Lack of Functional P110δ Affects Expression of Activation Marker CD80 but Does Not Influence Functions of Neutrophils
Abstract
1. Introduction
2. Results
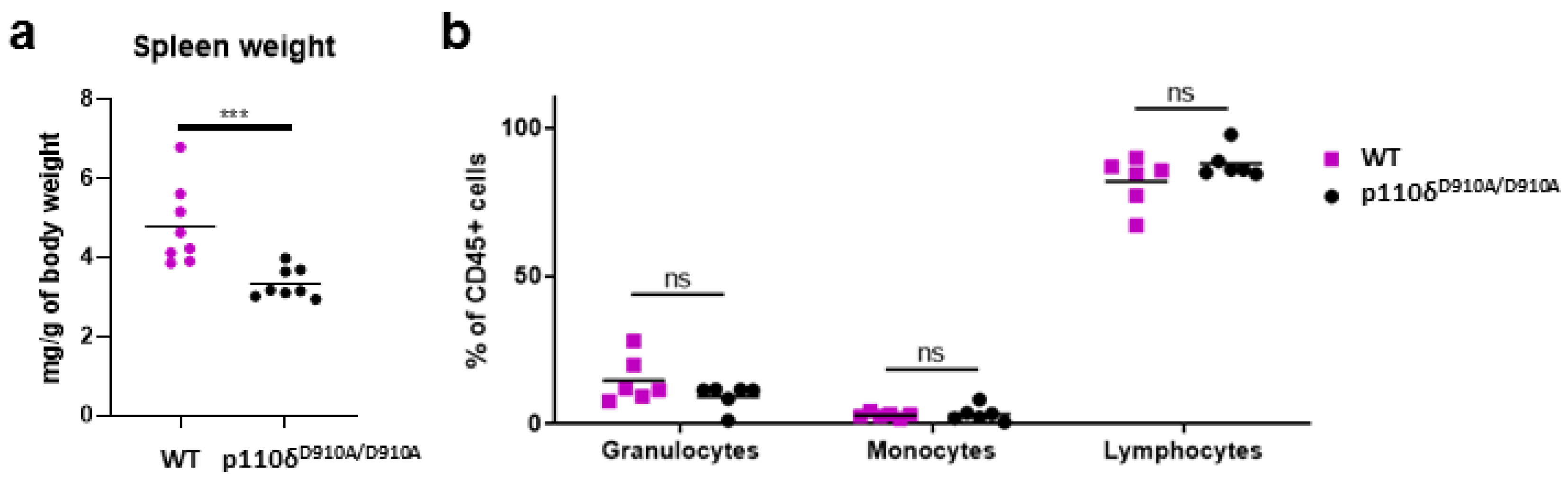
2.1. P110δD910A/D910A Mice Presented Unaltered White Blood Cell Percentages in Peripheral Blood
2.2. Neutrophils of P110δD910A/D910A Mice Were Characterized by Unaltered Granule Mobilization, Phagocytic Ability, ROS Synthesis and NET Formation
2.3. Neutrophils of P110δD910A/D910A Mice Revealed Unaltered Cell Survival In Vitro
2.4. Percentage of CD80- and CD86-Positive Cells Was Lower in P110δD910A/D910A Mice than in WT Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mice
4.3. Blood Collection and Staining
4.4. Cell Isolation
4.5. Analysis of Neutrophil Activation Markers
4.6. Degranulation Assay
4.7. Phagocytosis Assay
4.8. Neutrophil Extracellular Trap Formation Assays
4.9. Reactive Oxygen Species Formation Assay
4.10. Analysis of Cell Survival
4.11. Ex Vivo Stimulation of Cells and Intracellular Cytokine Staining
4.12. Bacterial Killing Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANCAs ATCC | Anti-neutrophil cytoplasmic antibodies American Type Culture Collection |
| BM | Bone marrow |
| DCs | Dendritic cells |
| FBS | Foetal bovine serum |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IFN | Interferon |
| IL | Interleukin |
| LB | Luria broth |
| LPS | Lipopolysaccharide |
| MFI | Median fluorescence intensity |
| NETs | Neutrophil extracellular traps |
| PAF | Platelet activating factor |
| PI3K | Phosphoinositide 3-kinases |
| PMA | Phorbol 12-myristate 13-acetate |
| ROS | Reactive oxygen species |
| RPMI | Roswell Park Memorial Institute |
| TNF | Tumour necrosis factor |
| WT | Wild type |
References
- Sadhu, C.; Dick, K.; Tino, W.T.; Staunton, D.E. Selective role of PI3K delta in neutrophil inflammatory responses. Biochem. Biophys. Res. Commun. 2003, 308, 764–769. [Google Scholar] [CrossRef]
- Hannigan, M.O.; Huang, C.K.; Wu, D.Q. Roles of PI3K in neutrophil function. Curr. Top. Microbiol. Immunol. 2004, 282, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.T.; Stephens, L.R. PI3K signalling in inflammation. Biochim. Biophys. Acta 2015, 1851, 882–897. [Google Scholar] [CrossRef]
- Chantry, D.; Vojtek, A.; Kashishian, A.; Holtzman, D.A.; Wood, C.; Gray, P.W.; Cooper, J.A.; Hoekstra, M.F. p110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J. Biol. Chem. 1997, 272, 19236–19241. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Bilancio, A.; Farjot, G.; Priddle, H.; Sancho, S.; Peskett, E.; Pearce, W.; Meek, S.E.; Salpekar, A.; Waterfield, M.D.; et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science 2002, 297, 1031–1034. [Google Scholar] [CrossRef]
- Rozman, S.; Yousefi, S.; Oberson, K.; Kaufmann, T.; Benarafa, C.; Simon, H.U. The generation of neutrophils in the bone marrow is controlled by autophagy. Cell Death Differ. 2015, 22, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Bucher, K.; Schmitt, F.; Mothes, B.; Blumendeller, C.; Schall, D.; Piekorz, R.; Hirsch, E.; Nurnberg, B.; Beer-Hammer, S. Deficiency of PI3-Kinase catalytic isoforms p110gamma and p110delta in mice enhances the IL-17/G-CSF axis and induces neutrophilia. Cell Commun. Signal. 2017, 15, 28. [Google Scholar] [CrossRef]
