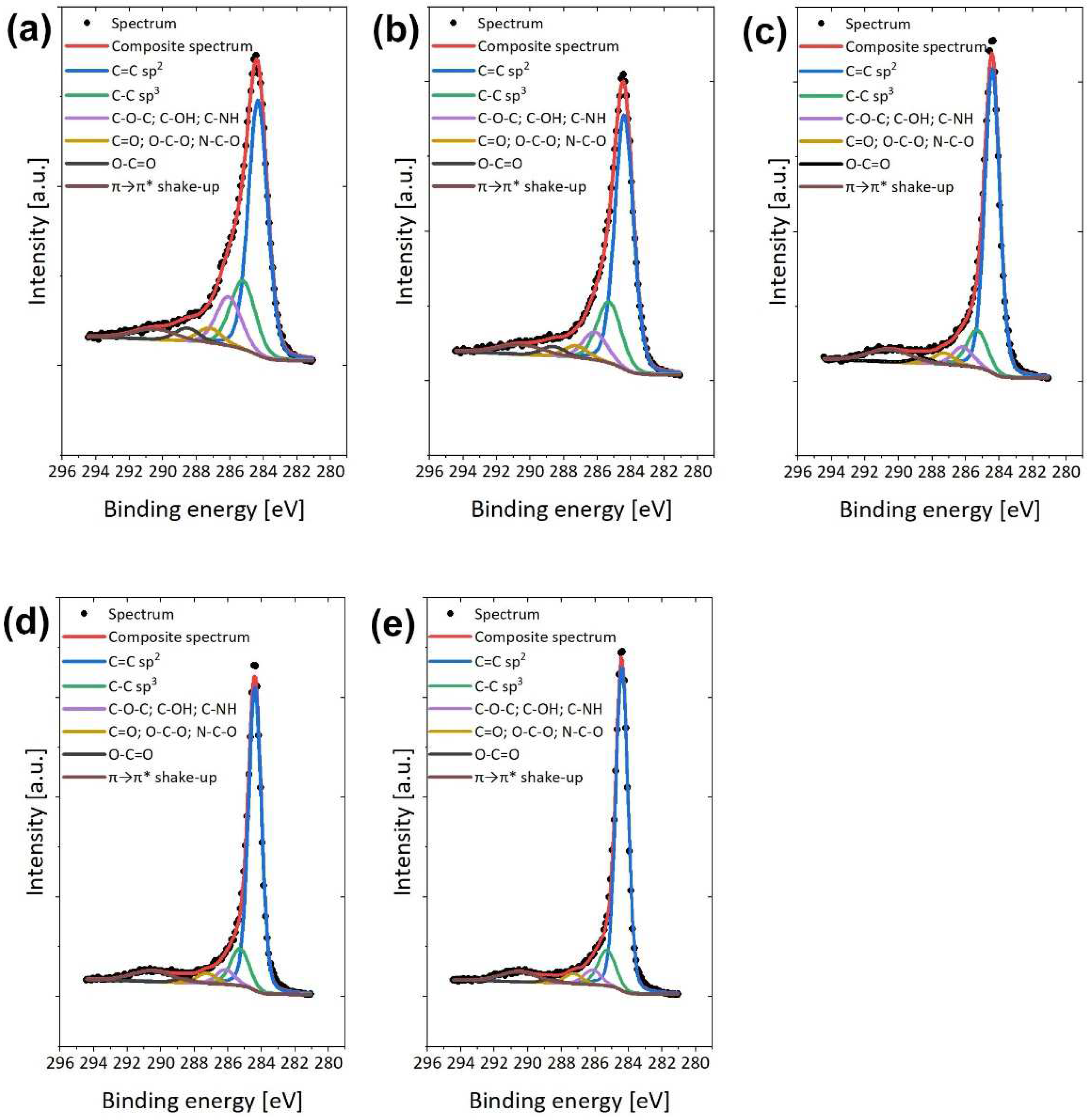
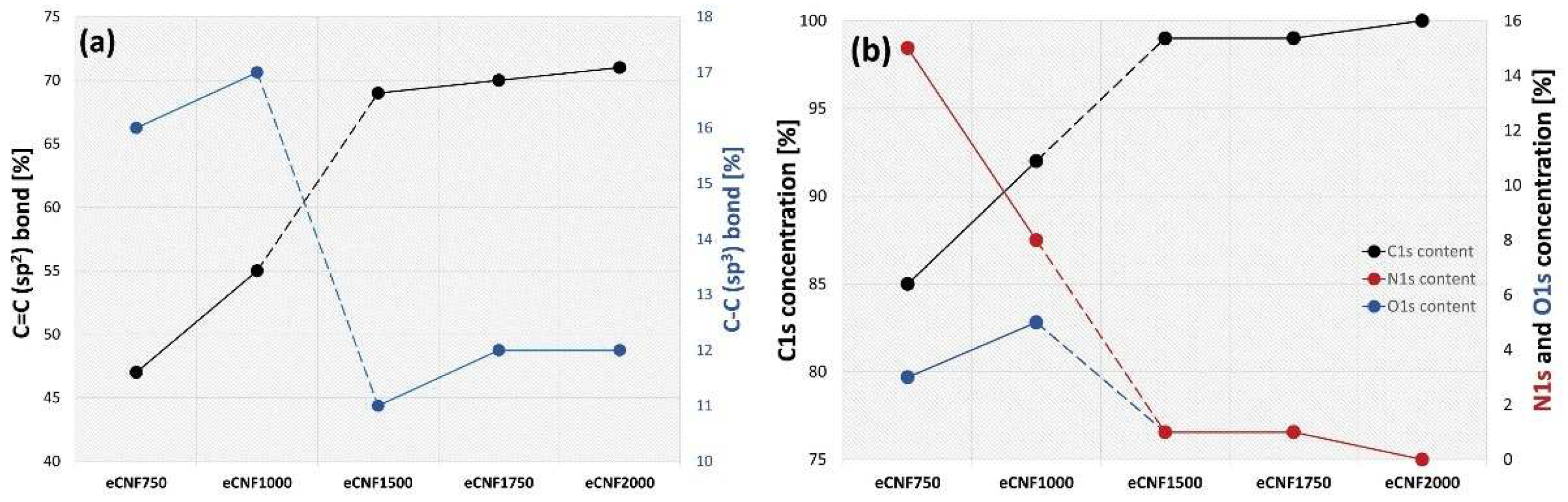
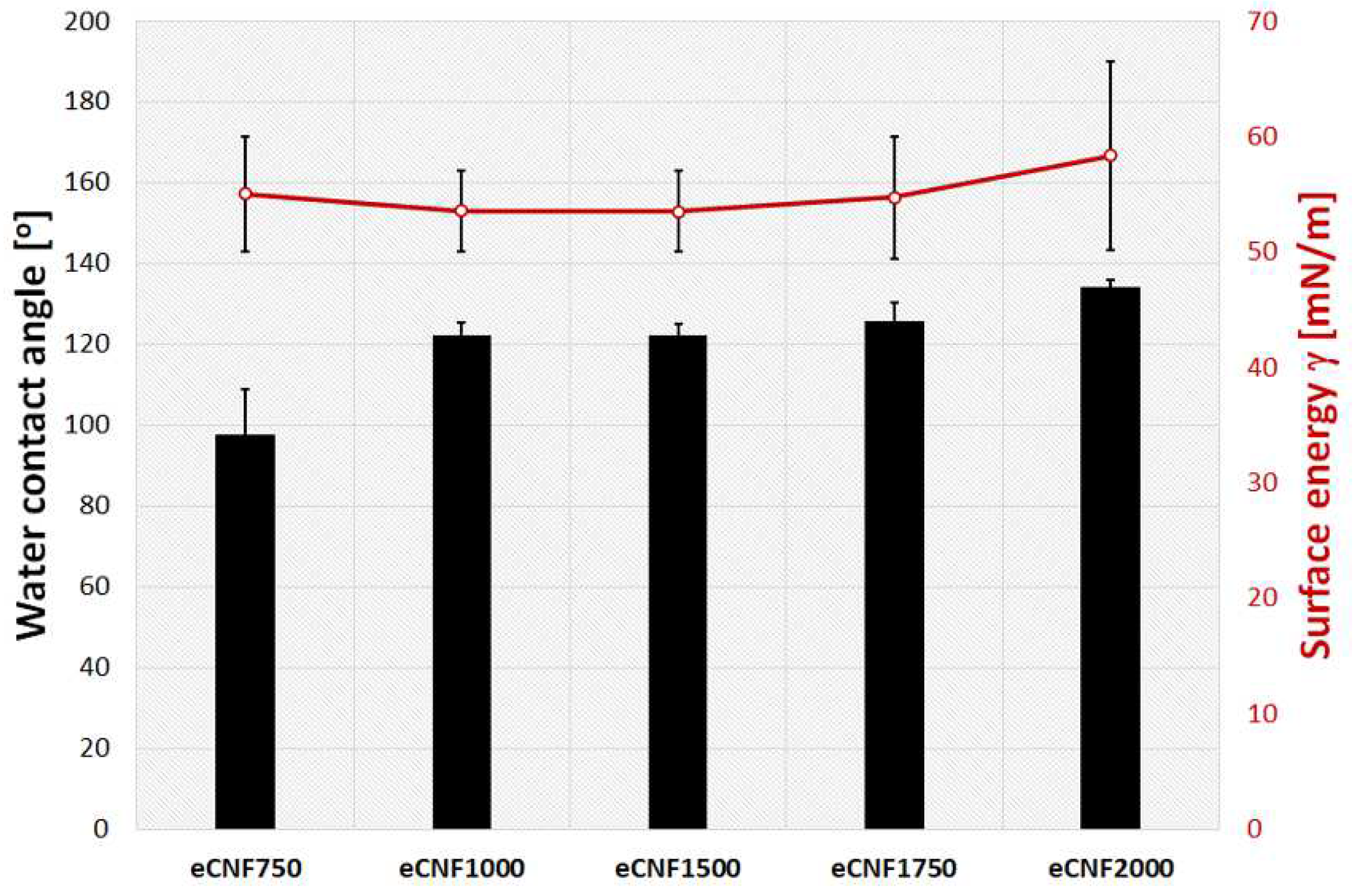
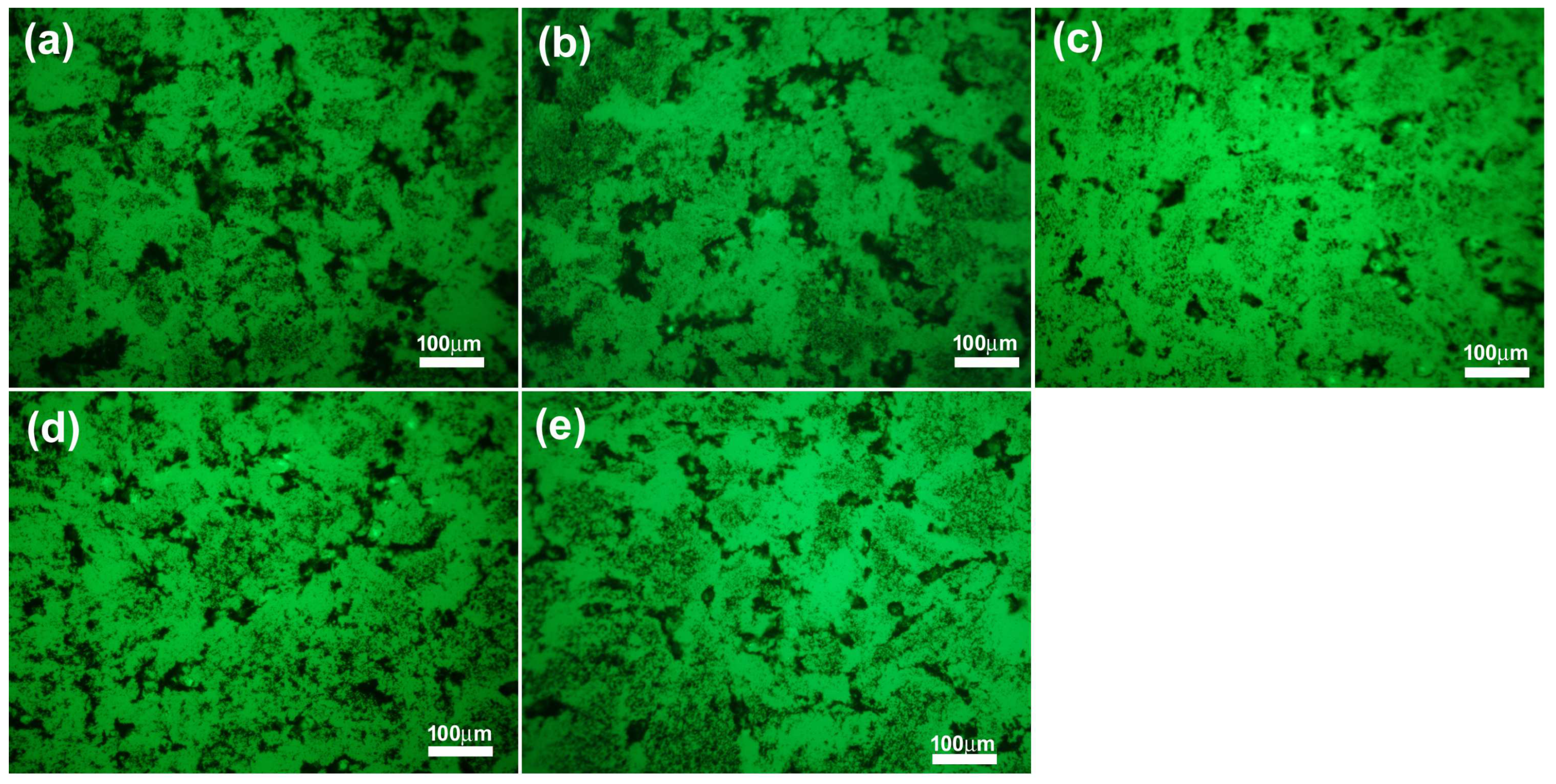
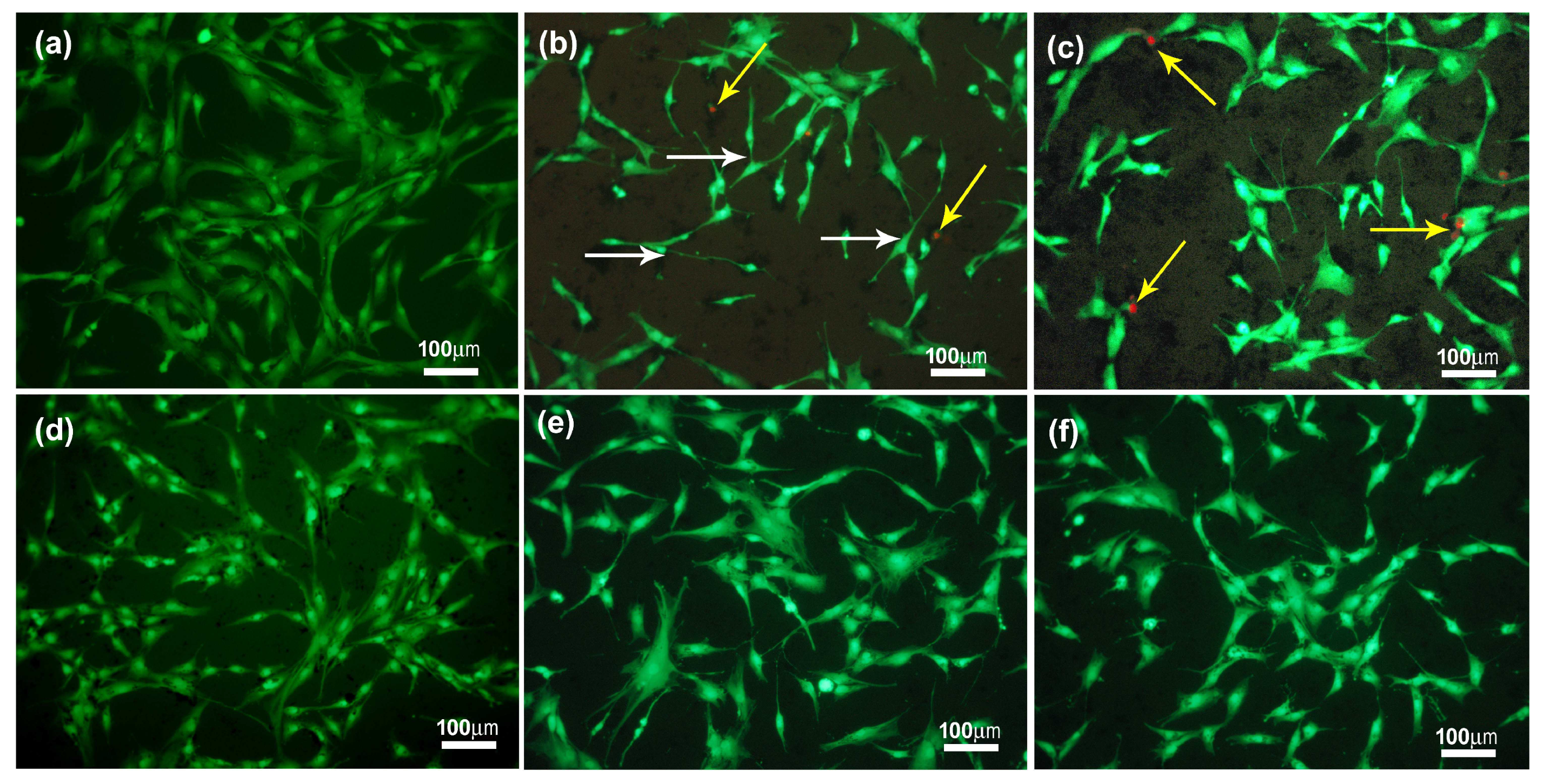
| Materials | Atomic Concentration (at.%) | ||
|---|---|---|---|
| C | O | N | |
| eCNF750 | 85.4 | 3.2 | 11.5 |
| eCNF1000 | 91.6 | 5.4 | 3.0 |
| eCNF1500 | 98.7 | 1.3 | - |
