Recent Advances in Managing Spinal Intervertebral Discs Degeneration
Abstract
:1. Introduction
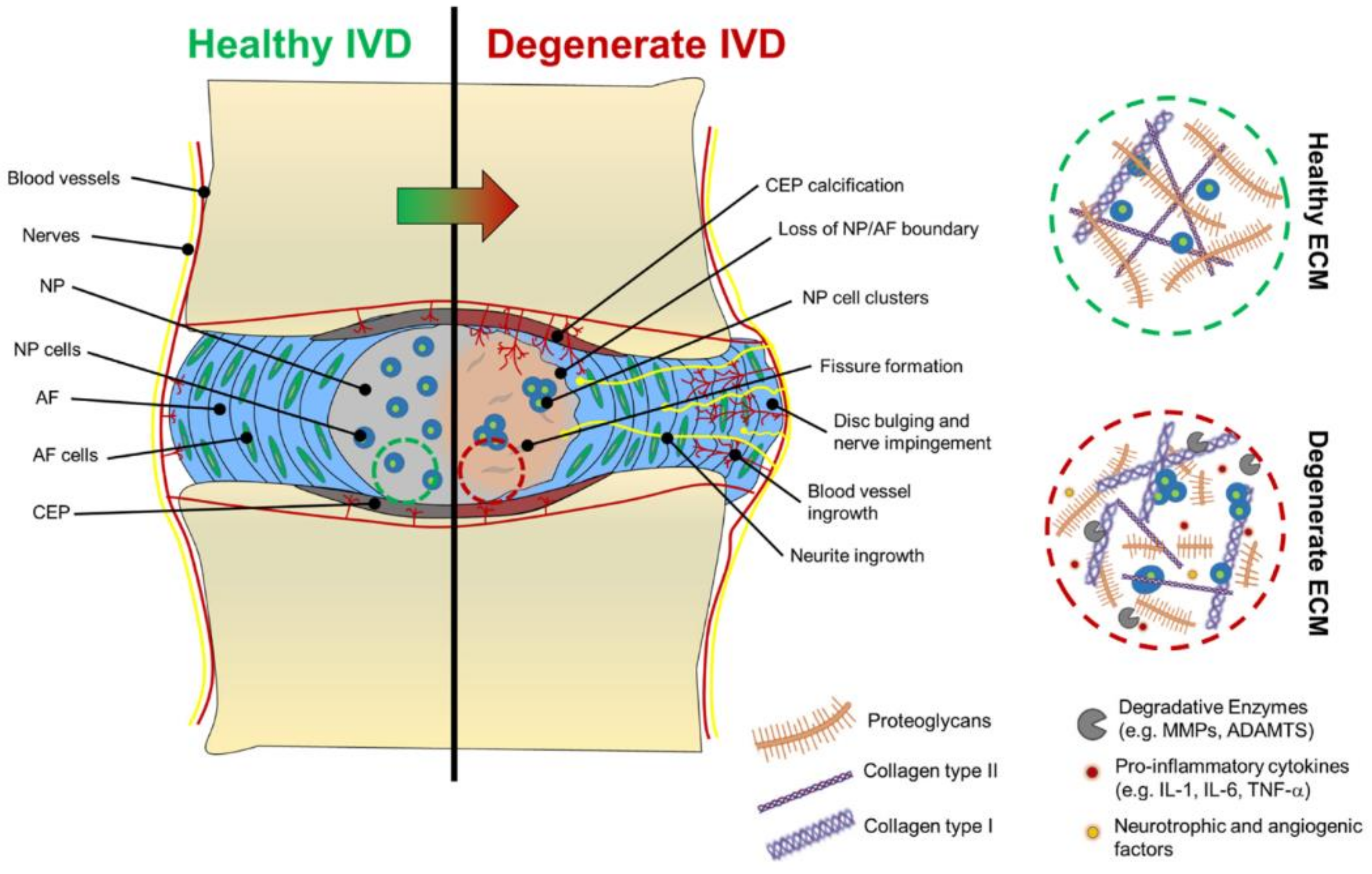
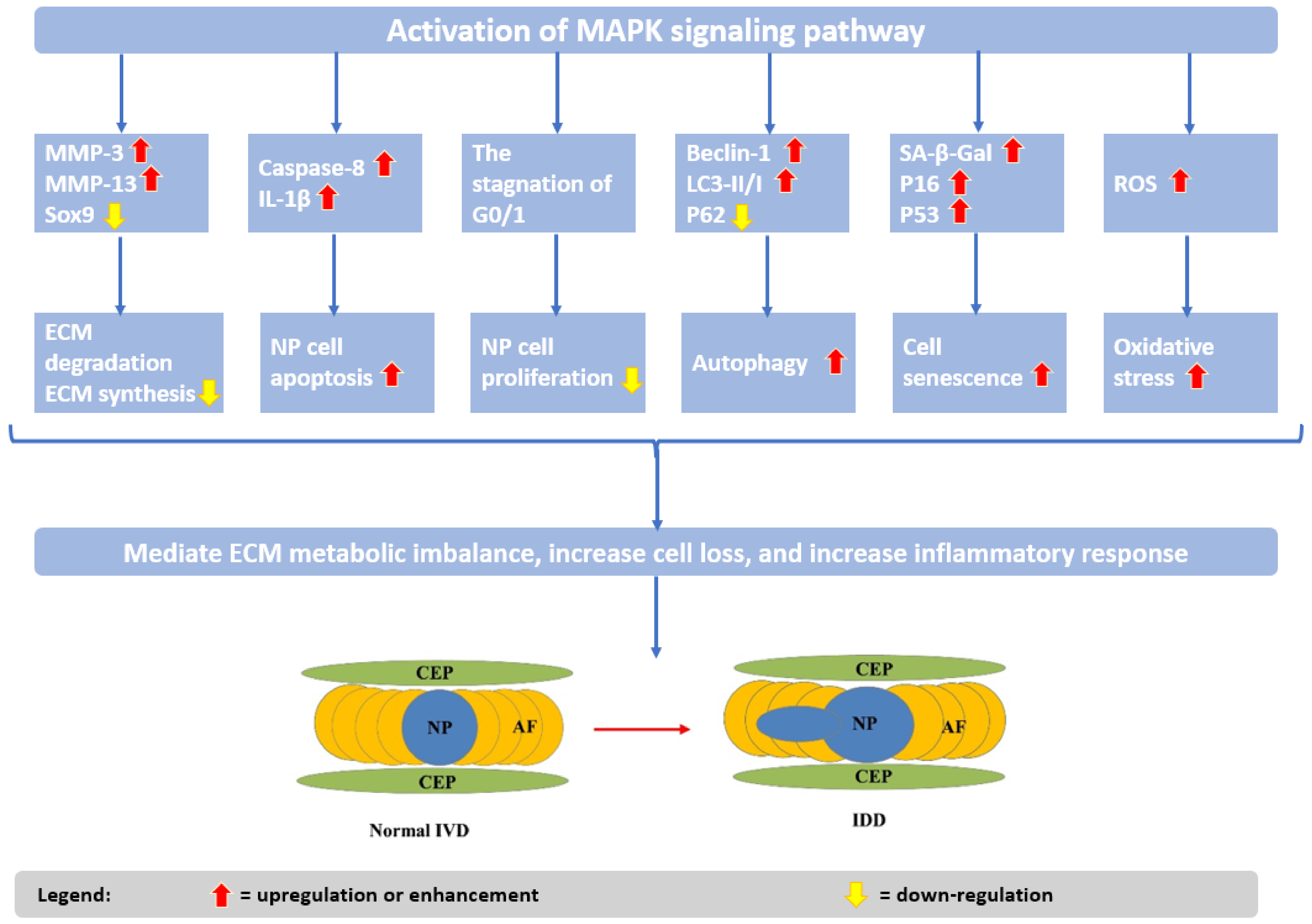
2. Pathogenesis and Etiology of Intervertebral Disc Degeneration
3. Strategies for Managing Intervertebral Disc Degeneration
3.1. Key Compounds in Intervertebral Disc Repair
3.1.1. Melatonin
3.1.2. Estrogen
3.1.3. Naringin
3.1.4. Icariin
3.1.5. Resveratrol
3.1.6. Quercetin
3.1.7. Berberine
3.1.8. Metformin
3.1.9. Vitamin D
3.1.10. Growth Factors
3.2. Cell-Based Strategies
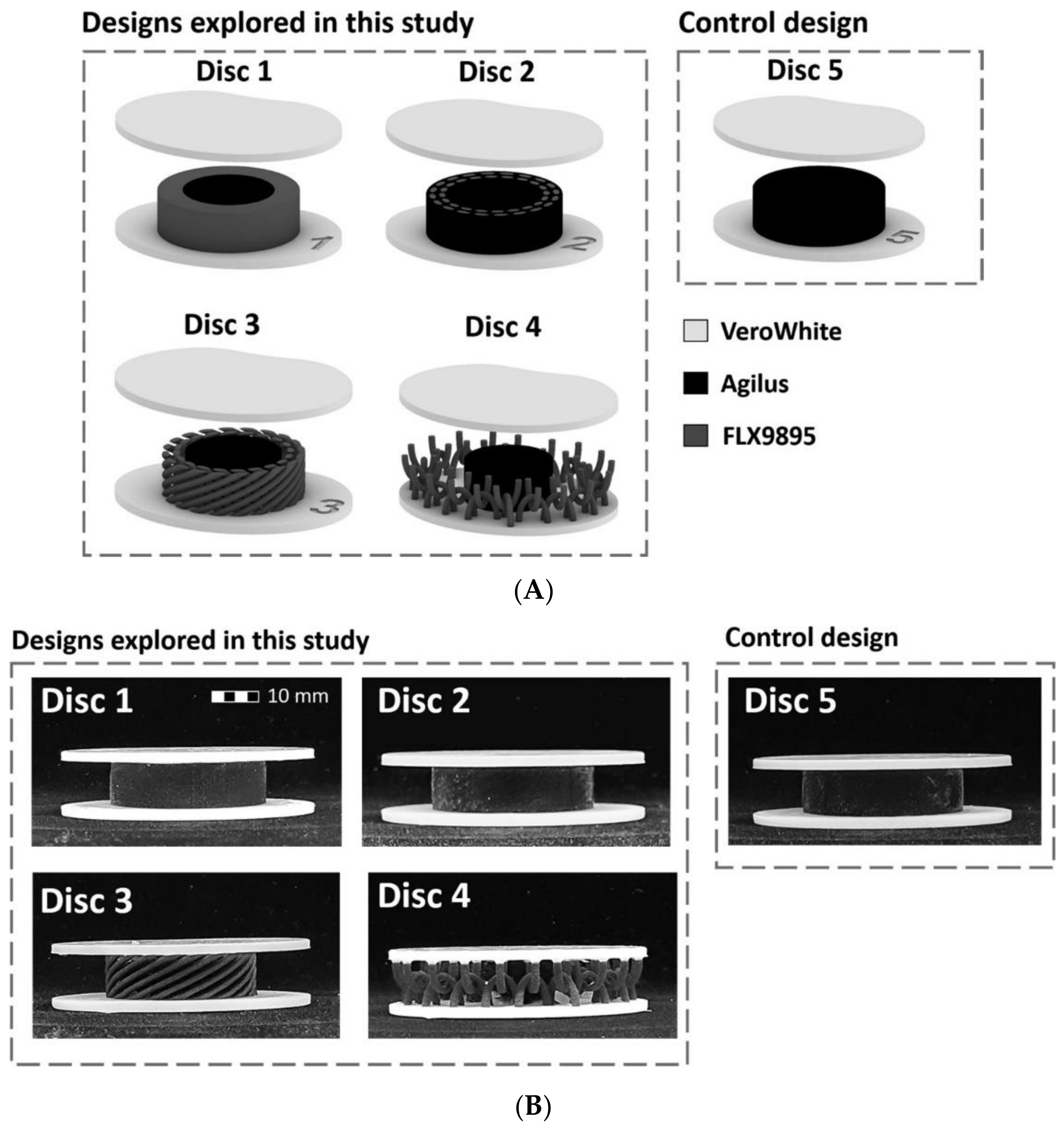
3.3. Artificial Intervertebral Discs
3.4. Other Emerging Strategies in Managing Intervertebral Disc Degeneration
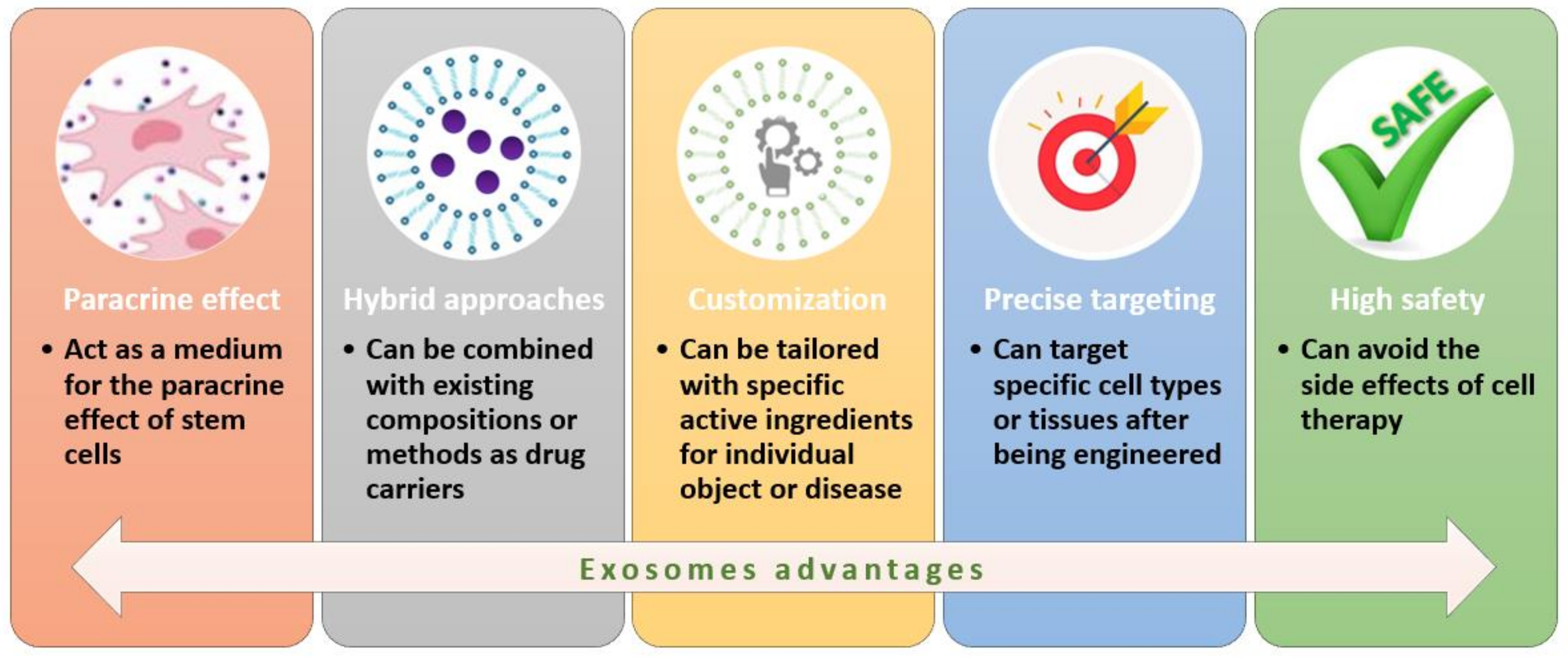
3.4.1. Exosomes
3.4.2. RNA Approaches
3.4.3. Platelet-Rich Plasma
3.4.4. Artificial Intelligence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | annulus fibrous |
| AI | artificial intelligence |
| AMPK | AMP-activated protein kinase |
| Bax | BCL-2-associated X protein |
| BC | bacterial cellulose |
| BCL-2 | B-cell lymphoma 2 |
| BMAC | bone marrow aspirate concentrate |
| BMP | bone morphogenetic protein |
| CCL-5 | C-C motif chemokine ligand 5 |
| CEP | cartilaginous endplates |
| cGAS | cyclic GMP–AMP |
| E2 | 17β-estradiol |
| ECM | extracellular matrix |
| ERK | extracellular signal-regulated kinase |
| ESC | embryonic stem cells |
| GAG | glycosaminoglycan |
| GDF-5 | growth differentiation factor 5 |
| GMP | good manufacturing practice |
| IDD | intervertebral disc degeneration |
| IFN-γ | interferon-gamma |
| IGF-1 | insulin-like growth factor 1 |
| IL | interleukin |
| iPSC | induced pluripotent stem cells |
| IVD | intervertebral disc |
| LBP | low back pain |
| LC3 | light chain 3 |
| MAPK | mitogen-activated protein kinase |
| ML | machine learning |
| MLK3 | mixed-lineage protein kinase 3 |
| MMP | matrix metalloproteinase |
| MSC | mesenchymal stem cells |
| mtROS | mitochondrial ROS |
| MVB | multivesicular bodies |
| NF-κB | nuclear factor kappa B |
| NLRP3 | NLR family pyrin domain containing 3 |
| NP | nucleus pulpous |
| Nrf-2 | nuclear factor erythroid 2–related factor 2 |
| p-Akt | phosphorylated Akt |
| PCL | poly(caprolactone) |
| PDGF | platelet-derived growth factor |
| P-GSK-3β | phospho-glycogen synthase kinase-3 beta |
| PHEMA | poly(2-hydroxyethylmethacrylate) |
| PI3K/Akt | phosphatidylinositol-3-kinase/protein kinase B |
| PLA | polylactic acid |
| P-mTOR | phospho-mammalian target of rapamycin |
| PRP | platelet-rich-plasma |
| PVA | polyvinyl alcohol |
| RANKL | receptor activators for nuclear factor-κB ligand |
| REC | rapidly expanding clone |
| rhGDF-5 | recombinant human GDF-5 |
| ROS | reactive oxygen species |
| SCN | stem cell niche |
| SDF-1 | stromal-cell-derived factor-1 |
| SERM | selective estrogen receptor modulators |
| SIRT1 | sirtuin 1 |
| Sox | SRY-Box Transcription Factor |
| Sphk2 | sphingosine kinase 2 |
| STING | stimulator of interferon genes |
| TDR | total disc replacement |
| TGF-β | transforming growth factor beta |
| TNF-α | tumor necrosis factor-alpha |
| TRAP | tartrate-resistant acid phosphatase |
References
- Smit, T.H.; Helder, M.N. 20—In vivo models of regenerative medicine in the spine. In Biomaterials for Spinal Surgery; Ambrosio, L., Tanner, E., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 582–607. [Google Scholar]
- Herger, N.; Bermudez-Lekerika, P.; Farshad, M.; Albers, C.E.; Distler, O.; Gantenbein, B.; Dudli, S. Should Degenerated Intervertebral Discs of Patients with Modic Type 1 Changes Be Treated with Mesenchymal Stem Cells? Int. J. Mol. Sci. 2022, 23, 2721. [Google Scholar] [CrossRef] [PubMed]
- Kasamkattil, J.; Gryadunova, A.; Martin, I.; Barbero, A.; Schären, S.; Krupkova, O.; Mehrkens, A. Spheroid-Based Tissue Engineering Strategies for Regeneration of the Intervertebral Disc. Int. J. Mol. Sci. 2022, 23, 2530. [Google Scholar] [CrossRef] [PubMed]
- Ekşi, M.Ş.; Turgut, V.U.; Berikol, G.; Özmen, B.B.; Huet, S.E.; Dinç, T.; Küçüksüleymanoğlu, D.; Orhun, Ö.; Özcan-Ekşi, E.E. Schmorl’s nodes could be associated with intervertebral disc degeneration at upper lumbar levels and end-plate disease at lower lumbar level in patients with low back pain. J. Clin. Neurosci. 2022, 100, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Millecamps, M.; Czerminski, J.T.; Mathieu, A.P.; Stone, L.S. Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model. Spine J. 2015, 15, 2524–2537. [Google Scholar] [CrossRef]
- Tendulkar, G.; Chen, T.; Ehnert, S.; Kaps, H.-P.; Nüssler, A.K. Intervertebral Disc Nucleus Repair: Hype or Hope? Int. J. Mol. Sci. 2019, 20, 3622. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Iwasaki, N.; Sudo, H. Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation. Cells 2022, 11, 602. [Google Scholar] [CrossRef]