- Hirsch, E.; Katanaev, V.L.; Garlanda, C.; Azzolino, O.; Pirola, L.; Silengo, L.; Sozzani, S.; Mantovani, A.; Altruda, F.; Wymann, M.P. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000, 287, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Irie-Sasaki, J.; Jones, R.G.; Oliveira-dos-Santos, A.J.; Stanford, W.L.; Bolon, B.; Wakeham, A.; Itie, A.; Bouchard, D.; Kozieradzki, I.; et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 2000, 287, 1040–1046. [Google Scholar] [CrossRef]
- Fumagalli, L.; Campa, C.C.; Germena, G.; Lowell, C.A.; Hirsch, E.; Berton, G. Class I phosphoinositide-3-kinases and SRC kinases play a nonredundant role in regulation of adhesion-independent and -dependent neutrophil reactive oxygen species generation. J. Immunol. 2013, 190, 3648–3660. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, A.M.; Davidson, K.; Anderson, K.E.; Ellson, C.D.; Crabbe, T.; Okkenhaug, K.; Vanhaesebroeck, B.; Turner, M.; Webb, L.; Wymann, M.P.; et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 2005, 106, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Kampe, M.; Lampinen, M.; Stolt, I.; Janson, C.; Stalenheim, G.; Carlson, M. PI3-kinase regulates eosinophil and neutrophil degranulation in patients with allergic rhinitis and allergic asthma irrespective of allergen challenge model. Inflammation 2012, 35, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Tang, Y.; Chen, J.; Li, J.; Wang, H.; Lu, M.; Gao, L.; Liu, F.; Chang, P.; Liu, X.; et al. Impaired airway epithelial barrier integrity was mediated by PI3Kdelta in a mouse model of lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol. 2021, 95, 107570. [Google Scholar] [CrossRef] [PubMed]
- Lodge, K.M.; Cowburn, A.S.; Li, W.; Condliffe, A.M. The Impact of Hypoxia on Neutrophil Degranulation and Consequences for the Host. Int. J. Mol. Sci. 2020, 21, 1183. [Google Scholar] [CrossRef]
- Hoenderdos, K.; Lodge, K.M.; Hirst, R.A.; Chen, C.; Palazzo, S.G.; Emerenciana, A.; Summers, C.; Angyal, A.; Porter, L.; Juss, J.K.; et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 2016, 71, 1030–1038. [Google Scholar] [CrossRef]
- Fensome, A.; Cunningham, E.; Prosser, S.; Tan, S.K.; Swigart, P.; Thomas, G.; Hsuan, J.; Cockcroft, S. ARF and PITP restore GTP gamma S-stimulated protein secretion from cytosol-depleted HL60 cells by promoting PIP2 synthesis. Curr. Biol. 1996, 6, 730–738. [Google Scholar] [CrossRef]
- Vieira, O.V.; Botelho, R.J.; Rameh, L.; Brachmann, S.M.; Matsuo, T.; Davidson, H.W.; Schreiber, A.; Backer, J.M.; Cantley, L.C.; Grinstein, S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell. Biol. 2001, 155, 19–25. [Google Scholar] [CrossRef]
- Tamura, N.; Hazeki, K.; Okazaki, N.; Kametani, Y.; Murakami, H.; Takaba, Y.; Ishikawa, Y.; Nigorikawa, K.; Hazeki, O. Specific role of phosphoinositide 3-kinase p110alpha in the regulation of phagocytosis and pinocytosis in macrophages. Biochem. J. 2009, 423, 99–108. [Google Scholar] [CrossRef]
- Yu, X.; Long, Y.C.; Shen, H.M. Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy 2015, 11, 1711–1728. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, E.; Martin-Cordero, L.; Hinchado, M.D.; Garcia, J.J.; Ortega, E. Role of phosphatidylinositol-3-kinase (PI3K), extracellular signal-regulated kinase (ERK) and nuclear transcription factor kappa β (NF-k β) on neutrophil phagocytic process of Candida albicans. Mol. Cell. Biochem. 2010, 333, 115–120. [Google Scholar] [CrossRef]
- Manda-Handzlik, A.; Bystrzycka, W.; Cieloch, A.; Glodkowska-Mrowka, E.; Jankowska-Steifer, E.; Heropolitanska-Pliszka, E.; Skrobot, A.; Muchowicz, A.; Ciepiela, O.; Wachowska, M.; et al. Nitric oxide and peroxynitrite trigger and enhance release of neutrophil extracellular traps. Cell. Mol. Life Sci. 2019, 77, 3059–3075. [Google Scholar] [CrossRef] [PubMed]
