Analysis of Silver Nanoparticles for the Treatment and Prevention of Nucleopolyhedrovirus Affecting Bombyx mori
Abstract
1. Introduction
2. Materials and Methods
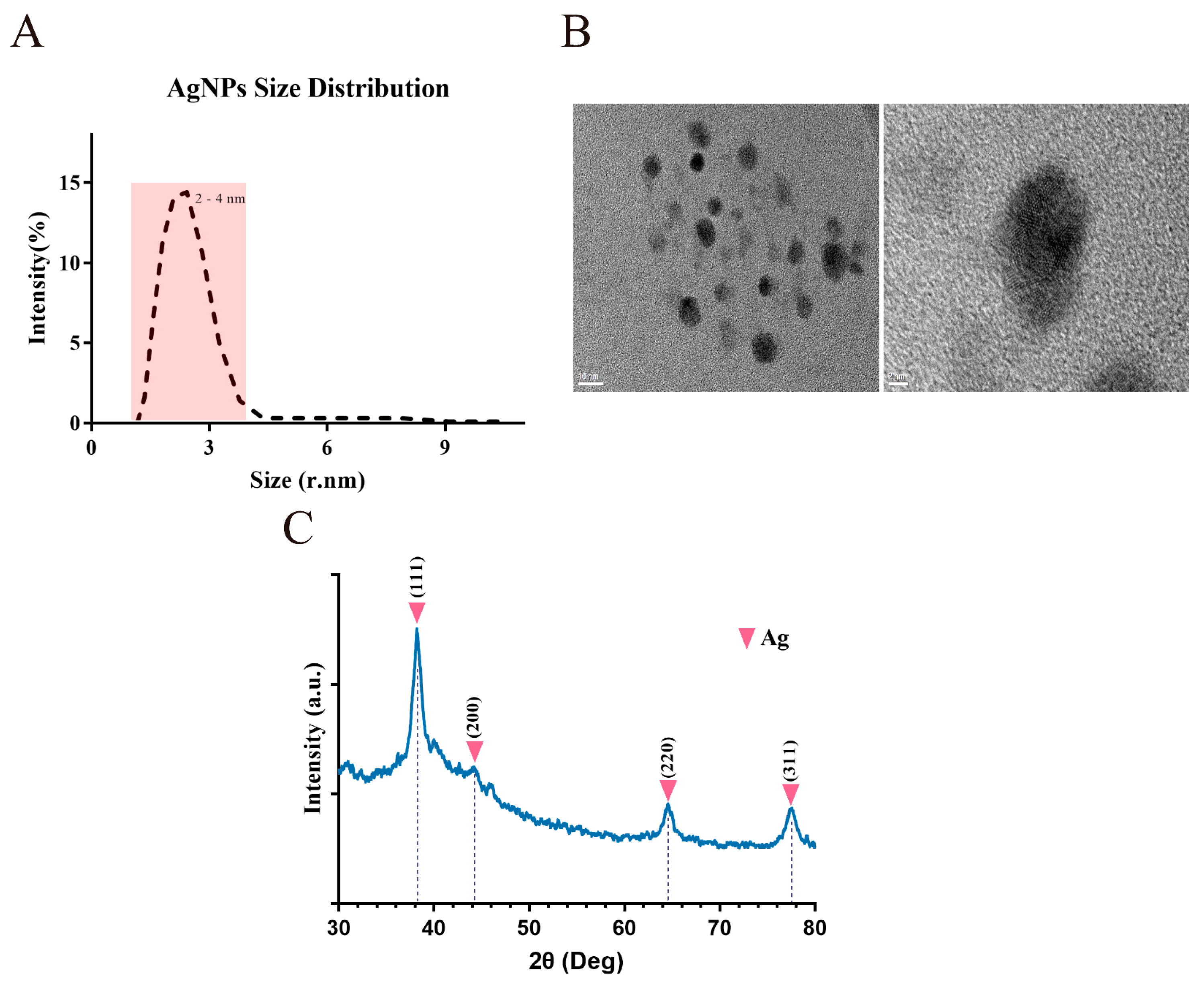
2.1. Synthesis and Characterization of the Nanometer Material
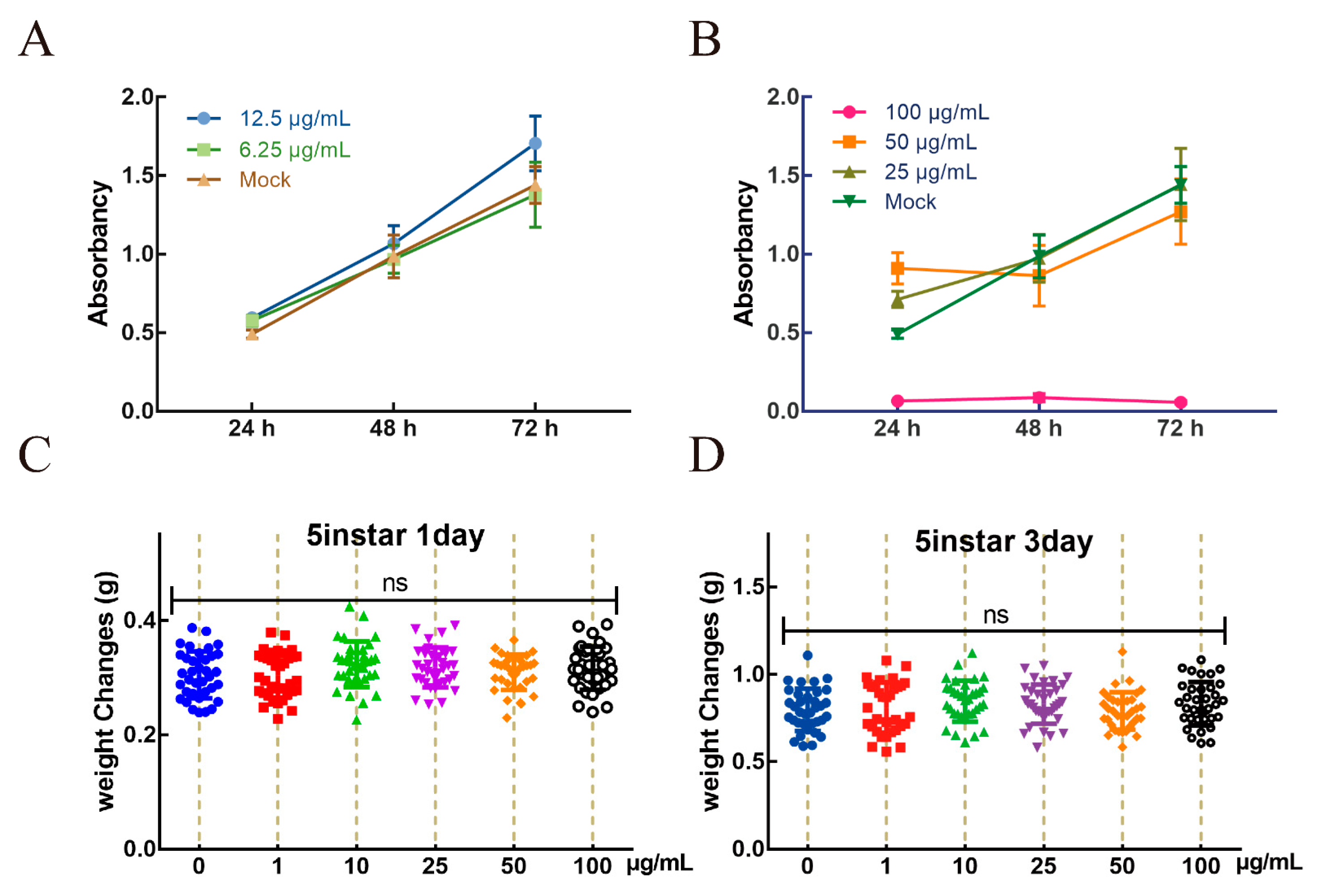
2.2. Determination of the Optimal Concentration of AgNPs
2.3. Effect of AgNPs on Growth Status of the Silkworm
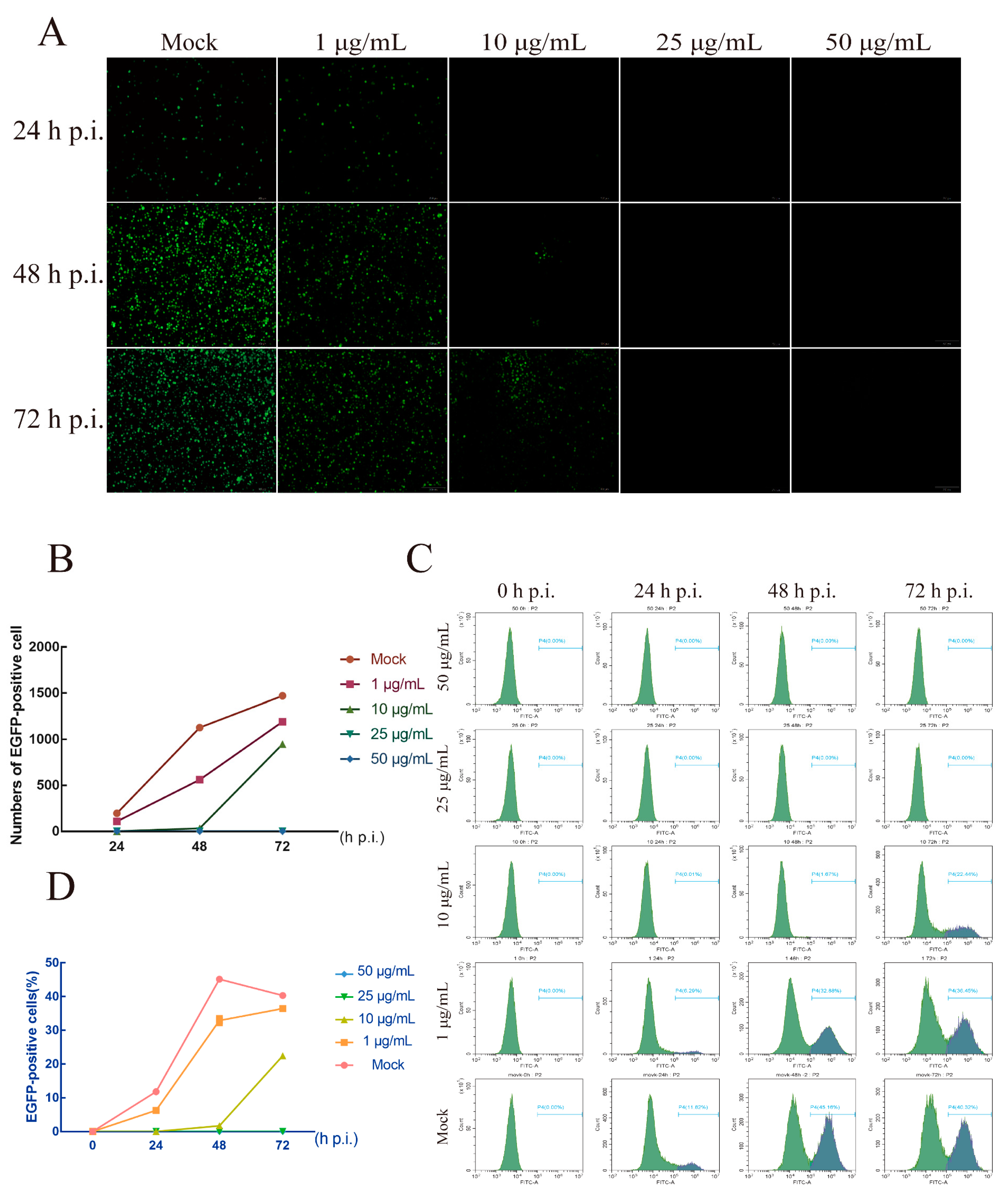
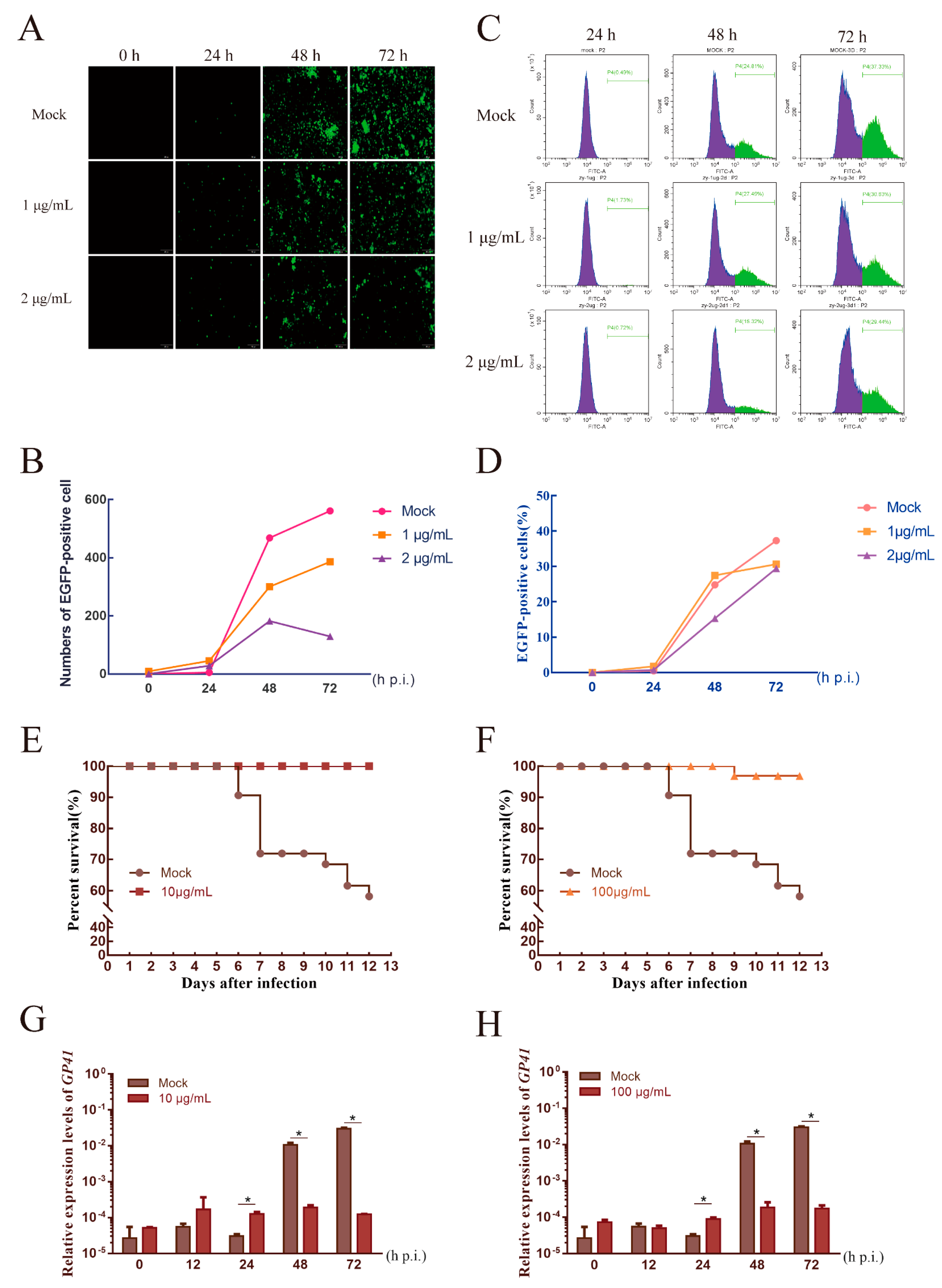
2.4. Influence of Morphology and Replication of BmNPV Treated by AgNPs
2.5. Fluorescence Observations
2.6. Flow Cytometry
2.7. Real-Time Fluorescent Quantitative PCR
2.8. Statistical Analysis
3. Results
3.1. BSA-Synthesis of Silver Nanoparticles
3.2. The Optimum Concentration of AgNPs In Vivo and In Vitro
3.3. The Effect of AgNPs on Morphology of BmNPV
3.4. AgNPs Can Inhibit BmNPV Multiplication
3.5. AgNPs as an Agent to Reduce the Infection Rate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Q.; Li, S.; Feng, Q. Advances in silkworm studies accelerated by the genome sequencing of Bombyx mori. Annu. Rev. Entomol. 2014, 59, 513–536. [Google Scholar] [CrossRef] [PubMed]
- Slack, J.; Arif, B.M. The Baculoviruses Occlusion-Derived Virus: Virion Structure and Function. Adv. Virus Res. 2006, 69, 99–165. [Google Scholar]
- Wang, X.Y.; Wu, K.H.; Pang, H.L.; Xu, P.Z.; Zhang, G.Z. Study on the Role of Cytc in Response to BmNPV Infection in Silkworm, Bombyx mori (Lepidoptera). Int. J. Mol. Sci. 2019, 20, 4325. [Google Scholar] [CrossRef]