- Ohnishi, T.; Iwasaki, N.; Sudo, H. Causes of and Molecular Targets for the Treatment of Intervertebral Disc Degeneration: A Review. Cells 2022, 11, 394. [Google Scholar] [CrossRef]
- Wang, C.; Guo, S.; Gu, Q.; Wang, X.; Long, L.; Xiao, C.; Xie, M.; Shen, H.; Li, S. Exosomes: A promising therapeutic strategy for intervertebral disc degeneration. Exp. Gerontol. 2022, 163, 111806. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Xiang, D.; Chen, Y.; Cui, Y.; Wang, S.; Liu, W. An Artificial PVA-BC Composite That Mimics the Biomechanical Properties and Structure of a Natural Intervertebral Disc. Materials 2022, 15, 1481. [Google Scholar] [CrossRef]
- Kamali, A.; Ziadlou, R.; Lang, G.; Pfannkuche, J.; Cui, S.; Li, Z.; Richards, R.G.; Alini, M.; Grad, S. Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions. Theranostics 2021, 11, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Kennon, J.C.; Awad, M.E.; Chutkan, N.; DeVine, J.; Fulzele, S. Current insights on use of growth factors as therapy for Intervertebral Disc Degeneration. Biomol. Concepts 2018, 9, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Cavagnaro, L.; Zanirato, A.; Divano, S.; Formica, C.; Formica, M.; Felli, L. What is the clinical evidence on regenerative medicine in intervertebral disc degeneration? Musculoskelet. Surg. 2017, 101, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Gloria, A.; De Santis, R.; Ambrosio, L.; Tanner, K.E. 1—Introduction to biomaterials for spinal surgery. In Biomaterials for Spinal Surgery; Ambrosio, L., Tanner, E., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 1–38. [Google Scholar]
- Gradišnik, L.; Maver, U.; Gole, B.; Bunc, G.; Voršič, M.; Ravnik, J.; Šmigoc, T.; Bošnjak, R.; Velnar, T. The Endplate Role in Degenerative Disc Disease Research: The Isolation of Human Chondrocytes from Vertebral Endplate—An Optimised Protocol. Bioengineering 2022, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Romaniyanto; Mahyudin, F.; Sigit Prakoeswa, C.R.; Notobroto, H.B.; Tinduh, D.; Ausrin, R.; Rantam, F.A.; Suroto, H.; Utomo, D.N.; Rhatomy, S. An update of current therapeutic approach for Intervertebral Disc Degeneration: A review article. Ann. Med. Surg. 2022, 77, 103619. [Google Scholar] [CrossRef]
- Dowdell, J.; Erwin, M.; Choma, T.; Vaccaro, A.; Iatridis, J.; Cho, S.K. Intervertebral Disk Degeneration and Repair. Neurosurgery 2017, 80, S46–S54. [Google Scholar] [CrossRef]
- Sakai, D.; Schol, J.; Watanabe, M. Clinical Development of Regenerative Medicine Targeted for Intervertebral Disc Disease. Medicina 2022, 58, 267. [Google Scholar] [CrossRef]
- Jin, L.-Y.; Song, X.-X.; Li, X.-F. The role of estrogen in intervertebral disc degeneration. Steroids 2020, 154, 108549. [Google Scholar] [CrossRef]
- Choi, Y.; Park, M.H.; Lee, K. Tissue Engineering Strategies for Intervertebral Disc Treatment Using Functional Polymers. Polymers 2019, 11, 872. [Google Scholar] [CrossRef] [Green Version]
- Kirnaz, S.; Capadona, C.; Wong, T.; Goldberg, J.L.; Medary, B.; Sommer, F.; McGrath, L.B., Jr.; Härtl, R. Fundamentals of Intervertebral Disc Degeneration. World Neurosurg. 2022, 157, 264–273. [Google Scholar] [CrossRef]
- Cheng, Z.; Xiang, Q.; Wang, J.; Zhang, Y. The potential role of melatonin in retarding intervertebral disc ageing and degeneration: A systematic review. Ageing Res. Rev. 2021, 70, 101394. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, C.; Hoyland, J.A.; Saiani, A. Self-Assembling Peptide Hydrogels as Functional Tools to Tackle Intervertebral Disc Degeneration. Gels 2022, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef]
- Monchaux, M.; Forterre, S.; Spreng, D.; Karol, A.; Forterre, F.; Wuertz-Kozak, K. Inflammatory processes associated with canine intervertebral disc herniation. Front. Immunol. 2017, 8, 1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.H.; Li, Z.P.; Liu, L.L.; Liu, H.; Xue, J.B. IL-17 in intervertebral disc degeneration: Mechanistic insights and therapeutic implications. Cell Biol. Int. 2022, 46, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, S.; Abdollahzade, S.; Sadr, M.; Mirbolouk, M.H.; Fattahi, E.; Khoshnevisan, A.; Rezaei, N. Association of interleukin 2, interleukin 12, and interferon-γ with intervertebral disc degeneration in Iranian population. BMC Med. Genet. 2020, 21, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Liao, H.-Y.; Bai, D.-Y.; Wang, Z.-Q.; Xie, X.-W. MAPK /ERK signaling pathway: A potential target for the treatment of intervertebral disc degeneration. Biomed. Pharmacother. 2021, 143, 112170. [Google Scholar] [CrossRef] [PubMed]
