Metabolomic Profiling of Angiotensin-II-Induced Abdominal Aortic Aneurysm in Ldlr−/− Mice Points to Alteration of Nitric Oxide, Lipid, and Energy Metabolisms
Abstract
1. Introduction
2. Results
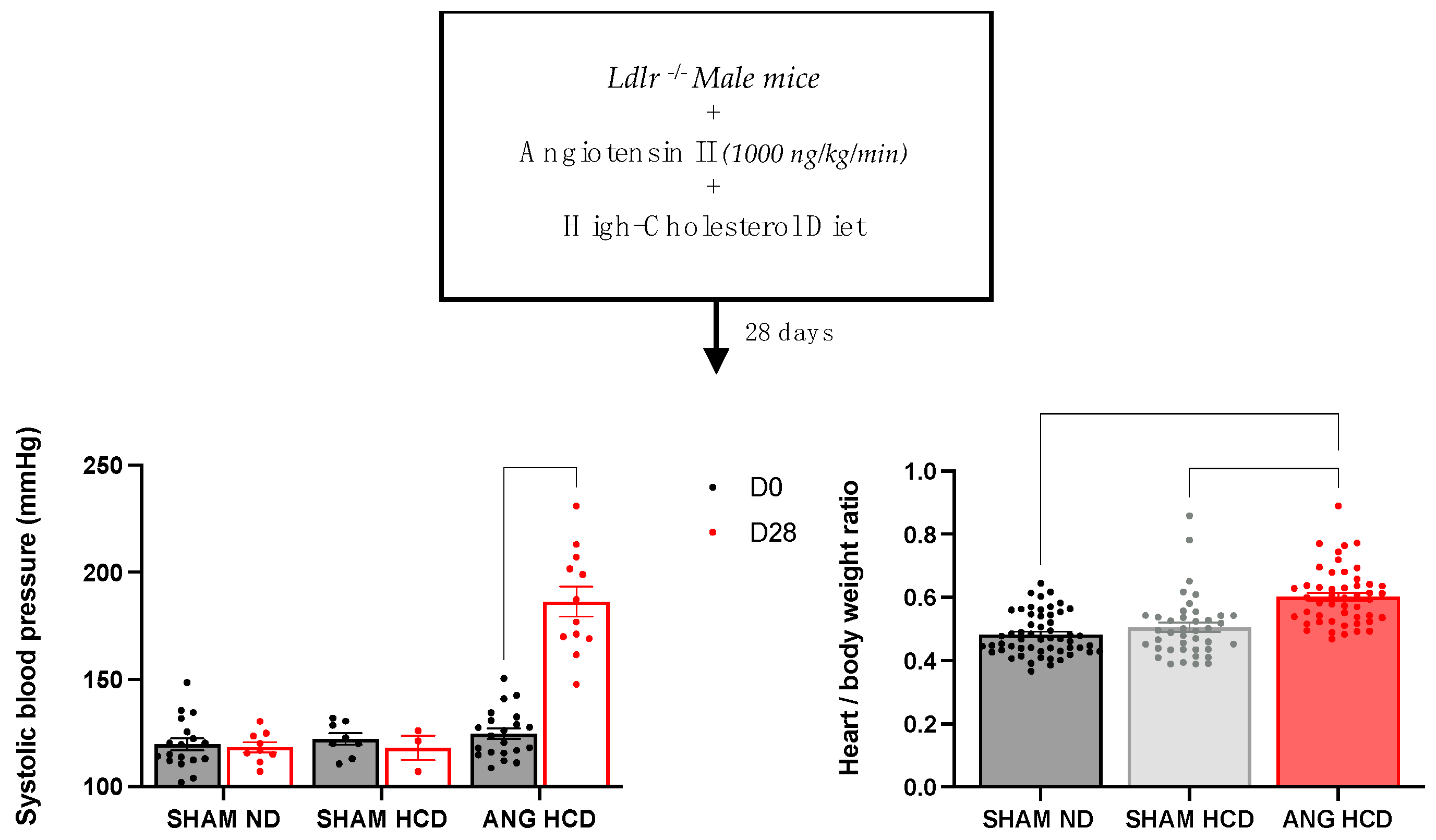
2.1. Hypertension Induces Cardiac Remodeling in Mice Fed a Cholesterol-Enriched Diet
2.2. Lipid Profile in Ldlr−/− Mice with or without Hypercholesterolemic Diet and Angiotensin II Treatment
2.3. Hypertrophic Remodelling with Lipid Deposition of Abdominal Aorta in Ldlr−/− Mice with High-Cholesterol Diet and Angiotensin II Treatment
2.4. Metabolomic Data Analysis
2.4.1. Analysis of Plasma and Aorta Metabolomes
2.4.2. Analysis of Plasma Block Metabolomic Signature of Aneurysmal Risk
2.4.3. Plasma Biomarker Research of Aortic Aneurysm
2.4.4. Analysis of the Aortic Block Metabolomic Signature of Aneurysmal Risk
3. Discussion
4. Materials and Methods
4.1. Animal Models and Diet
4.2. Hypertension Induction
4.3. Blood Pressure and Bodyweight Measurement
4.4. Sacrifice, Samples Extraction and Lipid Profil
4.5. Lipid Profile
4.6. Histomorphologic Analysis
4.7. Sample Preparation for Metabolomic Analysis
4.8. Mass Spectrometry Analysis of Plasma and Aortas Extracts
4.9. Statistical Analysis of Metabolomic Data
4.9.1. Univariate Analysis
4.9.2. Multivariate Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosures
Abbreviations
| 4A | α-aminoadipic acid |
| AAA | Abdominal aortic aneurysm |
| ADMA | Asymmetric dimethylarginine |
| ANG | Angiotensin II |
| BCAA | Branched-chain aminoacids |
| BCAT2 | Branched-chain amino acid transaminase 2 |
| C0 | Free carnitine |
| C2 | Acetylcarnitine |
| C3 | Propionylcarnitine |
| DDAH | Dimethylarginine dimethylaminohydrolase |
| DMA | Dimethylarginine |
| FDR | False discovery rate |
| Gln | Glutamine |
| Gly | Glycine |
| GLYAT | Glycine N-acyltransferase |
| HCD | High-cholesterol diet |
| HDLc | Circulating high-density lipoprotein |
| Ile | Isoleucine |
| LC | Liquid chromatography |
| LCAC | Long-chain acylcarnitine |
| LDLc | Circulating low-density lipoprotein |
| LDLr | Low-density lipoprotein receptor |
| Leu | Leucine |
| LysoPC | Lysophosphatidylcholine |
| Met | Methionine |
| MMP | Matrix metalloproteinase |
| MOCA | Multiblock orthogonal component analysis |
| NAD | Nicotinamide adenine dinucleotide |
| ND | Normal diet |
| NOS | Nitric oxide synthase |
| ODC | Ornithine decarboxylase |
| PC | Phosphatidylcholine |
| Phe | Phenylalanine |
| PLA1 | Phospholipase A1 |
| SAM | S-adenosyl methionine |
| SBP | Systolic blood pressure |
| SM | Sphingomyelin |
| SMSA | S-methyl-thioadenosine |
| t4 OH-Proline | Trans-4-hydroxyproline |
| TCA | Tricarboxylic acid |
| Thr | Threonine |
| TIMP | Tissue inhibitor of metalloproteinase |
| Val | Valine |
| VLDL | Very-low-density lipoprotein |
| VSMC | Vascular smooth muscle cell |
| α-KG | α-ketoglutarate |
References