- Jiang, L.; Goldsmith, M.R.; Xia, Q. Advances in the Arms Race between Silkworm and Baculovirus. Front. Immunol. 2021, 30, 628151. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Ding, X.Y.; Chen, Q.Y.; Zhang, K.X.; Li, M.W. Bmapaf-1 is Involved in the Response against BmNPV Infection by the Mitochondrial Apoptosis Pathway. Insects 2020, 11, 647. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef]
- Li, Z.; Song, G.; Bi, Y.; Gao, W.; Jiang, G. Occurrence and Distribution of Disinfection Byproducts in Domestic Wastewater Effluent, Tap Water, and Surface Water during the SARS-CoV-2 Pandemic in China. Environ. Sci. Technol. 2021, 55, 4103–4114. [Google Scholar] [CrossRef]
- Farouk, M.M.; El-Molla, A.; Salib, F.A.; Soliman, Y.A.; Shaalan, M. The Role of Silver Nanoparticles in a Treatment Approach for Multidrug-Resistant Salmonella Species Isolates. Int. J. Nanomed. 2020, 15, 6993–7011. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zheng, N.; Hu, C.; Huang, X.; Pan, M. Genetic bioengineering of overexpressed guanylate binding protein family BmAtlastin-n enhances silkworm resistance to Nosema bombycis. Int. J. Biol. Macromol. 2021, 172, 223–230. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, P.; Wang, G.; Cheng, T.; Yang, Q.; Jin, S.; Lin, P.; Xiao, Y.; Sun, Q.; Xia, Q. Comparison of factors that may affect the inhibitory efficacy of transgenic RNAi targeting of baculoviral genes in silkworm, Bombyx mori. Antivir. Res. 2013, 97, 255–263. [Google Scholar] [CrossRef]
