Hybrid Plasticizers Enhance Specificity and Sensitivity of an Electrochemical-Based Sensor for Cadmium Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening for the Primary Plasticizer
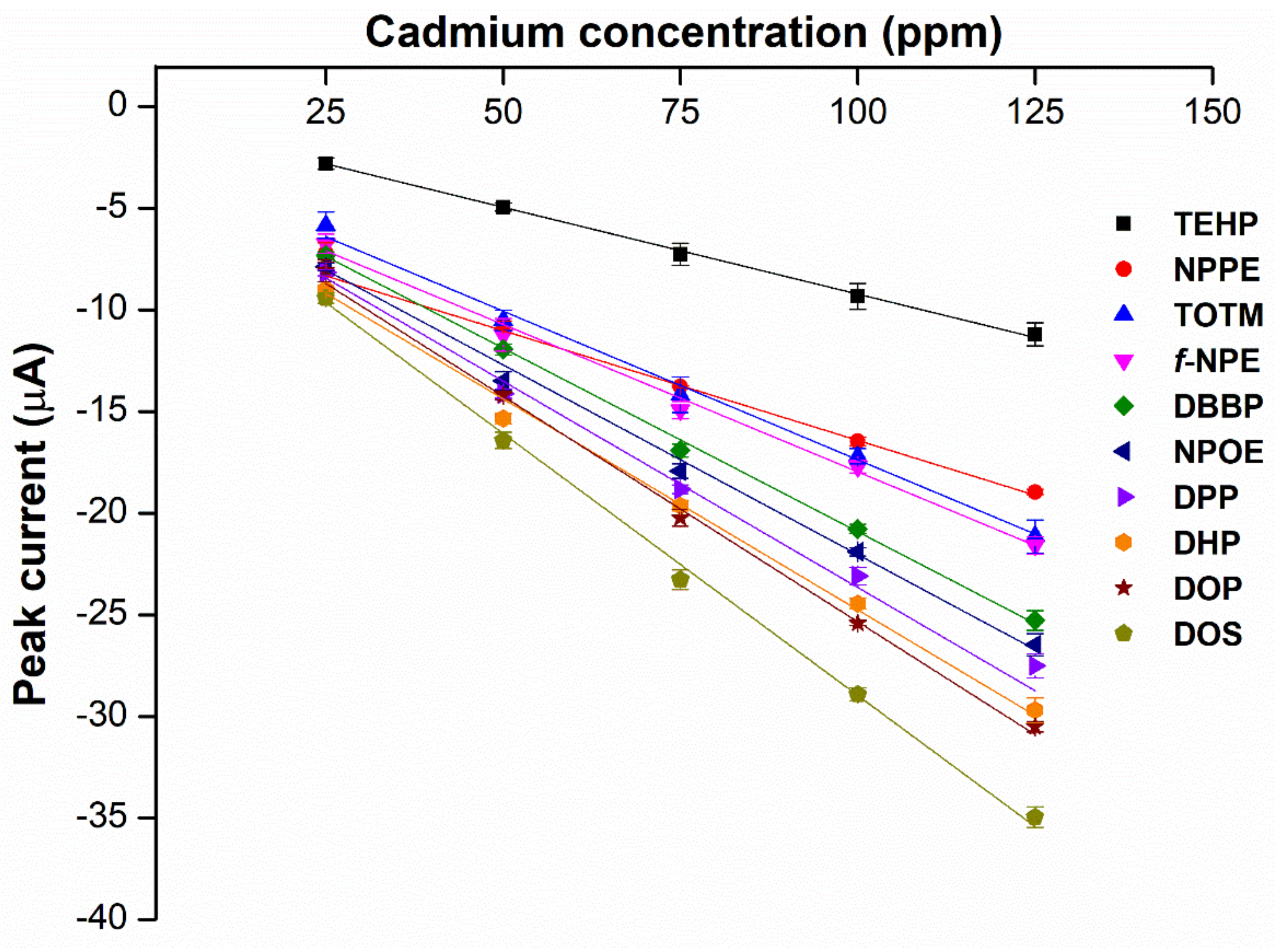
| No. | Plasticizer | Abbr. | Structure | Type | M.W. | Ɛ | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 2-fluorophenyl 2-nitrophenyl ether | f-NPE | 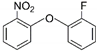 | Ether | 233.20 | 50.0 | [11] |
| 2 | 2-Nitrophenyl phenyl ether | NPPE | 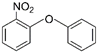 | Ether | 215.20 | 24.0 | [12] |
| 3 | 2-Nitrophenyl octyl ether | NPOE |  | Ether | 251.32 | 24.0 | [13] |
| 4 | Tris(2-ethylhexyl) phos-phate | TEHP |  | Phosphoric acid ester | 434.64 | 4.8 | [14] |
| 5 | Dibutyl butylphosphonate | DBBP | 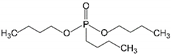 | Phosphoric acid ester | 250.31 | 4.6 | [14] |
| 6 | Dioctyl phthalate | DOP | 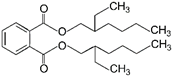 | Phthalate ester | 390.56 | 5.0 | [14] |
| 7 | Diheptyl phthalate | DHP | 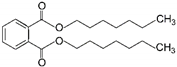 | Phthalate ester | 362.50 | ~4.0–9.0 | [15] |
| 8 | Diphenyl phthalate | DPP | 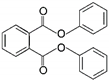 | Phthalate ester | 318.32 | ~4.0–9.0 | [15] |
| 9 | Tris(2-ethylhexyl) trimelli-tate | TOTM |  | Trimellitate ester | 546.79 | 4.9 | [16] |
| 10 | Bis(2-ethylhexyl) sebacate | DOS | 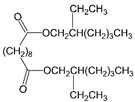 | Dibasic acid esters | 426.67 | 4.0 | [13] |
