Iron Speciation and Iron Binding Proteins in Arthrospira platensis Grown in Media Containing Different Iron Concentrations
Abstract
:1. Introduction
2. Results
2.1. Production of Arthrospira Platensis F&M-C256 Biomass and Biochemical Composition
2.2. Iron Bioaccumulation
2.3. Size Exclusion Chromatography (SEC) and Iron Speciation
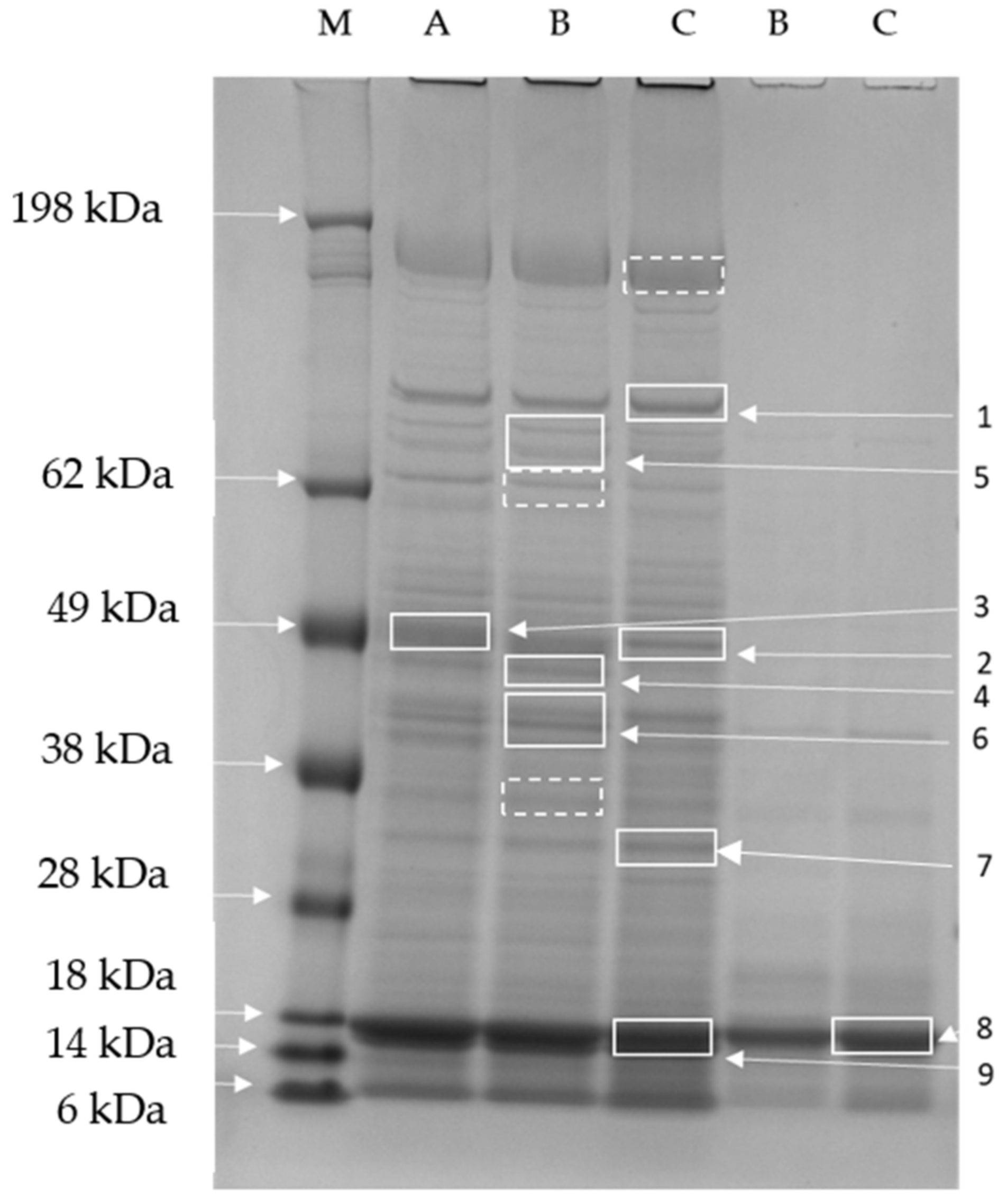
2.4. SDS-PAGE and Protein Identification Using Mass Spectrometry
3. Discussion
3.1. Production of A. platensis F&M-C256 Biomass and Biochemical Composition
3.2. Iron Bioaccumulation
3.3. Iron Speciation and Protein Identification
4. Materials and Methods
4.1. Production of Arthrospira platensis F&M-C256 Biomass
4.2. Pigments, Total Phenolic, and DPPH Analyses
4.3. Metal Analysis
4.4. Iron Speciation and Size Exclusion Chromatography (SEC)
4.5. SDS-PAGE
4.6. Protein Identification Using Mass Spectrometry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Anish, J.Z.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.K.S.; Bisen, P.S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, N.C. Forging a field: The golden age of iron biology. Blood 2008, 112, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranzler, C.; Rudolf, M.; Keren, N.; Schleiff, E. Iron in Cyanobacteria. Adv. Bot. Res. 2013, 65, 57–105. [Google Scholar] [CrossRef]
- Qiu, G.W.; Jiang, H.B.; Lis, H.; Li, Z.K.; Deng, B.; Shang, J.L.; Sun, C.Y.; Keren, N.; Qiu, B.S. A unique porin meditates iron-selective transport through cyanobacterial outer membranes. Environ. Microbiol. 2021, 23, 376–390. [Google Scholar] [CrossRef]
- Raven, J.A.; Evans, M.C.W.; Korb, R.E. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosyn. Res. 1999, 60, 111–150. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Price, N.M. The Biogeochemical Cycles of Trace Metals in the Oceans. Environ. Sci. 2003, 300, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Beck, K.L.; Conlon, C.A.; Kruger, R.; Coad, J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: A review. Nutrients 2014, 6, 3747–3776. [Google Scholar] [CrossRef] [Green Version]
- Mounicou, S.; Szpunar, J.; Lobinski, R. Metallomics: The concept and methodology. Chem. Soc. Rev. 2009, 38, 1119–1138. [Google Scholar] [CrossRef]
- Delrue, F.; Alaux, E.; Moudjaoui, L.; Gaignard, C.; Fleury, G.; Perilhou, A.; Richaud, P.; Petitjean, M.; Sassi, J.F. Optimization of Arthrospira platensis (Spirulina) Growth: From Laboratory Scale to Pilot Scale. Fermentation 2017, 3, 59. [Google Scholar] [CrossRef] [Green Version]
- Ismaiel, M.M.; Piercey-Normore, M.D.; Rampitsch, C. Proteomic analyses of the cyanobacterium Arthrospira (Spirulina) platensis under iron and salinity stress. Environ. Exp. Bot. 2018, 147, 63–74. [Google Scholar] [CrossRef]
- Xing, W.; Huang, W.M.; Li, D.H.; Liu, Y.D. Effect of iron on growth, pigment content, photosystem II efficiency, and siderophores production of Microcystis aeruginosa and Microcystis wesenbergii. Curr. Microbiol. 2007, 55, 94–98. [Google Scholar] [CrossRef]
