SOCS3 Ablation in Leptin Receptor-Expressing Cells Causes Autonomic and Cardiac Dysfunctions in Middle-Aged Mice despite Improving Energy and Glucose Metabolism
Abstract
:1. Introduction
2. Results
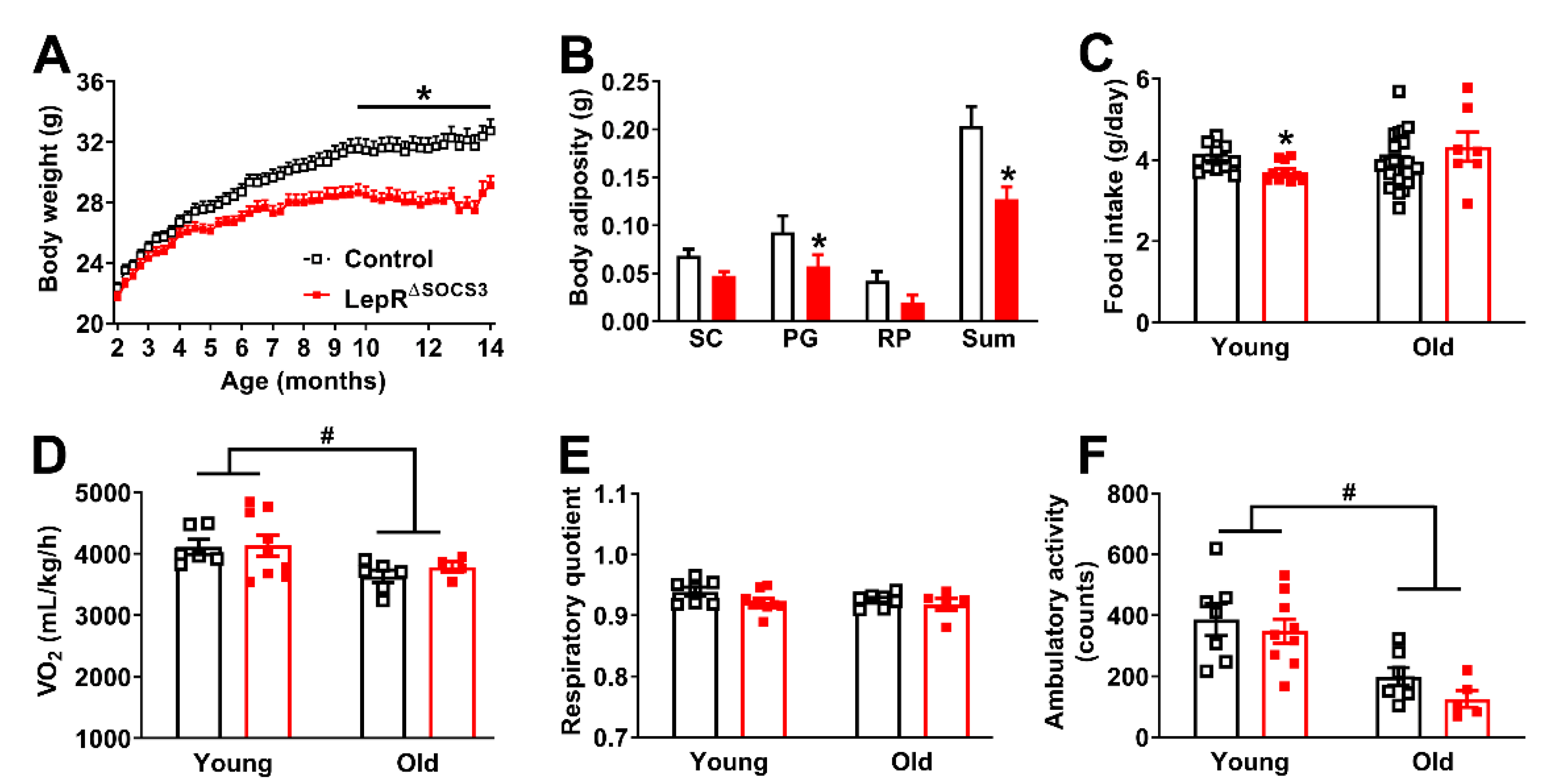