2.2. Effects of Hybrid Plasticizers on Cadmium Detection
| No. | Hybrid plasticizers (5:1) | Average M.W. | |
|---|---|---|---|
| Plasticizer A | Plasticizer B | ||
| 1 | DOS | NPOE | 397.45 |
| 2 | DOS | NPPE | 391.43 |
| 3 | DOS | DOP | 420.65 |
| 4 | DOS | f-NPE | 394.43 |
| 5 | DOS | DBBP | 397.28 |
| 6 | DOS | TEHP | 428.00 |
| 7 | DOS | DPP | 408.61 |
| 8 | DOS | TOTM | 446.69 |
| 9 | DOS | DHP | 415.98 |
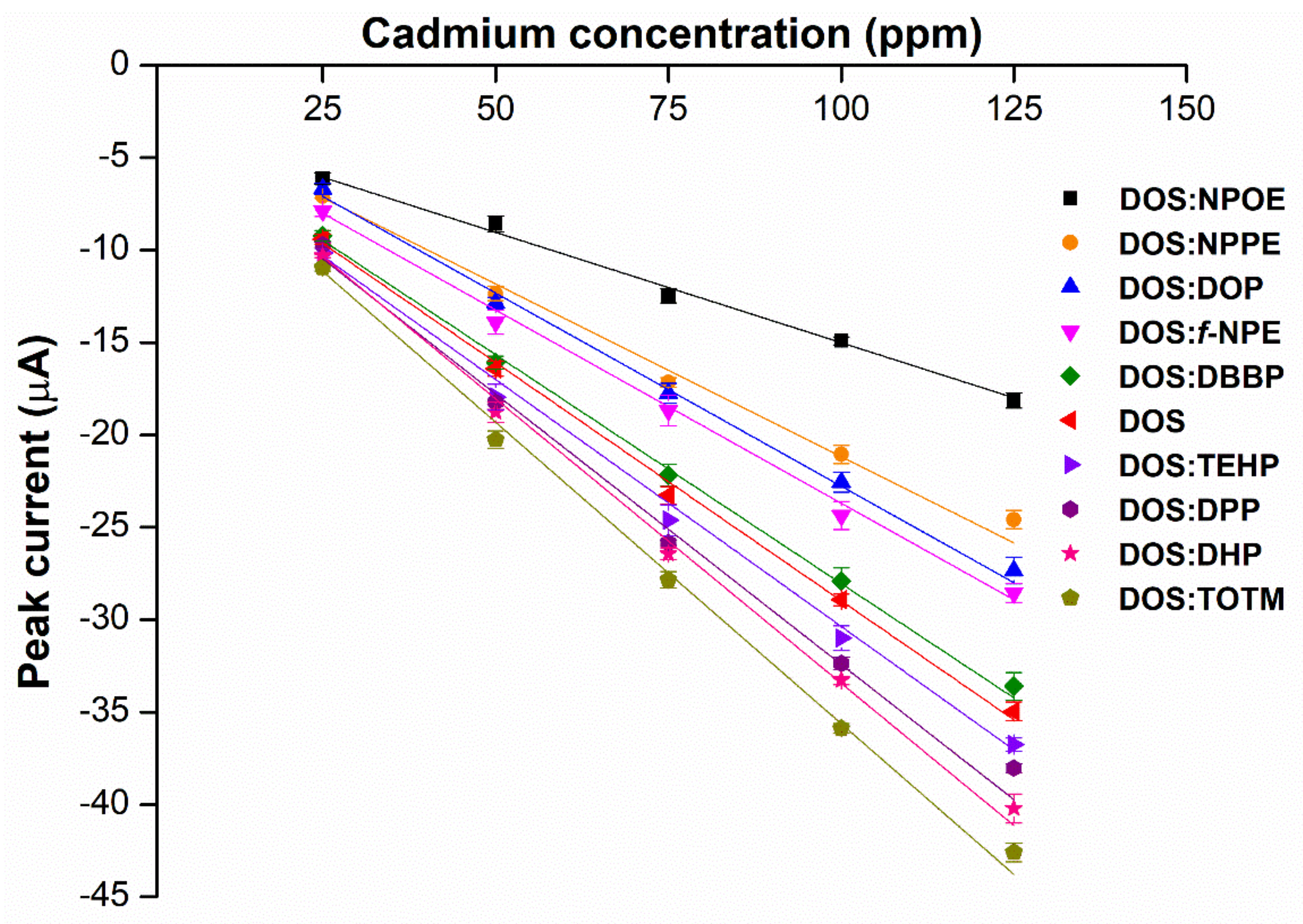
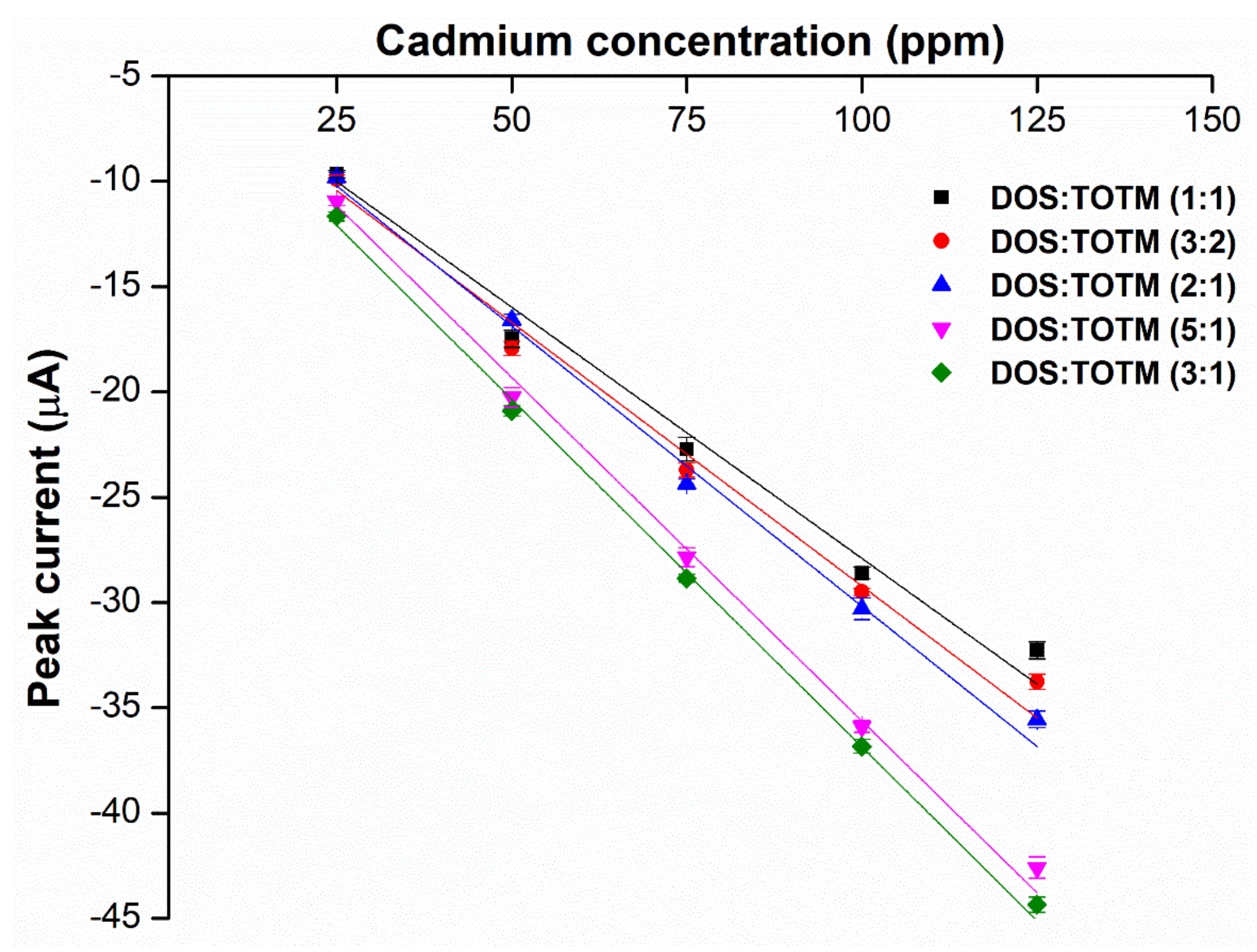
2.3. Effects of Weight Ratios of Hybrid Plasticizers on Sensor Performance
2.4. Choice of Supporting Electrolytes and pH
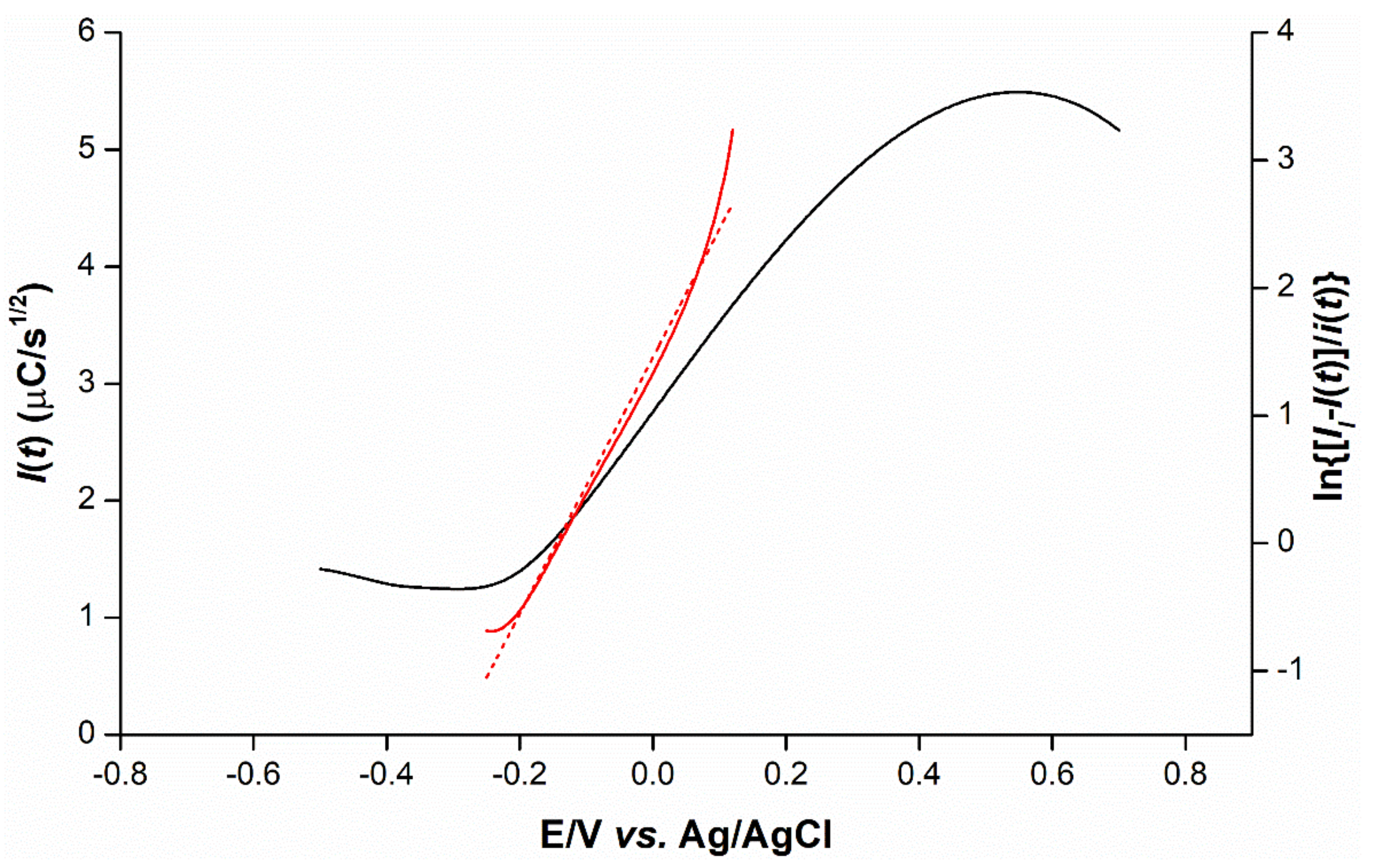
2.5. Mechanistic Determination
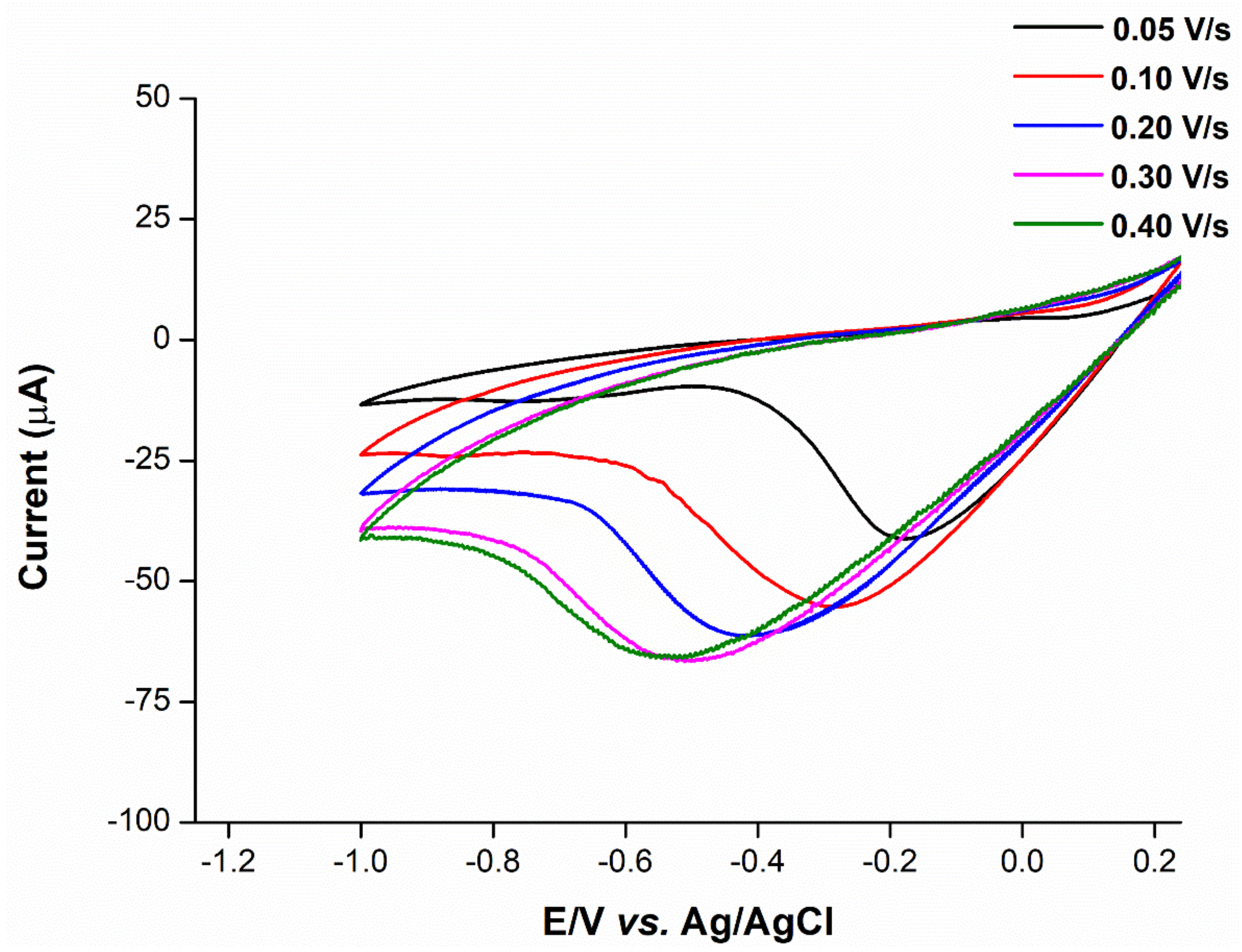
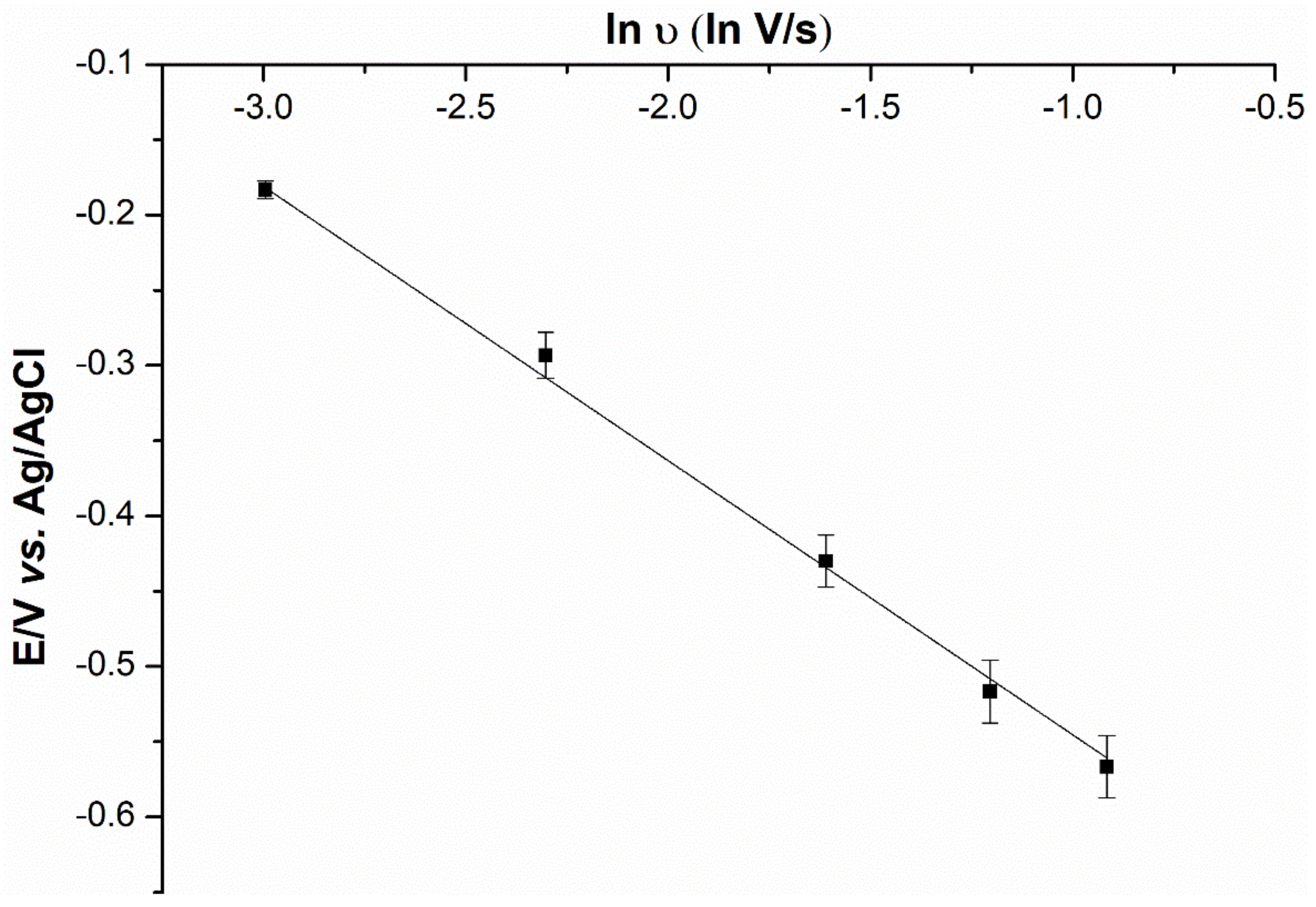
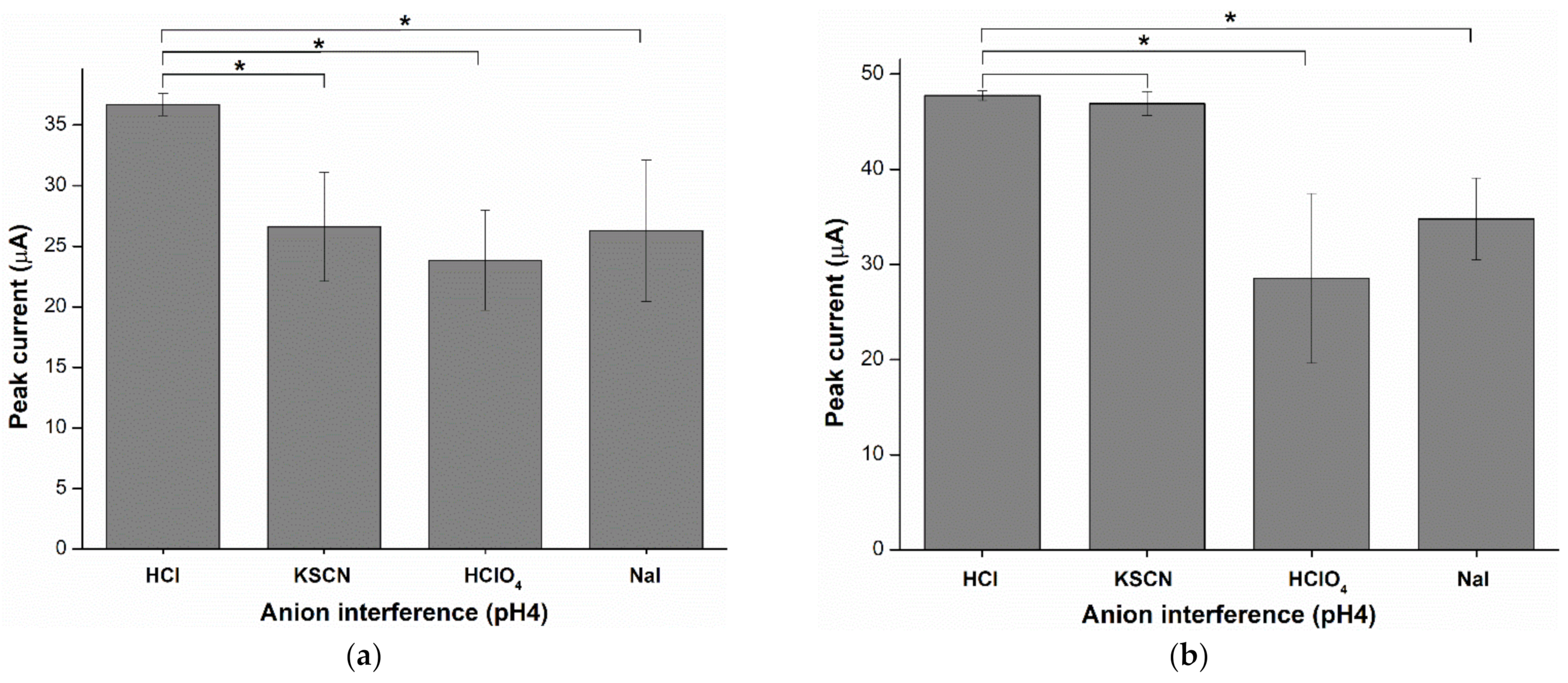
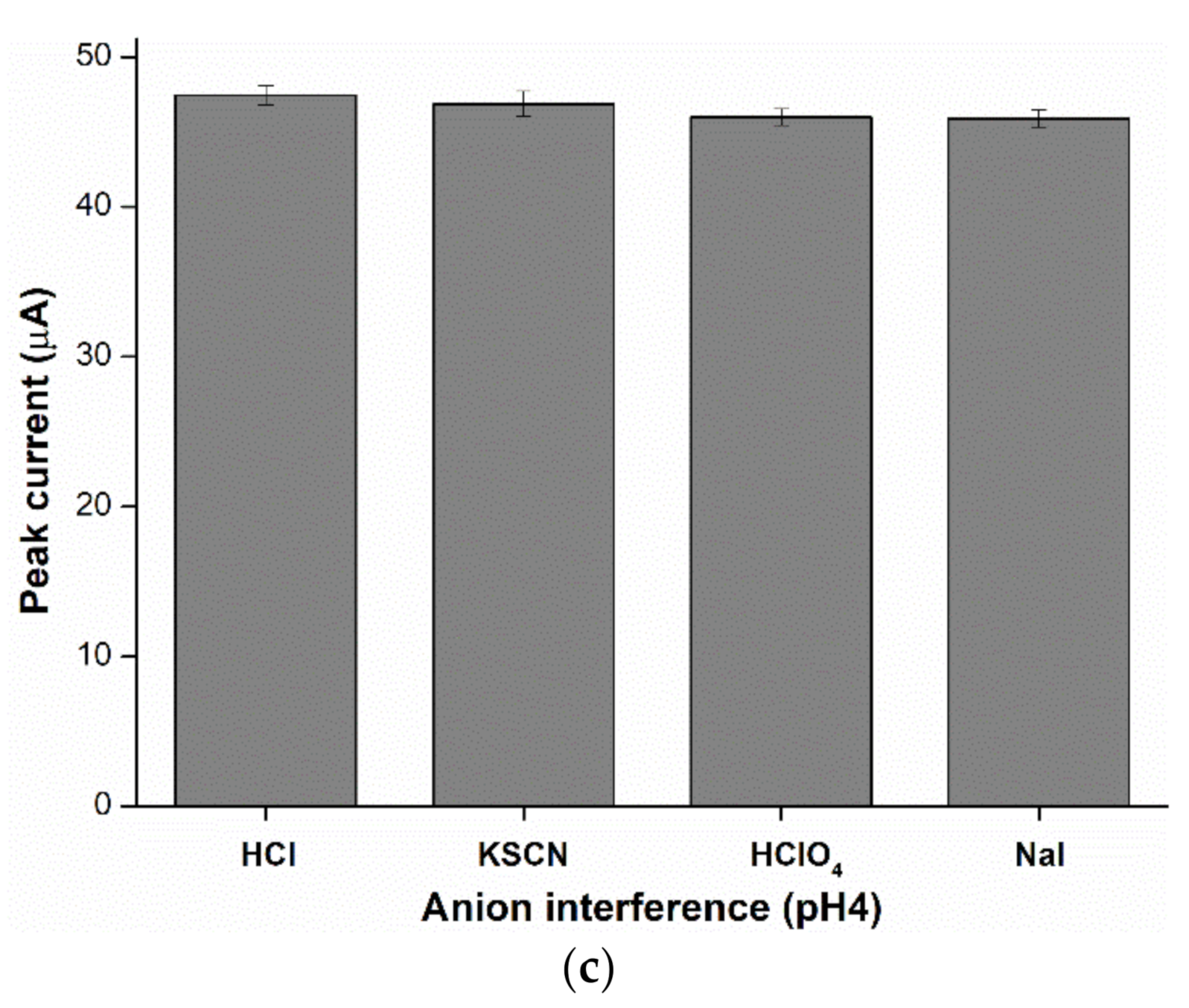
2.6. Anion Interference
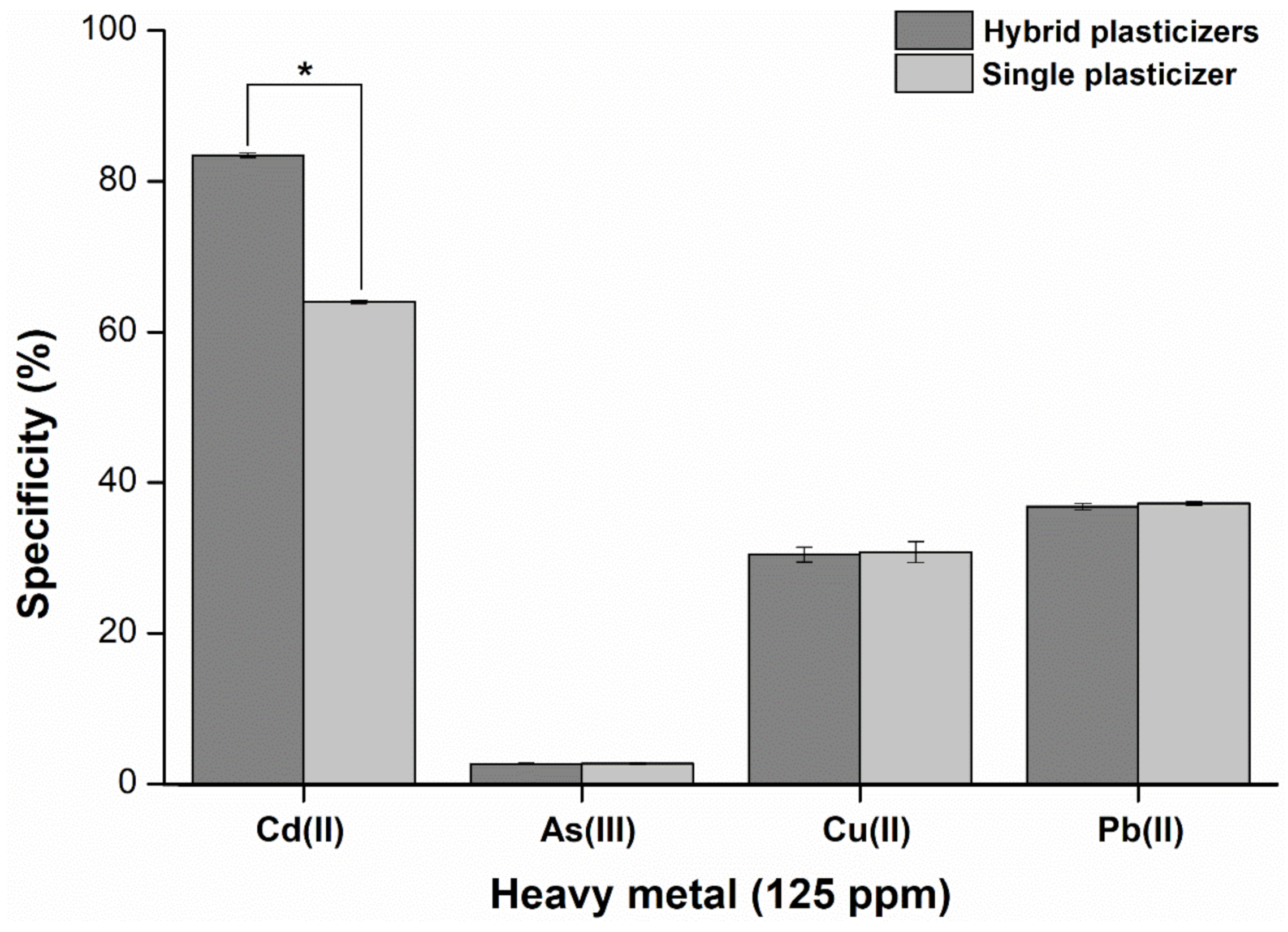
2.7. Specificity and Sensitivity of the Hybrid Plasticizer Sensor
2.8. Determination of Cadmium(II) in Water Samples
3. Materials and Methods
3.1. Reagents and Samples
3.2. Surface Cleaning and Membrane Preparation
3.3. Voltammetric Measurement
3.4. Semi-Integral Calculation of the Voltammetric Signals
3.5. Selection of a Primary Plasticizer for the Cadmium Sensor
3.6. Selection of Hybrid Plasticizers for the Cadmium Sensor
3.7. Optimization for Weight Ratios of the Hybrid Plasticizers