- Cherif, H.; Mannarino, M.; Pacis, A.S.; Ragoussis, J.; Rabau, O.; Ouellet, J.A.; Haglund, L. Single-Cell RNA-Seq Analysis of Cells from Degenerating and Non-Degenerating Intervertebral Discs from the Same Individual Reveals New Biomarkers for Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 23, 3993. [Google Scholar] [CrossRef]
- Tian, Y.; Ji, Y.; Mei, X.; Pan, J.; He, W.; Sun, J.; Wan, K.; Yang, H. Lower Plasma Melatonin in the Intervertebral Disk Degeneration Patients Was Associated with Increased Proinflammatory Cytokines. Clin. Interv. Aging 2021, 16, 215. [Google Scholar] [CrossRef]
- Turgut, M.; Uslu, S.; Uysal, A.; Yurtseven, M.E.; Üstün, H. Changes in vascularity of cartilage endplate of degenerated intervertebral discs in response to melatonin administration in rats. Neurosurg. Rev. 2003, 26, 133–138. [Google Scholar] [CrossRef]
- Cotrim, A.C.D.; Franca, E.L.; Franca, A.C.H.; Martins, J.S.; Silva, K.P.G.; Ghalfi, Y.C.; Machado, I.T.; Tozetti, I.A. Effect of Polyethylene Glycol Microspheres Adsorbed with Melatonin on Oxidative Stress and Viscosity of Cervical Mucus Samples Infected with Human Papillomavirus. Biointerface Res. Appl. Chem. 2020, 10, 6757–6772. [Google Scholar] [CrossRef]
- Zhang, Y.; He, F.; Chen, Z.; Su, Q.; Yan, M.; Zhang, Q.; Tan, J.; Qian, L.; Han, Y. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging 2019, 11, 10499–10512. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, G.; Liu, H.; Li, Z.; Pei, Y.; Wang, H.; Pan, H.; Cui, H.; Long, J.; Wang, J.; et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Zhou, Q.; Niu, J.; Wang, Y.; Yan, Q.; Wu, C.; Qian, J.; Yang, H.; Zou, J. Melatonin Protects Intervertebral Disc from Degeneration by Improving Cell Survival and Function via Activation of the ERK1/2 Signaling Pathway. Oxidat. Med. Cell. Longev. 2019, 2019, 5120275. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Du, J.; Li, X.; Lin, J.; Ni, L.; Zhu, P.; Zhou, H.; Kong, F.; Yang, H.; et al. Melatonin Attenuates Intervertebral Disk Degeneration via Maintaining Cartilaginous Endplate Integrity in Rats. Front. Physiol. 2021, 12, 672572. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Huo, Y.; Tian, T.; Yang, D.; Ma, L.; Yang, S.; Ding, W. 17β-Estradiol alleviates intervertebral disc degeneration by inhibiting NF-κB signal pathway. Life Sci. 2021, 284, 119874. [Google Scholar] [CrossRef]
- Lou, C.; Chen, H.; Mei, L.; Yu, W.; Zhu, K.; Liu, F.; Chen, Z.; Xiang, G.; Chen, M.; Weng, Q. Association between menopause and lumbar disc degeneration: An MRI study of 1566 women and 1382 men. Menopause 2017, 24, 1136–1144. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, F.; Ma, J.; Ding, W. Intervertebral disc ageing and degeneration: The antiapoptotic effect of oestrogen. Ageing Res. Rev. 2020, 57, 100978. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.-D.; Huo, X.-W.; Yang, D.-L.; Ma, L.; Ding, W.-Y. 17β-Estradiol inhibits intervertebral disc degeneration by down-regulating MMP-3 and MMP-13 and up-regulating type II collagen in a rat model. Artif. Cells Nanomed. Biotechnol. 2018, 46, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Delgado, B.J.; Lopez-Ojeda, W. Estrogen. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2020. [Google Scholar]
- Writing Group for the Women’s Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal WomenPrincipal Results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef] [Green Version]
- An, K.-C. Selective estrogen receptor modulators. Asian Spine J. 2016, 10, 787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.S.; Jordan, V.C. Selective estrogen receptor modulators (SERMs): Mechanisms of anticarcinogenesis and drug resistance. Mutat. Res. 2005, 591, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, A.C.; Silva, A.M.; Santos, M.S.; Sardão, V.A. Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. J. Steroid Biochem. Mol. Biol. 2014, 143, 61–71. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Gautam, P. Response Surface Optimization and Impact of Immobilized Enzymes Naringianse and Tannase on the Quality Parameters of Citrus maxima Juice. Biointerface Res. Appl. Chem. 2021, 11, 11535–11552. [Google Scholar] [CrossRef]
- Li, N.; Whitaker, C.; Xu, Z.; Heggeness, M.; Yang, S.-Y. Therapeutic effects of naringin on degenerative human nucleus pulposus cells for discogenic low back pain. Spine J. 2016, 16, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Lin, J.; Jin, H.; Wang, K.; Yan, Y.; Wang, J.; Wu, C.; Nisar, M.; Tian, N.; et al. Therapeutic Potential of Naringin for Intervertebral Disc Degeneration: Involvement of Autophagy Against Oxidative Stress-Induced Apoptosis in Nucleus Pulposus Cells. Am. J. Chin. Med. 2018, 46, 1561–1580. [Google Scholar] [CrossRef]