- Kuzmik, G.A.; Sang, A.X.; Elefteriades, J.A. Natural history of thoracic aortic aneurysms. J. Vasc. Surg. 2012, 56, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Williams, C.; Sweeting, M.J.; Jacomelli, J.; Summers, L.; Stevenson, A.; Lees, T.; Earnshaw, J.J. Safety of Men with Small and Medium Abdominal Aortic Aneurysms Under Surveillance in the NAAASP. Circulation 2019, 139, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, V.; Jacob, M.P.; Houard, X.; Rossignol, P.; Plissonnier, D.; Angles-Cano, E.; Michel, J.B. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am. J. Pathol. 2002, 161, 1701–1710. [Google Scholar] [CrossRef]
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.; van Keulen, J.W.; Rantner, B.; et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41 (Suppl. S1), S1–S58. [Google Scholar] [CrossRef]
- Michel, J.B.; Thaunat, O.; Houard, X.; Meilhac, O.; Caligiuri, G.; Nicoletti, A. Topological determinants and consequences of adventitial responses to arterial wall injury. Arter. Thromb. Vasc. Biol. 2007, 27, 1259–1268. [Google Scholar] [CrossRef]
- Miyake, T.; Morishita, R. Pharmacological treatment of abdominal aortic aneurysm. Cardiovasc. Res. 2009, 83, 436–443. [Google Scholar] [CrossRef]
- Chiou, A.C.; Chiu, B.; Pearce, W.H. Murine aortic aneurysm produced by periarterial application of calcium chloride. J. Surg. Res. 2001, 99, 371–376. [Google Scholar] [CrossRef]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef]
- Bhamidipati, C.M.; Mehta, G.S.; Lu, G.; Moehle, C.W.; Barbery, C.; DiMusto, P.D.; Laser, A.; Kron, I.L.; Upchurch, G.R., Jr.; Ailawadi, G. Development of a novel murine model of aortic aneurysms using peri-adventitial elastase. Surgery 2012, 152, 238–246. [Google Scholar] [CrossRef]
- Ishibashi, S.; Goldstein, J.L.; Brown, M.S.; Herz, J.; Burns, D.K. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Investig. 1994, 93, 1885–1893. [Google Scholar] [CrossRef]
- Defesche, J.C. Low-density lipoprotein receptor--its structure, function, and mutations. Semin. Vasc. Med. 2004, 4, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Oppi, S.; Lüscher, T.F.; Stein, S. Mouse Models for Atherosclerosis Research-Which Is My Line? Front. Cardiovasc. Med. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Reddick, R.L.; Piedrahita, J.A.; Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992, 258, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Cassis, L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann. N. Y. Acad. Sci. 1999, 892, 108–118. [Google Scholar] [CrossRef]
- Hungerford, J.E.; Little, C.D. Developmental biology of the vascular smooth muscle cell: Building a multilayered vessel wall. J. Vasc. Res. 1999, 36, 2–27. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Nishimura, M.; Yamashita, A.; Matsuura, Y.; Okutsu, J.; Fukahori, A.; Hirata, T.; Nishizawa, T.; Ishii, H.; Maekawa, K.; Nakamura, E.; et al. Upregulated Kynurenine Pathway Enzymes in Aortic Atherosclerotic Aneurysm: Macrophage Kynureninase Downregulates Inflammation. J. Atheroscler. Thromb. 2021, 28, 1214–1240. [Google Scholar] [CrossRef]
- Doppler, C.; Arnhard, K.; Dumfarth, J.; Heinz, K.; Messner, B.; Stern, C.; Koal, T.; Klavins, K.; Danzl, K.; Pitterl, F.; et al. Metabolomic profiling of ascending thoracic aortic aneurysms and dissections—Implications for pathophysiology and biomarker discovery. PLoS ONE 2017, 12, e0176727. [Google Scholar] [CrossRef]