3.8. Selection of Supporting Electrolyte (SE) and Influence of pH
3.9. Effect of Scan Rate ()
3.10. Anion Interference
3.11. Specificity and Sensitivity of the Hybrid Plasticizer Sensor
3.12. Determination of Cadmium(II) in Water Samples
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zdrachek, E.; Bakker, E. Potentiometric sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Kabagambe, B.; Izadyar, A.; Amemiya, S. Stripping voltammetry of nanomolar potassium and ammonium ions using a valinomycin-doped double-polymer electrode. Anal. Chem. 2012, 84, 7979–7986. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.A.; Cuartero, M.; Bakker, E. Thin layer ionophore-based membrane for multianalyte ion activity detection. Anal. Chem. 2015, 87, 7729–7737. [Google Scholar] [CrossRef]
- Yuan, D.; Cuartero, M.; Crespo, G.A.; Bakker, E. Voltammetric thin-layer ionophore-based films: Part 1. experimental evidence and numerical simulations. Anal. Chem. 2017, 89, 586–594. [Google Scholar] [CrossRef]
- Sanchez, P.G.-R.A.C. Hybrid Materials, Functional Applications. An Introduction. In Functional Hybrid Materials, 6th ed.; Sanchez, P.G.-R.C., Ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Stauthamer, W.P.R.V.; Engbersen, J.F.J.; Verboom, W.; Reinhoudt, D.N. Influence of plasticizer on the selectivity of nitrate-sensitive CHEMFETs. Sens. Actuators B Chem. 1994, 17, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Mills, A. Effect of plasticizer viscosity on the sensitivity of an [Ru(bpy)32+(Ph4B−)2]-based optical oxygen sensor. Analyst 1998, 123, 1135–1140. [Google Scholar] [CrossRef]