- Nan, L.P.; Wang, F.; Ran, D.; Zhou, S.F.; Liu, Y.; Zhang, Z.; Huang, Z.N.; Wang, Z.Y.; Wang, J.C.; Feng, X.M.; et al. Naringin alleviates H2O2-induced apoptosis via the PI3K/Akt pathway in rat nucleus pulposus-derived mesenchymal stem cells. Connect. Tissue Res. 2020, 61, 554–567. [Google Scholar] [CrossRef]
- Devraj, V.M.; Vemuri, S.K.; Banala, R.R.; Gunda, S.K.; Av, G.R.; Gpv, S. Evaluation of Anti-inflammatory and Regenerative Efficiency of Naringin and Naringenin in Degenerated Human Nucleus Pulposus Cells: Biological and Molecular Modeling Studies. Asian Spine J. 2019, 13, 875–889. [Google Scholar] [CrossRef]
- Deng, X.; Chen, S.; Zheng, D.; Shao, Z.; Liang, H.; Hu, H. Icariin Prevents H2O2-Induced Apoptosis via the PI3K/Akt Pathway in Rat Nucleus Pulposus Intervertebral Disc Cells. Evid. Based Complement. Altern. Med. 2017, 2017, 2694261. [Google Scholar] [CrossRef] [Green Version]
- Hua, W.; Li, S.; Luo, R.; Wu, X.; Zhang, Y.; Liao, Z.; Song, Y.; Wang, K.; Zhao, K.; Yang, S.; et al. Icariin protects human nucleus pulposus cells from hydrogen peroxide-induced mitochondria-mediated apoptosis by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165575. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Zhang, Y.; Wu, X.; Kang, L.; Tu, J.; Zhao, K.; Li, S.; Wang, K.; Song, Y.; Luo, R. Icariin attenuates interleukin-1β-induced inflammatory response in human nucleus pulposus cells. Curr. Pharm. Des. 2017, 23, 6071–6078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, F.; Feng, Y.; Zhang, S.; Xie, C.; Huang, H.; Sang, C.; Hu, S.; Jiao, F.; Jiang, J.; et al. Icariin regulates stem cell migration for endogenous repair of intervertebral disc degeneration by increasing the expression of chemotactic cytokines. BMC Complement. Med. Ther. 2022, 22, 63. [Google Scholar] [CrossRef]
- Huo, Y.; Yang, D.; Lai, K.; Tu, J.; Zhu, Y.; Ding, W.; Yang, S. Antioxidant Effects of Resveratrol in Intervertebral Disk. J. Investig. Surg. Off. J. Acad. Surg. Res. 2022, 35, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, L.; Zhuo, N.; Shen, J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem. Biophys. Res. Commun. 2017, 493, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, R.D.; Lawrence, A.B.; Oluyomi, A.S. COVID 19: Resveratrol as a Potential Supplement to Mitigate the Cardiotoxicity Associated with Chloroquine and Hydroxychloroquine Treatment. Biointerface Res. Appl. Chem. 2021, 11, 11172–11186. [Google Scholar] [CrossRef]
- Kakalij, R.M.; Gangarapu, K.; Kumar, B.D.; Diwan, P.V. In Silico analysis of piperine, resveratrol, and vanillic acid on NF-kappa B p65 protein expression. Biointerface Res. Appl. Chem. 2017, 7, 1927–1930. [Google Scholar]
- Noormand, F.; Kermani, A.S.; Raviz, E.K.; Esmaeilpour, K.; Golshani, M.; Bashiri, H.; Kalantaripour, T.P.; Asadi-Shekaari, M. Investigating the neuroprotective effects of Resveratrol on encephalopathy induced by bile duct ligation in male rats. Biointerface Res. Appl. Chem. 2020, 10, 5512–5515. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.; Xu, J.; Wu, X.; Guo, Z.; Fan, L.; Song, R.; Wang, J.; Wei, L.; Teng, H. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Biosci. Rep. 2018, 38, BSR20171454. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhang, Q.; Song, L. Resveratrol enhances matrix biosynthesis of nucleus pulposus cells through activating autophagy via the PI3K/Akt pathway under oxidative damage. Biosci. Rep. 2018, 38, BSR20180544. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Guo, R.; Shang, J.; Zhang, B.; Dai, M. Resveratrol inhibits TNF-α-induced matrix degradation via the p38/MAPK and Akt pathways in human nucleus pulposus cells. Int. J. Clin. Exp. Med. 2017, 10, 2764–2772. [Google Scholar]
- Jiang, Y.; Xie, Z.; Yu, J.; Fu, L. Resveratrol inhibits IL-1β-mediated nucleus pulposus cell apoptosis through regulating the PI3K/Akt pathway. Biosci. Rep. 2019, 39, BSR20190043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Guo, X.; Zhang, F.; Zheng, L.; Ding, W.; Yang, S. Resveratrol Combined with 17β-Estradiol Prevents IL-1β Induced Apoptosis in Human Nucleus Pulposus Via The PI3K/AKT/Mtor and PI3K/AKT/GSK-3β Pathway. J. Investig. Surg. 2021, 34, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, W.; Abulizi, Y.; Xu, T.; Cao, R.; Xun, C.; Zhang, J.; Sheng, W. Quercetin Alleviates Intervertebral Disc Degeneration by Modulating p38 MAPK-Mediated Autophagy. BioMed Res. Int. 2021, 2021, 6631562. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B. Quercetin and its role in chronic diseases. Drug Discov. Mother Nat. 2016, 929, 377–387. [Google Scholar]