- Lieberg, J.; Wanhainen, A.; Ottas, A.; Vähi, M.; Zilmer, M.; Soomets, U.; Björck, M.; Kals, J. Metabolomic Profile of Abdominal Aortic Aneurysm. Metabolites 2021, 11, 555. [Google Scholar] [CrossRef]
- Ciborowski, M.; Teul, J.; Martin-Ventura, J.L.; Egido, J.; Barbas, C. Metabolomics with LC-QTOF-MS permits the prediction of disease stage in aortic abdominal aneurysm based on plasma metabolic fingerprint. PLoS ONE 2012, 7, e31982. [Google Scholar] [CrossRef]
- Ren, Y.; Tang, Q.; Liu, W.; Tang, Y.; Zhu, R.; Li, B. Serum Biomarker Identification by Mass Spectrometry in Acute Aortic Dissection. Cell Physiol. Biochem. 2017, 44, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, R.; Zhang, T.; Liu, F.; Zhang, W.; Wang, G.; Gu, G.; Han, Q.; Xu, D.; Yao, C.; et al. Identification of Lysophosphatidylcholines and Sphingolipids as Potential Biomarkers for Acute Aortic Dissection via Serum Metabolomics. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, F.; Luo, M.; Chen, Q.; Liu, X.; Zhang, Y.; Zhu, G.; Chen, W.; Li, T.; Shu, C.; et al. Metabolomic Profile Reveals That Ceramide Metabolic Disturbance Plays an Important Role in Thoracic Aortic Dissection. Front. Cardiovasc. Med. 2022, 9, 826861. [Google Scholar] [CrossRef]
- Jeong, T.; Schissel, S.L.; Tabas, I.; Pownall, H.J.; Tall, A.R.; Jiang, X. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J. Clin. Investig. 1998, 101, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Reyes, J.A.; Arellano-Plancarte, A.; Castillo-Hernandez, J.R. Angiotensin II and the development of insulin resistance: Implications for diabetes. Mol. Cell Endocrinol. 2009, 302, 128–139. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef] [PubMed]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- ter Veld, F.; Primassin, S.; Hoffmann, L.; Mayatepek, E.; Spiekerkoetter, U. Corresponding increase in long-chain acyl-CoA and acylcarnitine after exercise in muscle from VLCAD mice. J. Lipid Res. 2009, 50, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, C.P.; van der Sluis, R.; Erasmus, E.; van Dijk, A.A. Glycine conjugation: Importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1139–1153. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Wu, J.; Wu, S.; Xu, G.; Wei, D. Essential Role of Nonessential Amino Acid Glutamine in Atherosclerotic Cardiovascular Disease. DNA Cell Biol. 2020, 39, 8–15. [Google Scholar] [CrossRef]
- Alipanah-Moghadam, R.; Molazadeh, L.; Jafari-Suha, Z.; Naghizadeh-Baghi, A.; Mohajeri, M.; Nemati, A. Glutamine supplementation can reduce some atherosclerosis markers after exhaustive exercise in young healthy males. Nutrition 2022, 94, 111506. [Google Scholar] [CrossRef] [PubMed]
- Zaric, B.L.; Radovanovic, J.N.; Gluvic, Z.; Stewart, A.J.; Essack, M.; Motwalli, O.; Gojobori, T.; Isenovic, E.R. Atherosclerosis Linked to Aberrant Amino Acid Metabolism and Immunosuppressive Amino Acid Catabolizing Enzymes. Front. Immunol. 2020, 11, 551758. [Google Scholar] [CrossRef] [PubMed]