| eCNF1750 | 99.4 | 0.7 | - |
| eCNF2000 | 99.6 | 0.4 | - |
| Sample | eCNF750 | eCNF1000 | eCNF1500 | eCNF1750 | eCNF2000 |
|---|---|---|---|---|---|
| Zeta potential ζ (mV) | −17.0 | −20.5 | −22.5 | −21.9 | −19.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowski, J.; Zambrzycki, M.; Smolka, W.; Panek, A.; Gubernat, M.; Czaja, P.; Marzec, M.; Fraczek-Szczypta, A. Influence of Heat Treatment of Electrospun Carbon Nanofibers on Biological Response. Int. J. Mol. Sci. 2022, 23, 6278. https://doi.org/10.3390/ijms23116278
Markowski J, Zambrzycki M, Smolka W, Panek A, Gubernat M, Czaja P, Marzec M, Fraczek-Szczypta A. Influence of Heat Treatment of Electrospun Carbon Nanofibers on Biological Response. International Journal of Molecular Sciences. 2022; 23(11):6278. https://doi.org/10.3390/ijms23116278
Chicago/Turabian StyleMarkowski, Jaroslaw, Marcel Zambrzycki, Wojciech Smolka, Agnieszka Panek, Maciej Gubernat, Paweł Czaja, Mateusz Marzec, and Aneta Fraczek-Szczypta. 2022. "Influence of Heat Treatment of Electrospun Carbon Nanofibers on Biological Response" International Journal of Molecular Sciences 23, no. 11: 6278. https://doi.org/10.3390/ijms23116278
APA StyleMarkowski, J., Zambrzycki, M., Smolka, W., Panek, A., Gubernat, M., Czaja, P., Marzec, M., & Fraczek-Szczypta, A. (2022). Influence of Heat Treatment of Electrospun Carbon Nanofibers on Biological Response. International Journal of Molecular Sciences, 23(11), 6278. https://doi.org/10.3390/ijms23116278