- Aljadaan, S.A.N.; Elias, R.S.; Al-Anssari, R.A. Investigation of the Antioxidant and Antibacterial Activity of Novel Quercetin Derivatives. Biointerface Res. Appl. Chem. 2020, 10, 7329–7336. [Google Scholar] [CrossRef]
- Sekar, A.; Soundhararajan, R.; Srinivasan, H. In silico Analysis of Quercetin and its Analogues Against Targeted Proteins. Biointerface Res. Appl. Chem. 2021, 11, 13695–13705. [Google Scholar] [CrossRef]
- Yousefi, M.; Shadnoush, M.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M. Encapsulation Systems for Delivery of Flavonoids: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13934–13951. [Google Scholar] [CrossRef]
- Wang, D.; He, X.; Wang, D.; Peng, P.; Xu, X.; Gao, B.; Zheng, C.; Wang, H.; Jia, H.; Shang, Q.; et al. Quercetin Suppresses Apoptosis and Attenuates Intervertebral Disc Degeneration via the SIRT1-Autophagy Pathway. Front. Cell Dev. Biol. 2020, 8, 613006. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, B.; Shi, Y.; Xie, C.; Huang, C.; Chen, B.; Zhang, H.; Zeng, G.; Liang, H.; Wu, Y.; et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthr. Cartil. 2021, 29, 413–422. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Wang, J.; Tang, C.; Khor, S.; Chen, J.; Chen, X.; Zhang, Z.; Tang, Q.; Wang, C.; et al. Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model. Int. J. Biol. Sci. 2018, 14, 682–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Cheke, R.S.; Narkhede, R.R.; Shinde, S.D.; Ambhore, J.P.; Jain, P.G. Natural Product Emerging as Potential SARS Spike Glycoproteins-ACE2 Inhibitors to Combat COVID-19 Attributed by In-Silico Investigations. Biointerface Res. Appl. Chem. 2021, 11, 10628–10639. [Google Scholar] [CrossRef]
- Luo, R.; Liao, Z.; Song, Y.; Yin, H.; Zhan, S.; Li, G.; Ma, L.; Lu, S.; Wang, K.; Li, S.; et al. Berberine ameliorates oxidative stress-induced apoptosis by modulating ER stress and autophagy in human nucleus pulposus cells. Life Sci. 2019, 228, 85–97. [Google Scholar] [CrossRef]
- Lu, L.; Hu, J.; Wu, Q.; An, Y.; Cui, W.; Wang, J.; Ye, Z. Berberine prevents human nucleus pulposus cells from IL-1β-induced extracellular matrix degradation and apoptosis by inhibiting the NF-κB pathway. Int. J. Mol. Med. 2019, 43, 1679–1686. [Google Scholar] [CrossRef]
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in aging and aging-related diseases: Clinical applications and relevant mechanisms. Theranostics 2022, 12, 2722–2740. [Google Scholar] [CrossRef]
- Umbarkar, R.P.; Mittal, A.; Charde, M.S. Validated Stability-Indicating Assay UHPLC Method for Simultaneous Analysis of Saxagliptin and Metformin in Fixed-Dose Combinations. Biointerface Res. Appl. Chem. 2022, 12, 2729–2744. [Google Scholar] [CrossRef]
- Chen, D.; Xia, D.; Pan, Z.; Xu, D.; Zhou, Y.; Wu, Y.; Cai, N.; Tang, Q.; Wang, C.; Yan, M.; et al. Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis. 2016, 7, e2441. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, F.; Deng, C.; He, F.; Zhang, Y.; Shen, H.; Chen, Z.; Qian, L. Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release. Aging 2019, 11, 10252–10265. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Cao, D.-Z.; Wang, Z.-H. Metformin alleviates intervertebral disc degeneration by upregulating MMP-1 expression via the KDM6A/SOX9/miR-202-3p/MMP-1 signaling pathway. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Ramanathan, R.; Firdous, A.; Dong, Q.; Wang, D.; Lee, J.; Vo, N.; Sowa, G. Investigation into the anti-inflammatory properties of metformin in intervertebral disc cells. JOR Spine 2022, e1197. [Google Scholar] [CrossRef]
- De Luca, P.; De Girolamo, L.; Perucca Orfei, C.; Viganò, M.; Cecchinato, R.; Brayda-Bruno, M.; Colombini, A. Vitamin D’s Effect on the Proliferation and Inflammation of Human Intervertebral Disc Cells in Relation to the Functional Vitamin D Receptor Gene FokI Polymorphism. Int. J. Mol. Sci. 2018, 19, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, T.; Shen, Z.; Hu, Z.; Yan, B. Vitamin D/VDR in the pathogenesis of intervertebral disc degeneration: Does autophagy play a role? Biomed. Pharmacother. 2022, 148, 112739. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-W.; Yi, Y.-Y.; Zhang, S.-B.; Hu, T.; Wang, S.-J.; Zhao, W.-D.; Wu, D.-S. Does vitamin D status influence lumbar disc degeneration and low back pain in postmenopausal women? A retrospective single-center study. Menopause 2020, 27, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cheng, S.; Zheng, T.; Ye, Y.; Ye, A.; Zhu, S.; Lin, X. Vitamin D retards intervertebral disc degeneration through inactivation of the NF-κB pathway in mice. Am. J. Transl. Res. 2019, 11, 2496–2506. [Google Scholar] [PubMed]
- Sun, Y.; Leung, V.Y.; Cheung, K.M. Clinical trials of intervertebral disc regeneration: Current status and future developments. Int. Orthop. 2019, 43, 1003–1010. [Google Scholar] [CrossRef]