- Liang, J.; Cheng, T.; Zhao, P.; Yang, Q.; Wang, G.; Jin, S.; Lin, P.; Xiao, Y.; Xia, Q.; Yuan, Y.A. Resistance to BmNPV via Overexpression of an Exogenous Gene Controlled by an Inducible Promoter and Enhancer in Transgenic Silkworm, Bombyx mori. PLoS ONE 2012, 7, e41838. [Google Scholar]
- Jiang, L.; Liu, W.; Guo, H.; Dang, Y.; Xia, Q. Distinct Functions of Bombyx mori Peptidoglycan Recognition Protein 2 in Immune Responses to Bacteria and Viruses. Front. Immunol. 2019, 10, 776. [Google Scholar] [CrossRef]
- Wesierska, M.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Dudek, J.; Polkowska-Motrenko, H. Audinot, Silver ions are responsible for memory impairment induced by oral administration of silver nanoparticles. Toxicol. Lett. 2018, 290, 133–144. [Google Scholar] [CrossRef]
- He, R.; Ren, F.; Chen, F. Embedded silver nanoparticles in KTP crystal produced by ion implantation. Mater. Lett. 2017, 193, 158–160. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles aginst HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvan, S.; Ashokkumar, T.; Govindaraju, K. Microscopy based studies on the interaction of bio-based silver nanoparticles with Bombyx mori Nuclear Polyhedrosis virus. J. Virol. Methods 2017, 242, 58–66. [Google Scholar] [CrossRef]