- Belay, A. Mass Culture of Spirulina Outdoors—The Earthrise Farms Experience (Chapter 8). In Spirulina Platensis Arthrospira: Physiology, Cell-Biology and Biotechnology, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar] [CrossRef]
- Perfiliev, Y.D.; Tambiev, K.; Konnychev, M.A.; Skalny, A.V.; Lobakova, E.S.; Kirpichnikov, M.P. Mössbauer spectroscopic study of transformations of iron species by the cyanobacterium Arthrospira platensis (formerly Spirulina platensis). J. Trace Elem. Med. Biol. 2018, 48, 105–110. [Google Scholar] [CrossRef]
- Cepoi, L.; Chiriac, T.; Rudi, L.; Djur, S.; Zosim, L.; Bulimaga, V.; Batir, L.; Elenciuc, D.; Rudic, V. Spirulina as a Raw Material for Products Containing Trace Elements (Chapter 19). In Recent Advances in Trace Elements, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and toxic elements in commercial microalgal food supplements. J. Appl. Phycol. 2019, 31, 3567–3579. [Google Scholar] [CrossRef] [Green Version]
- Wild, K.J.; Steinga, H.; Rodehutscord, M. Variability in nutrient composition and in vitro crude protein digestibility of 16 microalgae products. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1306–1319. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for iron. EFSA J. 2015, 13, 4254. [Google Scholar] [CrossRef]
- Puyfoulhoux, G.; Rouanet, J.M.; Besançon, P.; Baroux, B.; Baccou, J.C.; Caporiccio, B. Iron Availability from Iron-Fortified Spirulina by an in Vitro Digestion/Caco-2 Cell Culture Model. J. Agri. Food Chem. 2001, 49, 1625–1629. [Google Scholar] [CrossRef]
- Selmi, C.; Leung, P.S.C.; Fischer, L.; German, B.; Yang, C.Y.; Kenny, T.P.; Cysewski, G.R.; Gershwin, M.E. The effects of Spirulina on anemia and immune function in senior citizens. Cell. Mol. Immunol. 2011, 8, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Hunnicutt, J.; He, K.; Xun, P. Dietary iron intake and body iron stores are associated with risk of coronary heart disease in a meta-analysis of prospective cohort studies. J. Nutr. 2014, 144, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods. 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef]
- Bermejo, P.; Piñero, E.; Villar, Á.M. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chem. 2008, 110, 436–445. [Google Scholar] [CrossRef]
- Bhat, V.B.; Madyastha, K.M. C-Phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, J.; Ji, Y.; Park, S.; Do, H.; Hwang, C.; Lee, B.; Holzapfel, W. Impact of bubble size on growth and CO2 uptake of Arthrospira (Spirulina) platensis KMMCC CY-007. Bioresour. Technol. 2014, 170, 310–315. [Google Scholar] [CrossRef]
- Vorphal, M.A.; Bruna, C.; Wandersleben, T.; Dagnino-Leone, J.; Lobos-González, F.; Uribe, E.; Martìnez-Oyanedel, J.; Bunster, M. Molecular and functional characterization of ferredoxin NADP(H) oxidoreductase from Gracilaria chilensis and its complex with ferredoxin. Biol. Res. 2017, 50, 39. [Google Scholar] [CrossRef] [Green Version]