- Loughran, M.J.; Hunt, J.A. 18—Stem cells for disc regeneration. In Biomaterials for Spinal Surgery; Ambrosio, L., Tanner, E., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 536–562. [Google Scholar]
- Dong, Z.; Chen, S.; Wang, L.; Qi, P.; Wei, L. Fabrication of Flower-stacked structured microparticles encapsulated with Stem cells and Growth Factor to the potential treatment of Intervertebral Disc Degeneration. Process Biochem. 2022, 119, 39–47. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.H.; Kim, J.-S.; Kim, H.Y.; Lee, H.-C.; Byun, J.-H.; Lee, J.-H.; Kim, N.-H.; Oh, S.H. Intervertebral Disc Regeneration Using Stem Cell/Growth Factor-Loaded Porous Particles with a Leaf-Stacked Structure. Biomacromolecules 2020, 21, 4795–4805. [Google Scholar] [CrossRef] [PubMed]
- Ukeba, D.; Yamada, K.; Suyama, T.; Lebl, D.R.; Tsujimoto, T.; Nonoyama, T.; Sugino, H.; Iwasaki, N.; Watanabe, M.; Matsuzaki, Y.; et al. Combination of ultra-purified stem cells with an in situ-forming bioresorbable gel enhances intervertebral disc regeneration. eBioMedicine 2022, 76, 103845. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Lin, X.; Wang, D.; Wang, J.; Shang, Q.; He, X.; Wu, K.; Zhao, B.; Peng, P.; Wang, H.; et al. Injectable hydrogel with nucleus pulposus-matched viscoelastic property prevents intervertebral disc degeneration. J. Orthop. Transl. 2022, 33, 162–173. [Google Scholar] [CrossRef]
- Wolff, M.; Shillington, J.M.; Rathbone, C.; Piasecki, S.K.; Barnes, B. Injections of concentrated bone marrow aspirate as treatment for Discogenic pain: A retrospective analysis. BMC Musculoskelet. Disord. 2020, 21, 135. [Google Scholar] [CrossRef] [PubMed]
- El-Kadiry, A.E.-H.; Lumbao, C.; Rafei, M.; Shammaa, R. Autologous BMAC Therapy Improves Spinal Degenerative Joint Disease in Lower Back Pain Patients. Front. Med. 2021, 8, 622573. [Google Scholar] [CrossRef]
- Gloria, A.; De Santis, R.; Ambrosio, L.; Tanner, K.E. 8—Artificial intervertebral discs. In Biomaterials for Spinal Surgery; Ambrosio, L., Tanner, E., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 295–312. [Google Scholar]
- Bhattacharya, S.; Roy, S.; Rana, M.; Banerjee, S.; Karmakar, S.K.; Biswas, J.K. Biomechanical performance of a modified design of dynamic cervical implant compared to conventional ball and socket design of an artificial intervertebral disc implant: A finite element study. J. Mech. Med. Biol. 2019, 19, 1950017. [Google Scholar] [CrossRef]
- Lee, H.; Phillips, J.B.; Hall, R.M.; Tipper, J.L. Neural cell responses to wear debris from metal-on-metal total disc replacements. Eur. Spine J. 2020, 29, 2701–2712. [Google Scholar] [CrossRef] [Green Version]
- Lee, H. Spinal Cord Cellular Response to Wear Debris from Metal-on-Metal total Disc Replacements. Ph.D. Thesis, University of Leeds, Leeds, UK, 2016. [Google Scholar]
- Guo, X.; Liu, Y.; Shang, H. Silk fibroin/nano hydroxyapatite composite combined with icariin can promote the proliferation and differentiation of bone marrow mesenchymal stem cells into nucleus pulposus like cells. Chin. J. Tissue Eng. Res. 2022, 26, 3528. [Google Scholar]
- Wu, D.; Tan, J.; Yao, L.; Tian, J.; Luo, B.; Li, L.; Zhou, C.; Lu, L. Customized composite intervertebral disc scaffolds by integrated 3D bioprinting for therapeutic implantation. Compos. Part A Appl. Sci. Manuf. 2021, 147, 106468. [Google Scholar] [CrossRef]
- Du, L.; Yang, Q.; Zhang, J.; Zhu, M.; Ma, X.; Zhang, Y.; Wang, L.; Xu, B. Engineering a biomimetic integrated scaffold for intervertebral disc replacement. Mater. Sci. Eng. C 2019, 96, 522–529. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Clienti, C.; Corallo, D. Design of a new intervertebral disc prosthesis. Mater. Today Proc. 2019, 7, 529–536. [Google Scholar] [CrossRef]
- Zhu, M.; Tan, J.; Liu, L.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Construction of biomimetic artificial intervertebral disc scaffold via 3D printing and electrospinning. Mater. Sci. Eng. C 2021, 128, 112310. [Google Scholar] [CrossRef]
- Yu, Z.; Voumard, B.; Shea, K.; Stanković, T. Exploration of the influence of different biomimetic designs of 3D printed multi-material artificial spinal disc on the natural mechanics restoration. Mater. Des. 2021, 210, 110046. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Xia, C.; Zeng, Z.; Fang, B.; Tao, M.; Gu, C.; Zheng, L.; Wang, Y.; Shi, Y.; Fang, C.; Mei, S.; et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic. Biol. Med. 2019, 143, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhang, Y.; Liu, W.; Ni, W.; Huang, X.; Yuan, J.; Zhao, B.; Xiao, H.; Xue, F. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J. Cell. Mol. Med. 2020, 24, 11742–11754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, Y.; Liu, L.; Wang, H.; Shen, P.; Yang, H. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: Therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle 2020, 19, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gong, J.; Wang, Z.; Liu, Y.; Cao, J.; Qin, J.; Zuo, R.; Zhang, H.; Wang, S.; Zhao, P.; et al. Injectable cartilage matrix hydrogel loaded with cartilage endplate stem cells engineered to release exosomes for non-invasive treatment of intervertebral disc degeneration. Bioact. Mater. 2022, 15, 29–43. [Google Scholar] [CrossRef]