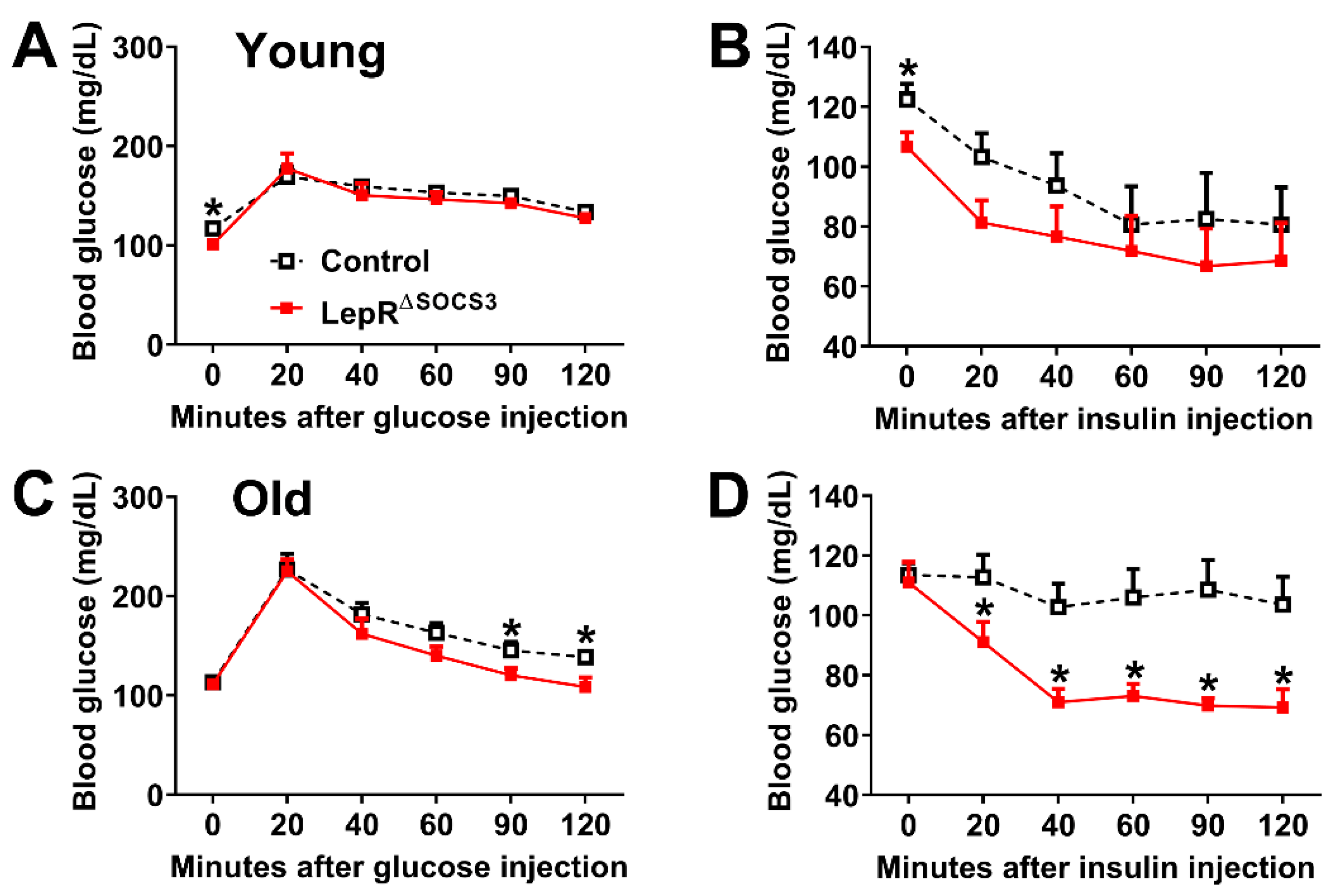
2.1. SOCS3 Ablation in LepR-Expressing Cells Improves Energy and Glucose Homeostasis in Aging Mice
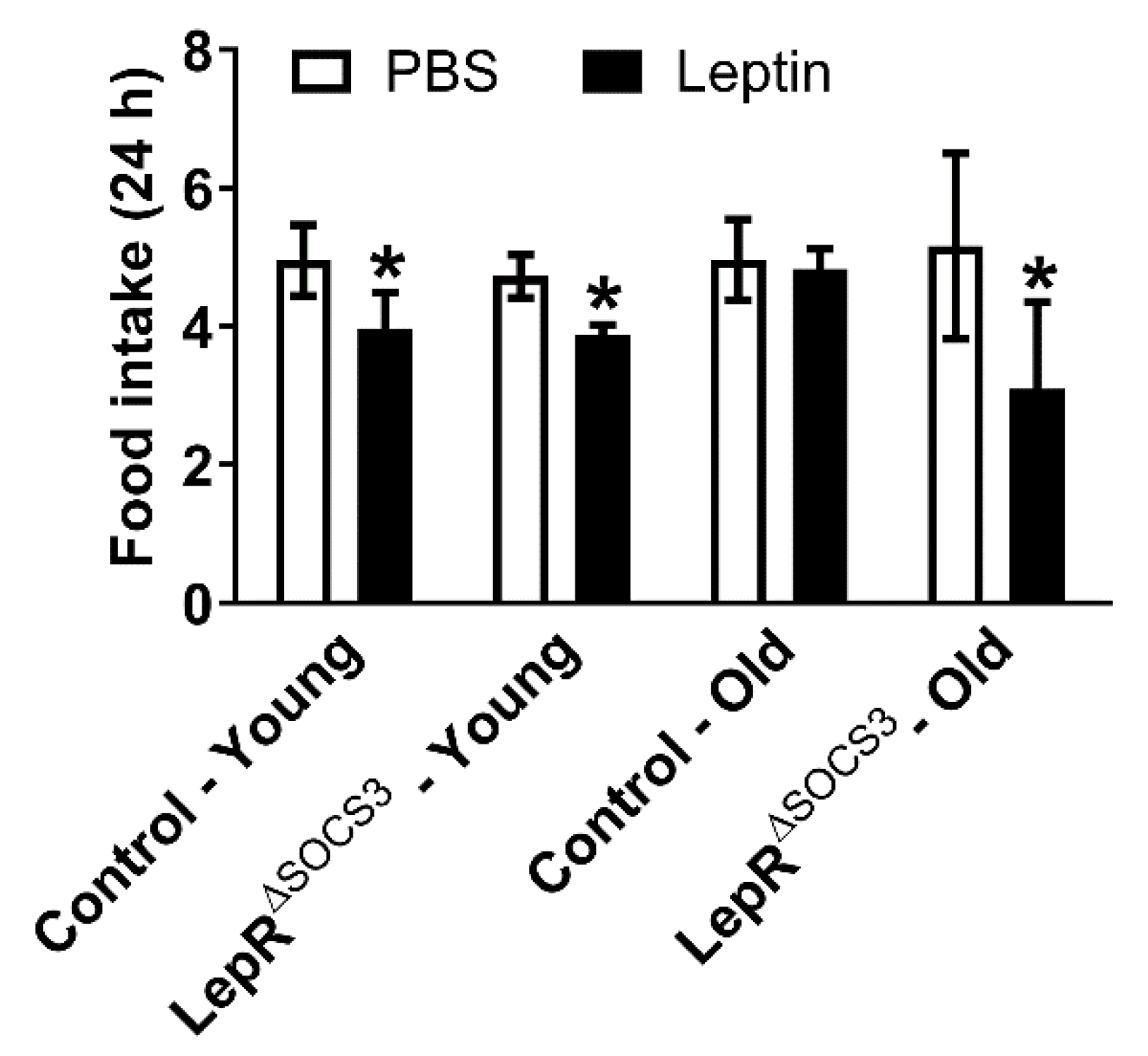
2.2. Increased Leptin Sensitivity in Middle-Aged LepR∆SOCS3 Mice