- Morsy, F.M.; Nakajima, M.; Yoshida, T.; Fujiwara, T.; Sakamoto, T.; Wada, K. Subcellular localization of ferredoxin-NADP+ oxidoreductase in phycobilisome retaining oxygenic photosysnthetic organisms. Photosyn. Res. 2008, 95, 73–85. [Google Scholar] [CrossRef]
- Qiu, G.W.; Lou, W.J.; Sun, C.Y.; Yang, N.; Li, Z.K.; Li, D.L.; Zang, S.S.; Fu, F.X.; Hutchins, D.A.; Jiang, H.B.; et al. Outer membrane iron uptake pathways in the model cyanobacterium Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2018, 84, e01512-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sannasimuthu, A.; Kumaresan, V.; Anilkumar, S.; Pasupuleti, M.; Ganesh, M.R.; Mala, K.; Paray, B.A.; Al-Sadoon, M.K.; Albeshr, M.F.; Arockiaraj, J. Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic. Biol. Med. 2019, 135, 198–209. [Google Scholar] [CrossRef]
- Rendón, J.L.; Calcagno, M.; Mendoza-Hernández, G.; Ondarza, R.N. Purification, properties, and oligomeric structure of glutathione reductase from the cyanobacterium Spirulina maxima. Arch. Biochem. Biophys. 1986, 248, 215–223. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Chen, W.; Li, D.; Zhao, X.; Wang, X.; Li, A.; Bao, Q. Identification of differentially expressed proteins of Arthrospira (Spirulina) plantensis-YZ under salt-stress conditions by proteomics and qRT-PCR analysis. Proteome Sci. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, A.; Boussiba, S.; Napoleone, V.; Hohlberg, A. Recovery of c-phycocyanin from the cyanobacterium Spirulina maxima. J. Appl. Phycol. 1989, 1, 325–331. [Google Scholar] [CrossRef]
- Bennett, A.; Bogobad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1973, 167, 191–194. [Google Scholar] [CrossRef]
- Parsons, T.R.; Strickland, J.D.H. Discussion of spectrophotometric determination of marine-plant pigments, with revised equations for ascertaining chlorophylls and carotenoids. J. Mar. Res. 1963, 21, 155–163. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]





| [Fe] Nominal Concentration (mg L−1) | [Fe] Culture Medium at Time Zero (mg L−1) | Chlorophyll a (% d.w.) | Carotenoids (% d.w.) | Phycocyanin (% d.w.) | Antioxidant Activity (% RSA) | Phenolics (mg GAE g−1) | [Fe] Biomass (mg g−1 dw) | [Fe] Algal Pellet (mg g−1 dw) | [Fe] Algal Cytosolic Extract (µg mL−1) |
|---|---|---|---|---|---|---|---|---|---|
| [Fe] 1 | 0.84 c ± 0.04 | 0.50 c ± 0.05 | 0.08 a ± 0.03 | 5.16 c ± 0.14 | 82.29 a ± 0.57 | 23.93 a ± 1.22 | 0.35 c ± 0.02 | 0.056 c ± 0.003 | 1.53 b ± 0.11 |
| [Fe] 5 | 3.44 b ± 0.18 | 0.66 b ± 0.10 | 0.08 a ± 0.01 | 7.81 a ± 0.48 | 73.83 b ± 1.57 | 20.26 a ± 0.77 | 1.28 b ± 0.03 | 0.160 b ± 0.020 | 3.68 a ± 0.42 |
| [Fe] 10 | 6.90 a ± 0.42 | 0.90 a ± 0.16 | 0.11 a ± 0.04 | 6.10 b ± 0.90 | 72.69 b ± 0.57 | 20.49 a ± 1.14 | 2.34 a ± 0.10 | 0.248 a ± 0.021 | 4.23 a ± 0.16 |