- Mihali, C.; Vaum, N. Use of plasticizers for electrochemical sensors. In Recent Advances in Plasticizers; Luqman, M., Ed.; InTech: London, UK, 2012; pp. 125–140. [Google Scholar]
- Wilson, A.S. Plasticisers: Principles and Practice, 1st ed.; CRC Press, Institute of Materials: London, UK, 1995. [Google Scholar]
- Khaled, E.; Kamel, M.S.; Hassan, H.N.; Haroun, A.A.; Youssef, A.M.; Aboul-Enein, H.Y. Novel multi walled carbon nanotubes/β-cyclodextrin based carbon paste electrode for flow injection potentiometric determination of piroxicam. Talanta 2012, 97, 96–102. [Google Scholar] [CrossRef]
- Kozlowski, C.; Walkowiak, W. Applicability of liquid membranes in chromium(VI) transport with amines as ion carriers. J. Membr. Sci. 2005, 266, 143–150. [Google Scholar] [CrossRef]
- Zook, J.M.; Langmaier, J.; Lindner, E. Current-polarized ion-selective membranes: The influence of plasticizer and lipophilic background electrolyte on concentration profiles, resistance, and voltage transients. Sens. Actuators B Chem. 2009, 136, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Jain, R.; Hamdan, A.J.; Agarwal, S.; Bharti, A.K. A novel ion selective sensor for promethium determination. Anal. Chim. Acta 2010, 681, 27–32. [Google Scholar] [CrossRef]