- Zhang, S.; Song, S.; Zhuang, Y.; Hu, J.; Cui, W.; Wang, X.; Zhao, Z.; Liu, X.; Sun, Z. Role of microRNA-15a-5p/Sox9/NF-κB axis in inflammatory factors and apoptosis of murine nucleus pulposus cells in intervertebral disc degeneration. Life Sci. 2021, 277, 119408. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, D.; Wang, Y.; Miao, Z.; Chen, Z.; Lin, Z.; Lin, J.; Huang, C.; Pan, L.; Wang, L.; et al. Targeting STING attenuates ROS induced intervertebral disc degeneration. Osteoarthr. Cartil. 2021, 29, 1213–1224. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, H.; Zhu, Y.; Zhao, C.; Wang, S.; Zheng, Y.; Xie, Z.; Jin, Y.; Song, H.; Yang, L.; et al. Injectable self-healing hydrogel with siRNA delivery property for sustained STING silencing and enhanced therapy of intervertebral disc degeneration. Bioact. Mater. 2022, 9, 29–43. [Google Scholar] [CrossRef]
- Banala, R.R.; Vemuri, S.K.; Dar, G.H.; Palanisamy, V.; Penkulinti, M.; Surekha, M.V.; Gurava Reddy, A.V.; Nalam, M.R.; Subbaiah, G.P.V. Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD). Spine J. 2019, 19, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Xiao, L.; Wang, C.; Liu, C.; Zhang, Y.; Ding, B.; Gao, D.; Lu, Y.; Xu, H. Circ_0022382 ameliorated intervertebral disc degeneration by regulating TGF-β3 expression through sponge adsorption of miR-4726-5p. Bone 2022, 154, 116185. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, M.; Ke, S.; Zhang, Y.; Xu, G.; Li, Z. Effect of Platelet-Rich Plasma on Intervertebral Disc Degeneration In Vivo and In Vitro: A Critical Review. Oxidat. Med. Cell. Longev. 2020, 2020, 8893819. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Jeyaraman, M.; Chellamuthu, G.; Jeyaraman, N.; Jain, R.; Khanna, M. Does the Intradiscal Injection of Platelet Rich Plasma Have Any Beneficial Role in the Management of Lumbar Disc Disease? Glob. Spine J. 2021, 12, 503–514. [Google Scholar] [CrossRef]
- Muthalaly, R.G.; Evans, R.M. Applications of Machine Learning in Cardiac Electrophysiology. Arrhythm. Electrophysiol. Rev. 2020, 9, 71–77. [Google Scholar] [CrossRef]
- Mostafa, F.; Hasan, E.; Williamson, M.; Khan, H. Statistical Machine Learning Approaches to Liver Disease Prediction. Livers 2021, 1, 23. [Google Scholar] [CrossRef]
- Nakamura, T.; Sasano, T. Artificial intelligence and cardiology: Current status and perspective. J. Cardiol. 2022, 79, 326–333. [Google Scholar] [CrossRef]
- Busnatu, S.; Niculescu, A.G.; Bolocan, A.; Petrescu, G.E.D.; Paduraru, D.N.; Nastasa, I.; Lupusoru, M.; Geanta, M.; Andronic, O.; Grumezescu, A.M.; et al. Clinical Applications of Artificial Intelligence—An Updated Overview. J. Clin. Med. 2022, 11, 2265. [Google Scholar] [CrossRef]
- Pana, M.; Vasilescu, E.I.; Busnatu, S.S.; Andrei, C.; Popescu, N.A.; Sinescu, C.J. Prediction of congestive heart failure in patients using artificial intelligence: Proof of concept. Eur. Heart J. 2021, 42, 2547. [Google Scholar] [CrossRef]
- Pana, M.A.; Busnatu, S.S.; Serbanoiu, L.I.; Vasilescu, E.; Popescu, N.; Andrei, C.; Sinescu, C.J. Reducing the Heart Failure Burden in Romania by Predicting Congestive Heart Failure Using Artificial Intelligence: Proof of Concept. Appl. Sci. 2021, 11, 1728. [Google Scholar] [CrossRef]
- Amol Soin, M.D.; Megan Hirschbeck, M.D.; Michael Verdon, D.O.; Laxmaiah Manchikanti, M.D. A pilot study implementing a machine learning algorithm to use artificial intelligence to diagnose spinal conditions. Pain Physician 2022, 25, 171–178. [Google Scholar]
- Bradley, J.; Rajendran, S. Developing predictive models for early detection of intervertebral disc degeneration risk. Healthc. Anal. 2022, 2, 100054. [Google Scholar] [CrossRef]


| Compound | Relevant Effects for IDD | Proposed Mechanism(s) |
|---|---|---|
| Melatonin | Antioxidant properties Anti-inflammatory activity Cell arrest inhibition Aggrecan and collagen II upregulation Collagen X downregulation Vascular invasion inhibition Calcification mitigation Damage repair acceleration | Activation of ERK1/2 signaling pathway Inhibition of NF-κB pathway |