- Sayed-Ahmed, M.M.; Khattab, M.M.; Gad, M.Z.; Mostafa, N. L-carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic rabbits. Pharm. Res. 2001, 44, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.L.; Geng, Y.J.; Sukhova, G.K.; Whittemore, A.D.; Knox, J.; Libby, P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation 1999, 99, 96–104. [Google Scholar] [CrossRef]
- Golledge, J.; Mallat, Z.; Tedgui, A.; Norman, P.E. Serum secreted phospholipase A2 is associated with abdominal aortic aneurysm presence but not progression. Atherosclerosis 2011, 216, 458–460. [Google Scholar] [CrossRef]
- Bouchareb, R.; Boulanger, M.C.; Tastet, L.; Mkannez, G.; Nsaibia, M.J.; Hadji, F.; Dahou, A.; Messadeq, Y.; Arsenault, B.J.; Pibarot, P.; et al. Activated platelets promote an osteogenic programme and the progression of calcific aortic valve stenosis. Eur. Heart J. 2019, 40, 1362–1373. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, Y.; Yang, G.; He, Z.; Zhang, H.; Peng, Z.; Peng, W.; Guo, T.; Zeng, M.; Ding, N.; et al. Lysophosphatidic Acid May Be a Novel Biomarker for Early Acute Aortic Dissection. Front. Surg. 2021, 8, 789992. [Google Scholar] [CrossRef]
- Durante, W. Role of arginase in vessel wall remodeling. Front. Immunol. 2013, 4, 111. [Google Scholar] [CrossRef]
- Fiedler, L. The DDAH/ADMA pathway is a critical regulator of NO signalling in vascular homeostasis. Cell Adh. Migr. 2008, 2, 149–150. [Google Scholar] [CrossRef][Green Version]
- Forte, A.; Balistreri, C.R.; De Feo, M.; Della Corte, A.; Hellstrand, P.; Persson, L.; Nilsson, B.O. Polyamines and microbiota in bicuspid and tricuspid aortic valve aortopathy. J. Mol. Cell Cardiol. 2019, 129, 179–187. [Google Scholar] [CrossRef]
- Lizarbe, T.R.; Tarín, C.; Gómez, M.; Lavin, B.; Aracil, E.; Orte, L.M.; Zaragoza, C. Nitric oxide induces the progression of abdominal aortic aneurysms through the matrix metalloproteinase inducer EMMPRIN. Am. J. Pathol. 2009, 175, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, W.; Tian, M.; Chen, H.; Li, C.; Zhang, L.; Yang, Y.; Wang, J.; Ji, M.; Yang, C.; et al. The short-term safety and effectiveness of a new distal perforating stent graft in Type B aortic dissection: A retrospective study. BMC Cardiovasc. Disord. 2021, 21, 457. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, A.; Matsui, I.; Hamano, T.; Ishimoto, T.; Katou, Y.; Takehana, K.; Inoue, K.; Kusunoki, Y.; Mori, D.; Nakano, C.; et al. Dietary L-lysine prevents arterial calcification in adenine-induced uremic rats. J. Am. Soc. Nephrol. 2014, 25, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Kools, J.J.; Taylor, W.R. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation 2001, 103, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.W.; Cassis, L.A.; Daugherty, A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arter. Thromb. Vasc. Biol. 2003, 23, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.G.; Martin-McNulty, B.; Sukovich, D.A.; Freay, A.; Halks-Miller, M.; Thinnes, T.; Loskutoff, D.J.; Carmeliet, P.; Dole, W.P.; Wang, Y.X. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ. Res. 2003, 92, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Brown, M.S.; Goldstein, J.L.; Gerard, R.D.; Hammer, R.E.; Herz, J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Investig. 1993, 92, 883–893. [Google Scholar] [CrossRef]