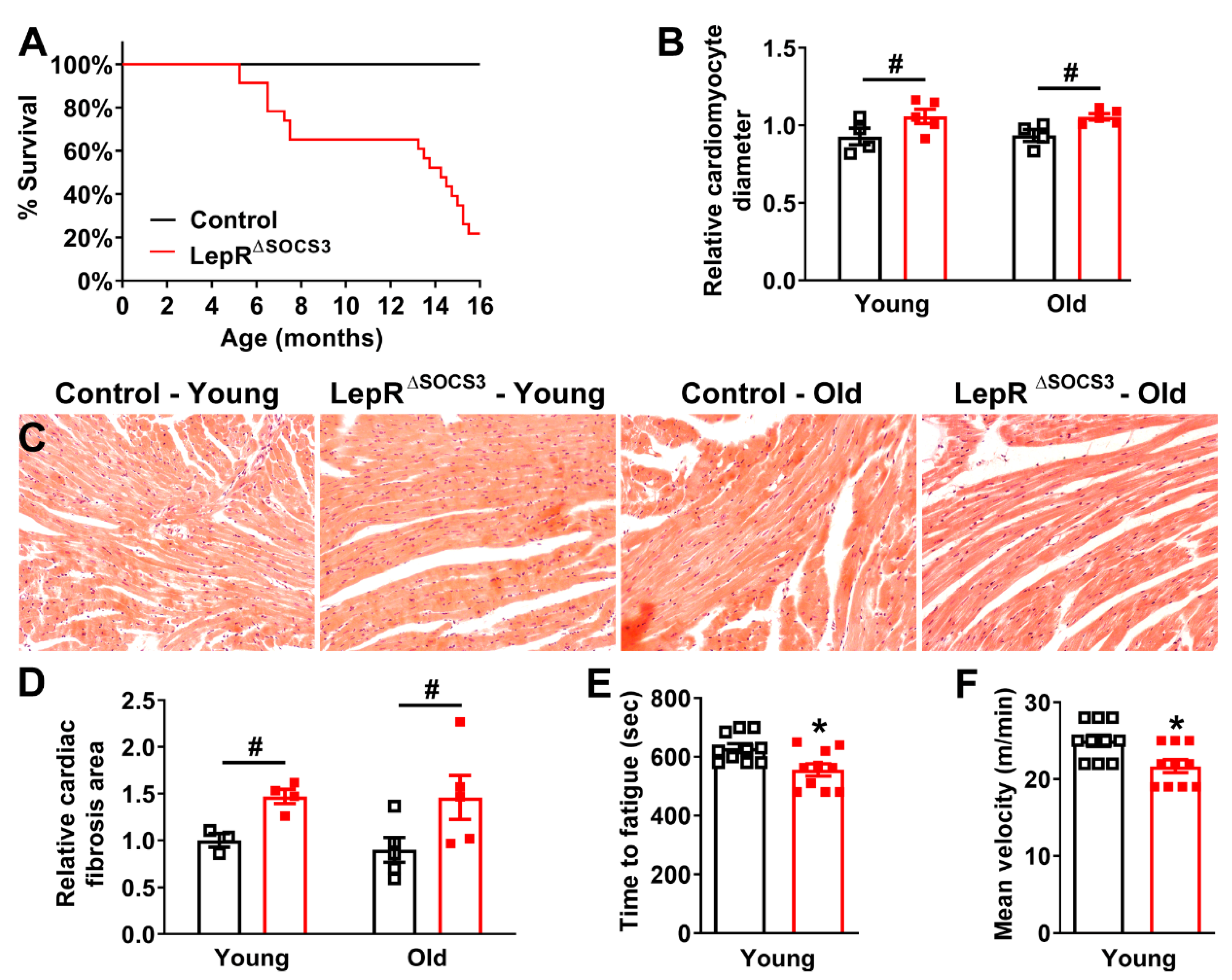
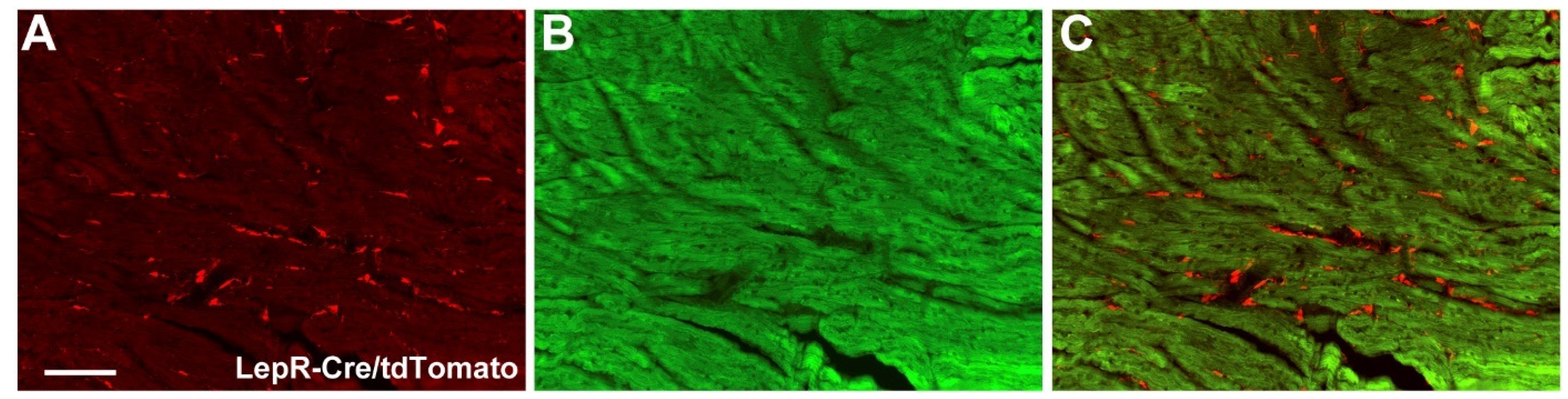
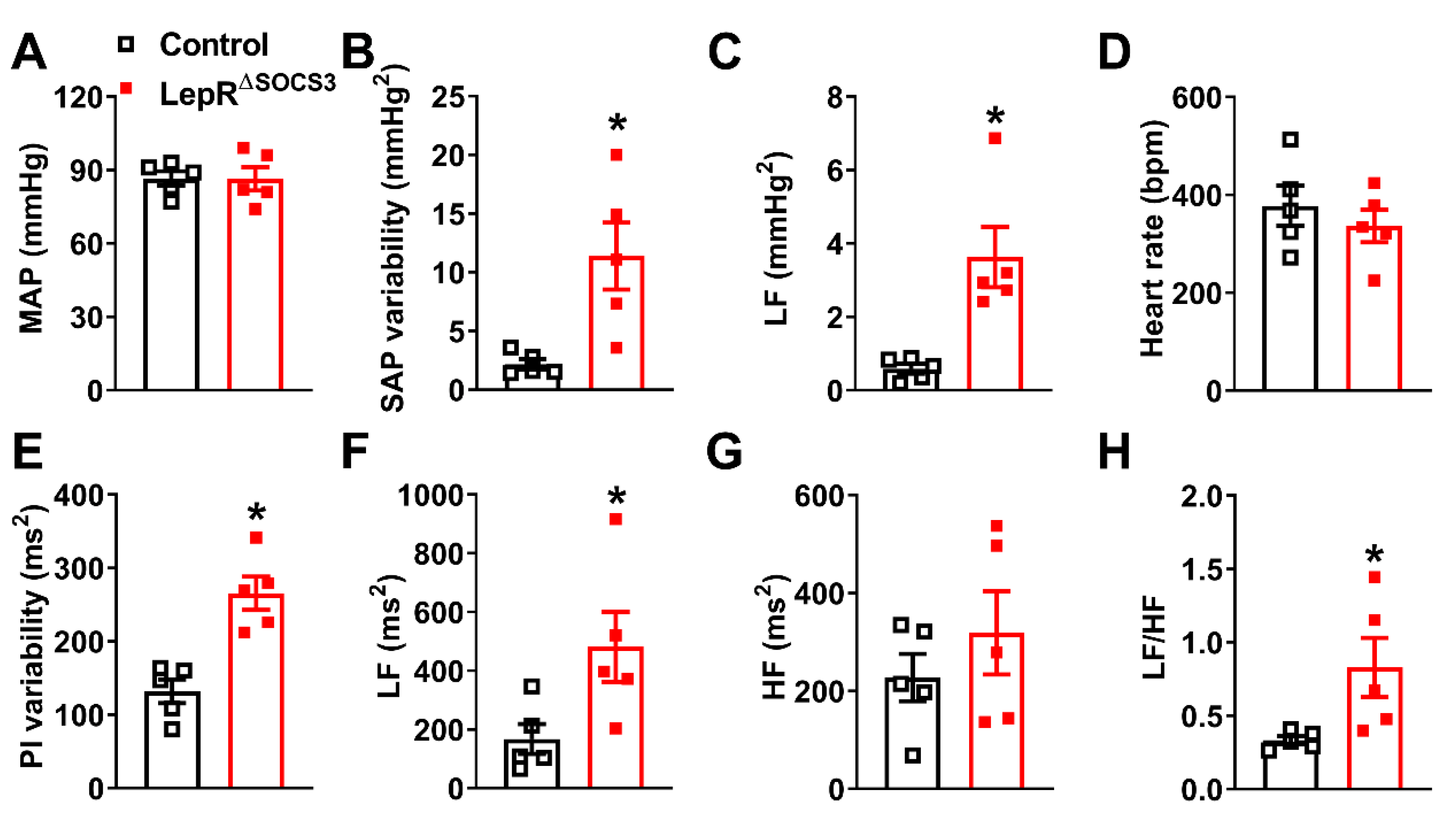
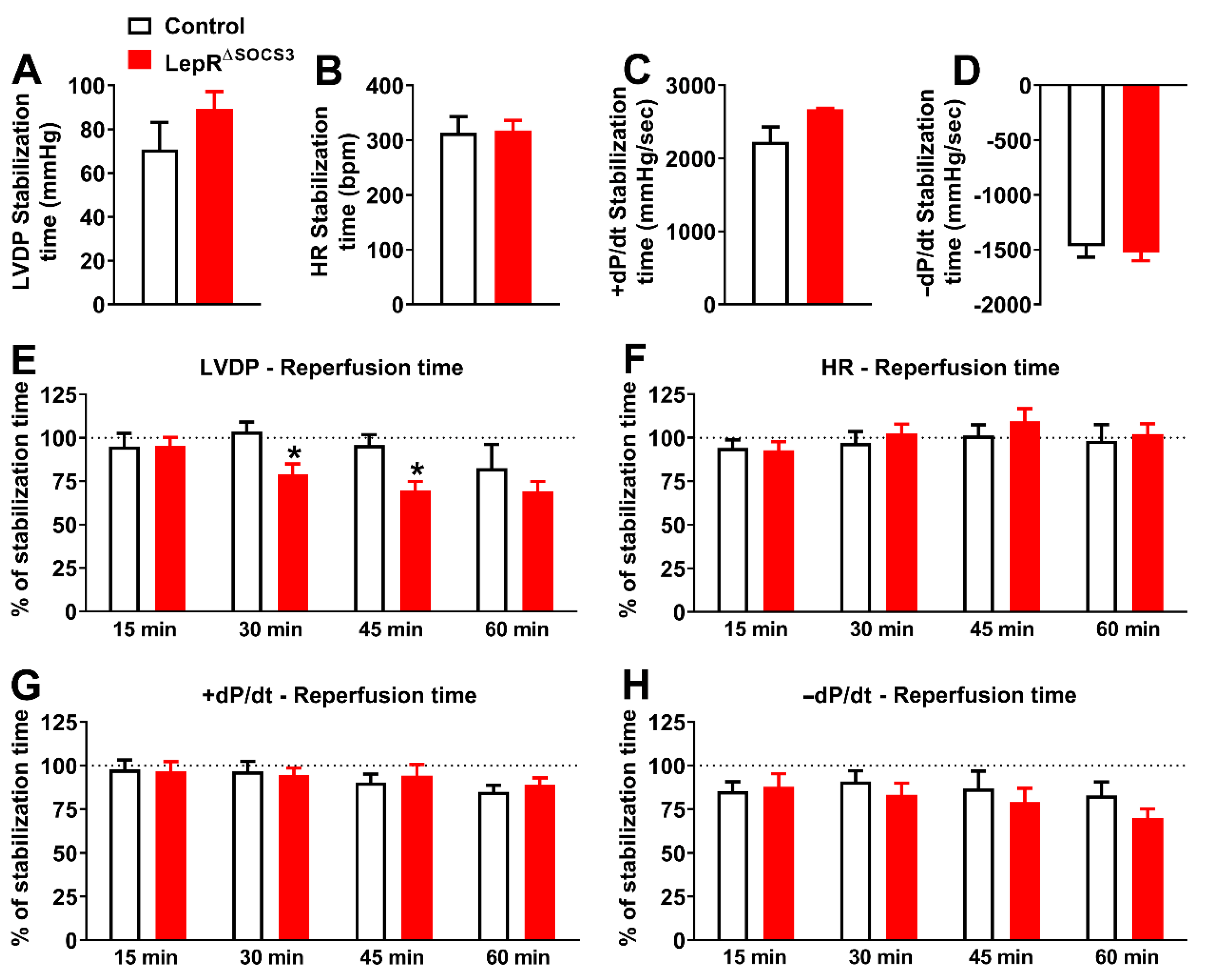
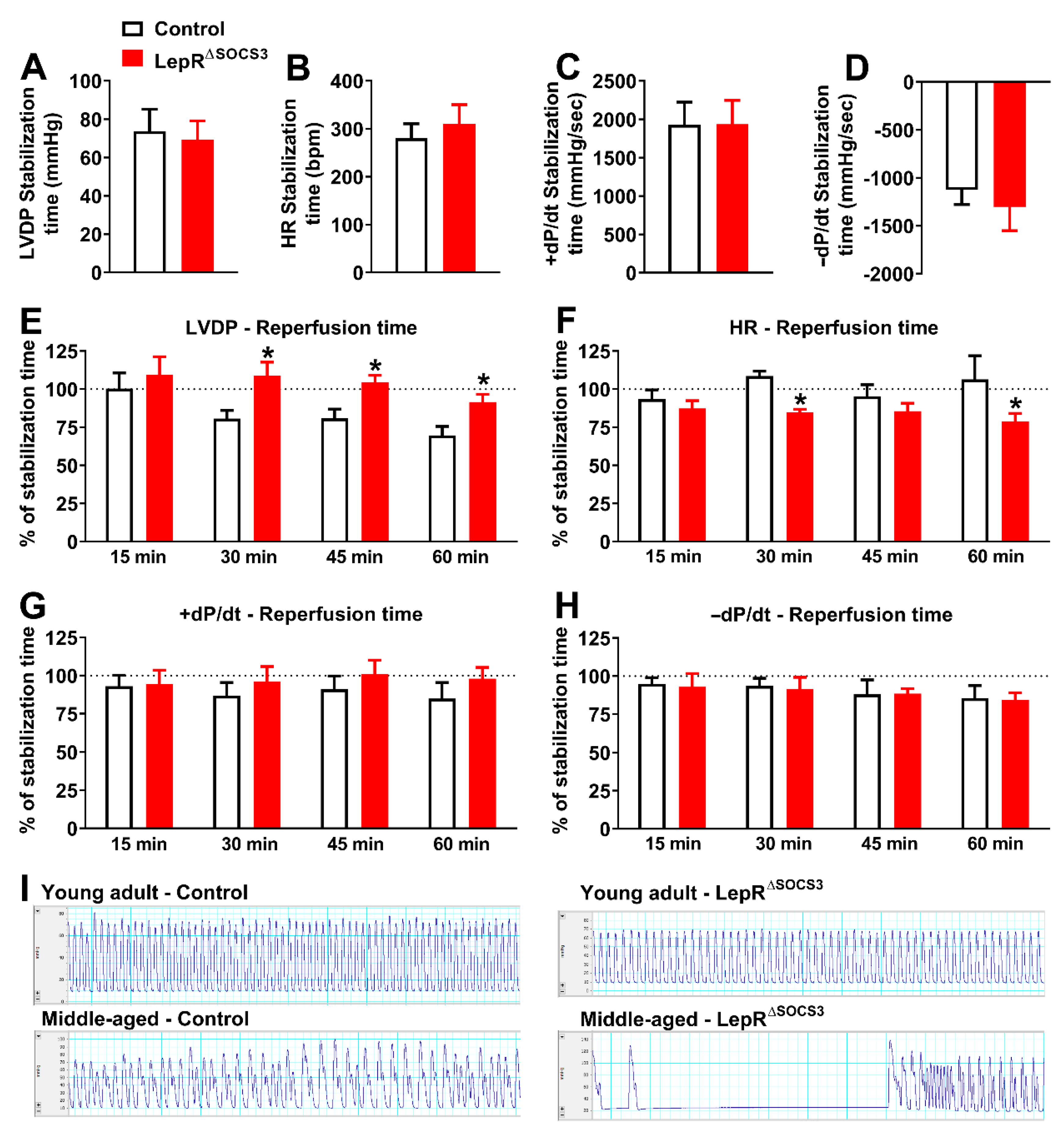