| Estrogen | Autophagy stimulant Antioxidant properties Anti-inflammatory activity Apoptosis inhibition Catabolism reduction Anabolism upregulation | Activation of PI3K/Akt pathway Inhibition of NF-κB pathway |
| Naringin | Autophagy stimulant Antioxidant properties Anti-inflammatory activity Apoptosis attenuation Upregulation of aggrecan, BMP-2, and Sox6 Downregulation of TNF-α and MMP3 | Activation of PI3K/Akt pathway |
| Icariin | Antioxidant properties Anti-inflammatory activity ECM preservation Stem cells recruitment Upregulation of chemotactic cytokines Downregulation of Caspase-3 and Bax | Inhibition of MAPK pathway Inhibition of NF-κB pathway |
| Resveratrol | Autophagy stimulant Antioxidant properties Anti-inflammatory activity Apoptosis attenuation Aggrecan and collagen II upregulation GAG production stimulation | Activation of PI3K/Akt pathway |
| Quercetin | Autophagy stimulant Antioxidant properties Anti-inflammatory activity Apoptosis inhibition ECM degradation prevention | Inhibition of p38 MAPK signaling pathway Inhibition of NF-κB pathway |
| Berberine | Autophagy stimulant Antioxidant properties Anti-inflammatory activity Apoptosis prevention ER stress modulation Inhibition of matrix-degrading enzymes production Upregulation of ECM-catabolic factors Downregulation of ECM-anabolic factors | Inhibition of NF-κB pathway |
| Metformin | Autophagy stimulant Anti-inflammatory activity Apoptosis attenuation Cellular senescence inhibition Reduction of hypermethylation level of SOX9 promoter Upregulation of anabolic genes Downregulation of catabolic genes | Inhibition of NF-κB pathway Blockage of HMGB1 translocation |
| Vitamin D | Antioxidant properties Anti-inflammatory activity Apoptosis inhibition Cellular senescence delay Aggrecan and collagen II upregulation Collagen X downregulation | Inhibition of NF-κB pathway |
| ClinicalTrials.Gov Identifier | Official Title | Intervention/ Treatment | Enrollment | Intervention Model | Phase | Status (as Reported until 10 May 2022) |
|---|---|---|---|---|---|---|
| NCT01158924 | A Phase I/IIa, Multicenter, Open-label, Clinical Trial to Evaluate the Safety, Tolerability and Preliminary Effectiveness of Single Administration Intradiscal rhGDF-5 for the Treatment of Early Stage Lumbar Disc Degeneration | Drug: Intradiscal rhGDF-5 | 40 participants | Single Group Assignment | Phase 1 Phase 2 | Completed |
| NCT00813813 | Phase I/II, Multicenter, Open-label, Single Administration, Dose Finding, Clinical Trial to Evaluate the Safety and Tolerability of Intradiscal rhGDF-5 for the Treatment of Early Stage Lumbar Disc Degeneration | Drug: Intradiscal rhGDF-5 | 32 participants | Single Group Assignment | Phase 1 Phase 2 | Completed |
| NCT01182337 | A Multicenter, Randomized, Double-blind, Placebo Controlled, Clinical Trial to Evaluate the Safety, Tolerability and Preliminary Effectiveness of Single Administration Intradiscal rhGDF-5 for the Treatment of Early Stage Lumbar Disc Degeneration | Drug: Intradiscal rhGDF-5 Drug: Vehicle control | 31 participants | Parallel Assignment | Phase 1 Phase 2 | Completed |
| NCT01124006 | A Multicenter, Randomized, Double-blind, Placebo Controlled, Clinical Trial to Evaluate the Safety, Tolerability and Preliminary Effectiveness of 2 Doses of Intradiscal rhGDF-5 (Single Administration) for the Treatment of Early Stage Lumbar Disc Degeneration | Drug: Intradiscal rhGDF-5 Other: Water for injection | 24 participants | Parallel Assignment | Phase 2 | Completed |
| NCT04816747 | Intradiscal and Intra-articular Injection of Autologous Platelet-Rich-Plasma (PRP) in Patients With Lumbar Degenerative Disc Disease and Facet Joint Syndrome: A Prospective, Single-arm, Open Label Clinical Trial | Biological: Autologous PRP | 50 participants (estimated) | Single Group Assignment | Phase 3 | Not yet recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costăchescu, B.; Niculescu, A.-G.; Teleanu, R.I.; Iliescu, B.F.; Rădulescu, M.; Grumezescu, A.M.; Dabija, M.G. Recent Advances in Managing Spinal Intervertebral Discs Degeneration. Int. J. Mol. Sci. 2022, 23, 6460. https://doi.org/10.3390/ijms23126460
Costăchescu B, Niculescu A-G, Teleanu RI, Iliescu BF, Rădulescu M, Grumezescu AM, Dabija MG. Recent Advances in Managing Spinal Intervertebral Discs Degeneration. International Journal of Molecular Sciences. 2022; 23(12):6460. https://doi.org/10.3390/ijms23126460
Chicago/Turabian StyleCostăchescu, Bogdan, Adelina-Gabriela Niculescu, Raluca Ioana Teleanu, Bogdan Florin Iliescu, Marius Rădulescu, Alexandru Mihai Grumezescu, and Marius Gabriel Dabija. 2022. "Recent Advances in Managing Spinal Intervertebral Discs Degeneration" International Journal of Molecular Sciences 23, no. 12: 6460. https://doi.org/10.3390/ijms23126460