- Romao, S.; Tejera, E.; Nytko, K.J.; Siler, U.; Munz, C.; Reichenbach, J. Defective nuclear entry of hydrolases prevents neutrophil extracellular trap formation in patients with chronic granulomatous disease. J. Allergy Clin. Immunol. 2015, 136, 1703–1706.e5. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Stojkov, D.; Oberson, K.; Yousefi, S.; Simon, H.U. Neither eosinophils nor neutrophils require ATG5-dependent autophagy for extracellular DNA trap formation. Immunology 2017, 152, 517–525. [Google Scholar] [CrossRef]
- DeSouza-Vieira, T.; Guimaraes-Costa, A.; Rochael, N.C.; Lira, M.N.; Nascimento, M.T.; Lima-Gomez, P.S.; Mariante, R.M.; Persechini, P.M.; Saraiva, E.M. Neutrophil extracellular traps release induced by Leishmania: Role of PI3Kgamma, ERK, PI3Ksigma, PKC, and [Ca2+]. J. Leukoc. Biol. 2016, 100, 801–810. [Google Scholar] [CrossRef]
- Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, With Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Matsuyama, Y.; Araki, S.; Koizumi, A.; Kariya, Y.; Takasuga, S.; Eguchi, S.; Nakanishi, H.; Sasaki, J.; Sasaki, T. The effect and possible clinical efficacy of in vivo inhibition of neutrophil extracellular traps by blockade of PI3K-gamma on the pathogenesis of microscopic polyangiitis. Mod. Rheumatol. 2018, 28, 530–541. [Google Scholar] [CrossRef]
- Uno, J.K.; Rao, K.N.; Matsuoka, K.; Sheikh, S.Z.; Kobayashi, T.; Li, F.; Steinbach, E.C.; Sepulveda, A.R.; Vanhaesebroeck, B.; Sartor, R.B.; et al. Altered macrophage function contributes to colitis in mice defective in the phosphoinositide-3 kinase subunit p110delta. Gastroenterology 2010, 139, 1642–1653.e6. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; Filep, J.G. Modulation of Neutrophil Apoptosis and the Resolution of Inflammation through β2 Integrins. Front. Immunol. 2013, 4, 60. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Voyich, J.M.; Whitney, A.R.; DeLeo, F.R. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J. Leukoc. Biol. 2005, 78, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Juss, J.K.; Hayhoe, R.P.; Owen, C.E.; Bruce, I.; Walmsley, S.R.; Cowburn, A.S.; Kulkarni, S.; Boyle, K.B.; Stephens, L.; Hawkins, P.T.; et al. Functional redundancy of class I phosphoinositide 3-kinase (PI3K) isoforms in signaling growth factor-mediated human neutrophil survival. PLoS ONE 2012, 7, e45933. [Google Scholar] [CrossRef]
- Takashima, A.; Yao, Y. Neutrophil plasticity: Acquisition of phenotype and functionality of antigen-presenting cell. J. Leukoc. Biol. 2015, 98, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, G.P.; Ahmed, Z.; Perry, N.; Davison, M.; Lupton, A.; Young, B. Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation. Immunology 2005, 114, 354–368. [Google Scholar] [CrossRef]
- Son, Y.; Kim, B.Y.; Park, Y.C.; Eo, S.K.; Cho, H.R.; Kim, K. PI3K and ERK signaling pathways are involved in differentiation of monocytic cells induced by 27-hydroxycholesterol. Korean J. Physiol. Pharm. 2017, 21, 301–308. [Google Scholar] [CrossRef]