- Chen, L.; Meng, X.; Gu, J.; Fan, W.; Abdlli, N.; Peprah, F.A.; Wang, N.; Zhu, F.; Lü, P.; Ma, S.; et al. Silver nanoparticle toxicity in silkworms: Omics technologies for a mechanistic understanding. Ecotoxicol. Environ. Saf. 2019, 172, 388–395. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef]
- Zielewicz, J. A Review on Potential Role of Silver Nanoparticles and Possible Mechanisms of their Actions on Bacteria. Drug Res. 2017, 11, 70–76. [Google Scholar]
- Anna, K.; Dorota, K.; Bogumi, B. Action of Monomeric/Gemini Surfactants on Free Cells and Biofilm of Asaia lannensis. Molecules 2017, 22, 2036. [Google Scholar]
- Dee, S.; Deen, J.; Burns, D.; Douthit, G.; Pijoan, C. An assessment of sanitation protocols for commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 2004, 68, 208. [Google Scholar]
- Matteo, I.; Benedetta, M.; Sandra, C. Industrial Cooling Tower Disinfection Treatment to Prevent Legionella spp. Int. J. Environ. Res. Public Health 2017, 14, 1125. [Google Scholar]
- Hsueh, Y.H.; Lin, K.S.; Ke, W.J.; Hsieh, C.T.; Chiang, C.L.; Tzou, D.Y.; Liu, S.T. The Antimicrobial Properties of Silver Nanoparticles in Bacillus subtilis Are Mediated by Released Ag+ Ions. PLoS ONE 2015, 10, e0144306. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, I.V.; Farroukh, M.A.; Skomorokhova, E.A.; Rekstin, A.R.; Puchkova, L.V. Anti-Influenza Effect of Nanosilver in a Mouse Model. Vaccines 2020, 8, 679. [Google Scholar] [CrossRef]