- Graham, P.R. Phthalate ester plasticizers—Why and how they are used. Environ. Health Perspect. 1973, 3, 3–12. [Google Scholar]
- Uddin, Z.; Watanabe, M.; Shirai, H.; Hirai, T. Effects of plasticizers on novel electromechanical actuations with different poly(vinyl chloride) gels. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2119–2127. [Google Scholar] [CrossRef]
- Stark, T.; Choi, H.; Diebel, P. Influence of plasticizer molecular weight on plasticizer retention in PVC geomembrane. Geosynth. Int. 2005, 12, 99–110. [Google Scholar] [CrossRef]
- Gupta, K.C.; Jeanne D’Arc, M. Effect of concentration of ion exchanger, plasticizer and molecular weight of cyanocopolymers on selectivity and sensitivity of Cd(II) ion selective electrode. Talanta 2000, 52, 1087–1103. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ionselective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Santos, C.D.C.; Pimenta, T.C.; Thomasini, R.L.; Verly, R.M.; Franco, D.L.; Ferreira, L.F. Electropolymerization of phenol and aniline derivatives: Synthesis, characterization and application as electrochemical transducers. J. Electroanal. Chem. 2019, 846, 113163. [Google Scholar] [CrossRef]
- Guo, J.; Amemiya, S. Voltammetric heparin-selective electrode based on thin liquid membrane with conducting polymer-modified solid support. Anal. Chem. 2006, 78, 6893–6902. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, I. Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Samec, Z. Simple kinetic models of ion transfer across an interface between two immiscible electrolyte solutions. A comparison with experimental data. Electrochim. Acta 1998, 44, 85–90. [Google Scholar] [CrossRef]
- Grenness, M.; Oldham, K.B. Semiintegral electroanalysis. Theory and verification. Anal. Chem. 1972, 44, 1121–1129. [Google Scholar] [CrossRef]
- Grdeń, M. Semi-differential analysis of irreversible voltammetric peaks. J. Solid State Electrochem. 2017, 21, 1045–1058. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W.; Jing, G.; Cui, T. Trace determination of arsenite with an ionophore-coated selective micro sensor. IEEE Sens. J. 2018, 18, 4364–4371. [Google Scholar] [CrossRef]
- Shamsipur, M.; Sahari, S.; Payehghadr, M.; Alizadeh, K. Flow injection potentiometric determination of Cd2+ ions using a coated graphite plasticized PVC-membrane electrode based on 1,3-bis(2-cyanobenzene)triazene. Acta Chim. Slov. 2011, 58, 555–562. [Google Scholar] [PubMed]
- Khamjumphol, U.; Watchasit, S.; Suksai, C.; Janrungroatsakul, W.; Boonchiangma, S.; Tuntulani, T.; Ngeontae, W. New polymeric membrane cadmium(II)-selective electrodes using tripodal amine based ionophores. Anal. Chim. Acta 2011, 704, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, A.; Tavakkoli, H.; Mombeni, T. Fabrication of a highly selective cadmium (II) sensor