| Band a | Entry Name b | Protein Full Name | MM (Da) c | Organism | Score d | Sign Pept e | Sign Seq f | Molecular Function | Biological Process |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A0A015IXE4_ 9GLOM | J-domain containing protein | 33,255 | Arthrospira platensis | 21 | 1 | 1 | DNA binding | |
| 2 | WP_035760125.1 | Iron-uptake porin | 67,484 | Arthrospira platensis | 59 | 6 | 6 | Porin activity | Carbohydrate transport |
| 3 | K1VSN7_ ARTPT | Carbohydrate-selective porin OprB | 68,358 | Arthrospira platensis | 119 | 13 | 12 | Porin activity | Carbohydrate transport |
| 4 | H1WGM5_ 9CYAN | Glutathione oxidoreductase | 48,615 | Arthrospira platensis | 155 | 10 | 8 | Oxidoreductase | Cell redox homeostasis—Cell oxidant detox |
| 5 | H1WMK8_ 9CYAN | Transketolase | 72,899 | Arthrospira platensis | 67 | 3 | 2 | Transferase, metal ion-binding | Pentose shunt |
| 6 | H1W8T2_ 9CYAN | Phosphoglycerate kinase | 42,306 | Arthrospira platensis | 213 | 12 | 8 | Transferase | Glycolysis |
| H1WJF3_ 9CYAN | Adenosylhomo cysteinase | 46,765 | Arthrospira platensis | 124 | 7 | 7 | Hydrolase | One-carbon metabolism | |
| H1WJ30_ 9CYAN | Sulfate adenylyltransferase | 44,594 | Arthrospira platensis | 92 | 6 | 6 | Transferase | Sulfate assimilation | |
| 7 | H1WF80_ 9CYAN | Ferredoxin NADP reductase | 45,396 | Arthrospira platensis | 74 | 7 | 6 | Oxidoreductase | Photosynthesis |
| 8–9 | A0A0C4VZ70_ ARTPT | CpcA protein | 17,703 | Arthrospira platensis | 682 | 34 | 8 | Chromophore | Photosynthesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isani, G.; Niccolai, A.; Andreani, G.; Dalmonte, T.; Bellei, E.; Bertocchi, M.; Tredici, M.R.; Rodolfi, L. Iron Speciation and Iron Binding Proteins in Arthrospira platensis Grown in Media Containing Different Iron Concentrations. Int. J. Mol. Sci. 2022, 23, 6283. https://doi.org/10.3390/ijms23116283
Isani G, Niccolai A, Andreani G, Dalmonte T, Bellei E, Bertocchi M, Tredici MR, Rodolfi L. Iron Speciation and Iron Binding Proteins in Arthrospira platensis Grown in Media Containing Different Iron Concentrations. International Journal of Molecular Sciences. 2022; 23(11):6283. https://doi.org/10.3390/ijms23116283
Chicago/Turabian StyleIsani, Gloria, Alberto Niccolai, Giulia Andreani, Thomas Dalmonte, Elisa Bellei, Martina Bertocchi, Mario R. Tredici, and Liliana Rodolfi. 2022. "Iron Speciation and Iron Binding Proteins in Arthrospira platensis Grown in Media Containing Different Iron Concentrations" International Journal of Molecular Sciences 23, no. 11: 6283. https://doi.org/10.3390/ijms23116283
APA StyleIsani, G., Niccolai, A., Andreani, G., Dalmonte, T., Bellei, E., Bertocchi, M., Tredici, M. R., & Rodolfi, L. (2022). Iron Speciation and Iron Binding Proteins in Arthrospira platensis Grown in Media Containing Different Iron Concentrations. International Journal of Molecular Sciences, 23(11), 6283. https://doi.org/10.3390/ijms23116283