- Blissard, G.W.; Theilmann, D.A. Baculovirus Entry and Egress from Insect Cells. Annu. Rev. Virol. 2018, 5, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Volodymyr, K.; Wang, Y.; Volodymyr, D. The bactericidal spectrum and virucidal effects of silver nanoparticles against the pathogens in sericulture. Open J. Anim. Sci. 2013, 3, 169–173. [Google Scholar] [CrossRef][Green Version]
- Zhou, W.; Zeng, C.; Liu, R.H.; Chen, J.; Wan, Y.J. Antiviral activity and specific modes of action of bacterial prodigiosin against Bombyx mori nucleopolyhedrovirus in vitro. Appl. Microbiol. Biotechnol. 2016, 100, 3979–3988. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, L.; Guo, H.; Sun, Q.; Wang, Y.; Xie, E.; Xia, Q. Screening of PI3K-Akt-targeting Drugs for Silkworm against Bombyx mori Nucleopolyhedrovirus. Molecules 2019, 24, 1260. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, J.; Xu, W.; Wang, H.; Kong, X.; Wu, X. Bombyx mori nucleopolyhedrovirus utilizes a clathrin and dynamin dependent endocytosis entry pathway into BmN cells. Virus Res. 2018, 253, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Arsenopoulou, Z.V.; Taitzoglou, I.A.; Molyvdas, P.A.; Gourgoulianis, K.I.; Hatzoglou, C.; Zarogiannis, S.G. Silver Nanoparticles Alter Cell Adhesion and Proliferation of Sheep Primary Mesothelial Cells. In Vivo 2018, 32, 109–112. [Google Scholar] [PubMed]