based on 1,13-bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane as a supramolecular ionophore. Mater. Sci. Eng. C 2014, 38, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.; Bateman, D.; Hauberg, S.; Wehbring, R. GNU Octave Version 6.4.0 Manual: A High-Level Interactive Language for Numerical Computations; 2021. [Google Scholar]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- The International Council for Harmonisation (ICH). Q2 (R1) Validation of Analytical Procedures: Text and Methodology; The International Council for Harmonisation (ICH): Geneva, Switzerland, 2005; Volume 1. [Google Scholar]





| No. | Electrode | Plasticizer | Ionophore | Response Time (s) | LOD (μM) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Gold | Hybrid plasticizers (DOS:TOTM) | Cd ionophore I | 30 | 0.8 | This work |
| 2 | Graphite | Benzyl acetate | 1,3-bis(2-cyanobenzene)triazene (L) | 2 | 8.0 | [27] |
| 3 | Ion selective electrode | DOS | 25,27-bis(ethyl-2-(bis(2- pyridylmethyl)aminomethyl)aniline)-26,28-dihydroxy p-tert-butylcalix[4] arene | 10 | 1.6 | [28] |
| 4 | Graphite | DOS | 1,13-bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane | 15 | 8.4 | [29] |
| Sample | Spiked Concentration (ppb) | Found Concentration 1 (ppb) | RSD (%) | Recovery (%) | p-Value 2 |
|---|---|---|---|---|---|
| Milli-Q water | 0 | <LOD | - | - | - |
| 500 | 498 ± 1.36 | 0.270 | 99.6 | ||