- Xue, Z.; Li, W.; Wang, H.; Huang, B.; Ge, Z.; Gu, C.; Liu, Y.; Zhang, K.; Yang, J.; Han, R.; et al. ZSTK474, a novel PI3K inhibitor, modulates human CD14+ monocyte-derived dendritic cell functions and suppresses experimental autoimmune encephalomyelitis. J. Mol. Med. 2014, 92, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Law, H.K.; Lau, Y.L. Insulin-like growth factor I promotes maturation and inhibits apoptosis of immature cord blood monocyte-derived dendritic cells through MEK and PI 3-kinase pathways. Pediatr. Res. 2003, 54, 919–925. [Google Scholar] [CrossRef][Green Version]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011, 13, 170–180. [Google Scholar] [CrossRef]
- Low, P.C.; Misaki, R.; Schroder, K.; Stanley, A.C.; Sweet, M.J.; Teasdale, R.D.; Vanhaesebroeck, B.; Meunier, F.A.; Taguchi, T.; Stow, J.L. Phosphoinositide 3-kinase delta regulates membrane fission of Golgi carriers for selective cytokine secretion. J. Cell. Biol. 2010, 190, 1053–1065. [Google Scholar] [CrossRef]
- Fortin, C.F.; Cloutier, A.; Ear, T.; Sylvain-Prevost, S.; Mayer, T.Z.; Bouchelaghem, R.; McDonald, P.P. A class IA PI3K controls inflammatory cytokine production in human neutrophils. Eur. J. Immunol. 2011, 41, 1709–1719. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Wang, S.; Lin, J.; Liang, J.; Xu, J. Azithromycin promotes alternatively activated macrophage phenotype in systematic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death Dis. 2018, 9, 1080. [Google Scholar] [CrossRef]
- Fujita, A.; Kan, O.K.; Tonai, K.; Yamamoto, N.; Ogawa, T.; Fukuyama, S.; Nakanishi, Y.; Matsumoto, K. Inhibition of PI3Kdelta Enhances Poly I:C-Induced Antiviral Responses and Inhibits Replication of Human Metapneumovirus in Murine Lungs and Human Bronchial Epithelial Cells. Front. Immunol. 2020, 11, 432. [Google Scholar] [CrossRef]
- Okeke, E.B.; Mou, Z.; Onyilagha, N.; Jia, P.; Gounni, A.S.; Uzonna, J.E. Deficiency of Phosphatidylinositol 3-Kinase delta Signaling Leads to Diminished Numbers of Regulatory T Cells and Increased Neutrophil Activity Resulting in Mortality Due to Endotoxic Shock. J. Immunol. 2017, 199, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, D.; Amini, P.; Oberson, K.; Sokollik, C.; Duppenthaler, A.; Simon, H.U.; Yousefi, S. ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J. Cell. Biol. 2017, 216, 4073–4090. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manda-Handzlik, A.; Mroczek, A.; Kuźmicka, W.; Cieloch, A.; Homoncik, Z.; Muchowicz, A.; Demkow, U.; Wachowska, M. Lack of Functional P110δ Affects Expression of Activation Marker CD80 but Does Not Influence Functions of Neutrophils. Int. J. Mol. Sci. 2022, 23, 6361. https://doi.org/10.3390/ijms23126361
Manda-Handzlik A, Mroczek A, Kuźmicka W, Cieloch A, Homoncik Z, Muchowicz A, Demkow U, Wachowska M. Lack of Functional P110δ Affects Expression of Activation Marker CD80 but Does Not Influence Functions of Neutrophils. International Journal of Molecular Sciences. 2022; 23(12):6361. https://doi.org/10.3390/ijms23126361
Chicago/Turabian StyleManda-Handzlik, Aneta, Agnieszka Mroczek, Weronika Kuźmicka, Adrianna Cieloch, Zuzanna Homoncik, Angelika Muchowicz, Urszula Demkow, and Małgorzata Wachowska. 2022. "Lack of Functional P110δ Affects Expression of Activation Marker CD80 but Does Not Influence Functions of Neutrophils" International Journal of Molecular Sciences 23, no. 12: 6361. https://doi.org/10.3390/ijms23126361
APA StyleManda-Handzlik, A., Mroczek, A., Kuźmicka, W., Cieloch, A., Homoncik, Z., Muchowicz, A., Demkow, U., & Wachowska, M. (2022). Lack of Functional P110δ Affects Expression of Activation Marker CD80 but Does Not Influence Functions of Neutrophils. International Journal of Molecular Sciences, 23(12), 6361. https://doi.org/10.3390/ijms23126361