- Dong, Z.; Wu, Q.; Long, J.; Lu, B.; Zheng, N.; Hu, C.; Chen, P.; Hu, N.; Lu, C.; Pan, M. Silver nanoparticles are effective in controlling microsporidia. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021, 125, 112106. [Google Scholar]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, B.; Dong, Z.; Wu, Q.; Guo, B.; Fang, W.; Hu, C.; Long, J.; Chen, P.; Lu, C.; Pan, M. Analysis of Silver Nanoparticles for the Treatment and Prevention of Nucleopolyhedrovirus Affecting Bombyx mori. Int. J. Mol. Sci. 2022, 23, 6325. https://doi.org/10.3390/ijms23116325
Deng B, Dong Z, Wu Q, Guo B, Fang W, Hu C, Long J, Chen P, Lu C, Pan M. Analysis of Silver Nanoparticles for the Treatment and Prevention of Nucleopolyhedrovirus Affecting Bombyx mori. International Journal of Molecular Sciences. 2022; 23(11):6325. https://doi.org/10.3390/ijms23116325
Chicago/Turabian StyleDeng, Boyuan, Zhanqi Dong, Qin Wu, Bingyu Guo, Wenxuan Fang, Congwu Hu, Jiangqiong Long, Peng Chen, Cheng Lu, and Minhui Pan. 2022. "Analysis of Silver Nanoparticles for the Treatment and Prevention of Nucleopolyhedrovirus Affecting Bombyx mori" International Journal of Molecular Sciences 23, no. 11: 6325. https://doi.org/10.3390/ijms23116325
APA StyleDeng, B., Dong, Z., Wu, Q., Guo, B., Fang, W., Hu, C., Long, J., Chen, P., Lu, C., & Pan, M. (2022). Analysis of Silver Nanoparticles for the Treatment and Prevention of Nucleopolyhedrovirus Affecting Bombyx mori. International Journal of Molecular Sciences, 23(11), 6325. https://doi.org/10.3390/ijms23116325