- Cassis, L.A.; Gupte, M.; Thayer, S.; Zhang, X.; Charnigo, R.; Howatt, D.A.; Rateri, D.L.; Daugherty, A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1660-5. [Google Scholar] [CrossRef]
- Owens, A.P., 3rd; Rateri, D.L.; Howatt, D.A.; Moore, K.J.; Tobias, P.S.; Curtiss, L.K.; Lu, H.; Cassis, L.A.; Daugherty, A. MyD88 deficiency attenuates angiotensin II-induced abdominal aortic aneurysm formation independent of signaling through Toll-like receptors 2 and 4. Arter. Thromb. Vasc. Biol. 2011, 31, 2813–2819. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Schmidt, S.; Schindler, M.; Eriksson, L. Block-wise exploration of molecular descriptors with Multi-block Orthogonal Component Analysis (MOCA). Mol. Inf. 2021, 41, 2100165. [Google Scholar] [CrossRef] [PubMed]








| Normal Diet (n = 7) | High-Cholesterol Diet (n = 7) | High-Cholesterol Diet + Angiotensin II Treatment (n = 2–5) | |
|---|---|---|---|
| Plasma cholesterol (mmol/L) | 13.96 ± 1.38 | 11.09 ± 2.03 | 23.4 ± 1.4 |
| Plasma triglycerids (mmol/L) | 4.58 ± 0.83 | 0.99 ± 0.1 * | 2.27 ± 1.07 |
| Plasma HDLc (mmol/L) | 1.06 ± 0.18 | 0.39 ± 0.08 | 0.94 ± 0.37 |
| Plasma LDLc (mmol/L) | 6.82 ± 1.29 | 10.24 ± 1.97 | 23.4 ± 1.041 * |
| LDLc/HDLc Ratio | 9.1 ± 3.16 | 29.84 ± 5.18 | 80.69 ± 32.76 |
| Total cholesterol/HDLc | 15.72 ± 2.679 | 32.43 ± 5.425 | 117 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao de la Barca, J.M.; Richard, A.; Robert, P.; Eid, M.; Fouquet, O.; Tessier, L.; Wetterwald, C.; Faure, J.; Fassot, C.; Henrion, D.; et al. Metabolomic Profiling of Angiotensin-II-Induced Abdominal Aortic Aneurysm in Ldlr−/− Mice Points to Alteration of Nitric Oxide, Lipid, and Energy Metabolisms. Int. J. Mol. Sci. 2022, 23, 6387. https://doi.org/10.3390/ijms23126387
Chao de la Barca JM, Richard A, Robert P, Eid M, Fouquet O, Tessier L, Wetterwald C, Faure J, Fassot C, Henrion D, et al. Metabolomic Profiling of Angiotensin-II-Induced Abdominal Aortic Aneurysm in Ldlr−/− Mice Points to Alteration of Nitric Oxide, Lipid, and Energy Metabolisms. International Journal of Molecular Sciences. 2022; 23(12):6387. https://doi.org/10.3390/ijms23126387
Chicago/Turabian StyleChao de la Barca, Juan Manuel, Alexis Richard, Pauline Robert, Maroua Eid, Olivier Fouquet, Lydie Tessier, Céline Wetterwald, Justine Faure, Celine Fassot, Daniel Henrion, and et al. 2022. "Metabolomic Profiling of Angiotensin-II-Induced Abdominal Aortic Aneurysm in Ldlr−/− Mice Points to Alteration of Nitric Oxide, Lipid, and Energy Metabolisms" International Journal of Molecular Sciences 23, no. 12: 6387. https://doi.org/10.3390/ijms23126387
APA StyleChao de la Barca, J. M., Richard, A., Robert, P., Eid, M., Fouquet, O., Tessier, L., Wetterwald, C., Faure, J., Fassot, C., Henrion, D., Reynier, P., & Loufrani, L. (2022). Metabolomic Profiling of Angiotensin-II-Induced Abdominal Aortic Aneurysm in Ldlr−/− Mice Points to Alteration of Nitric Oxide, Lipid, and Energy Metabolisms. International Journal of Molecular Sciences, 23(12), 6387. https://doi.org/10.3390/ijms23126387