| 600 | 592 ± 1.17 | 0.200 | 98.7 | - | |
| Drinking water | 0 | <LOD | - | - | - |
| 500 | 497 ± 2.20 | 0.440 | 99.3 | 1.000 | |
| 600 | 589 ± 2.51 | 0.430 | 98.2 | 0.362 | |
| Tap water | 0 | <LOD | - | - | - |
| 500 | 494 ± 3.47 | 0.700 | 98.7 | 0.231 | |
| 600 | 588 ± 2.78 | 0.470 | 97.9 | 0.146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruangsuj, P.; Wanthongcharoen, S.; Chaisriratanakul, W.; Bunjongpru, W.; Yamprayoonswat, W.; Jeamsaksiri, W.; Jumpathong, W.; Yasawong, M. Hybrid Plasticizers Enhance Specificity and Sensitivity of an Electrochemical-Based Sensor for Cadmium Detection. Int. J. Mol. Sci. 2022, 23, 6402. https://doi.org/10.3390/ijms23126402
Ruangsuj P, Wanthongcharoen S, Chaisriratanakul W, Bunjongpru W, Yamprayoonswat W, Jeamsaksiri W, Jumpathong W, Yasawong M. Hybrid Plasticizers Enhance Specificity and Sensitivity of an Electrochemical-Based Sensor for Cadmium Detection. International Journal of Molecular Sciences. 2022; 23(12):6402. https://doi.org/10.3390/ijms23126402
Chicago/Turabian StyleRuangsuj, Pattarawan, Suwanan Wanthongcharoen, Woraphan Chaisriratanakul, Win Bunjongpru, Wariya Yamprayoonswat, Wutthinan Jeamsaksiri, Watthanachai Jumpathong, and Montri Yasawong. 2022. "Hybrid Plasticizers Enhance Specificity and Sensitivity of an Electrochemical-Based Sensor for Cadmium Detection" International Journal of Molecular Sciences 23, no. 12: 6402. https://doi.org/10.3390/ijms23126402