2.3. Cardiac Abnormalities and Decreased Survival Rate in Aging LepR∆SOCS3 Mice
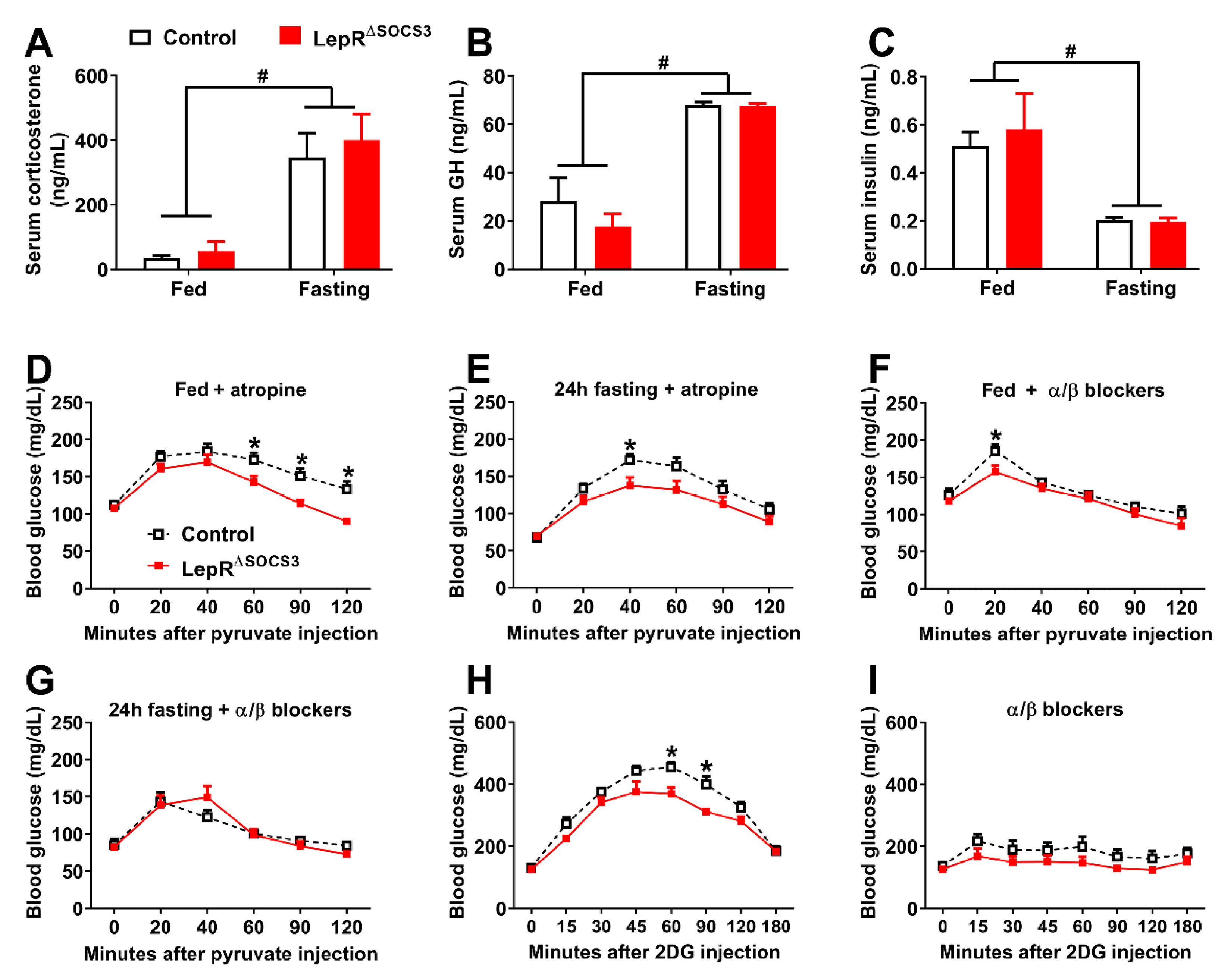
2.4. LepR∆SOCS3 Mice Exhibit Fasting-Induced Hypoglycemia That Is Associated with Reduced Gluconeogenesis
2.5. Possible Alterations in the Sympathetic Nervous System Explain the Impaired Gluconeogenesis and Counterregulatory Response of LepR∆SOCS3 Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Energy and Glucose Homeostasis
4.3. Tissue Collection and Processing
4.4. Incremental Treadmill Maximal Running Test
4.5. Leptin Sensitivity
4.6. Constant Flow Langendorff Preparation
4.7. Hemodynamic Recordings and Spectral Analysis
4.8. Glucose Responses to Fasting, Pyruvate and 2-Deoxy-D-Glucose
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Balland, E.; Cowley, M.A. New insights in leptin resistance mechanisms in mice. Front. Neuroendocr. 2015, 39, 59–65. [Google Scholar] [CrossRef]
- Andreoli, M.F.; Donato, J.; Cakir, I.; Perello, M. Leptin resensitisation: A reversion of leptin-resistant states. J. Endocrinol. 2019, 241, R81–R96. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Lobo, A.M.; Donato, J., Jr. The role of leptin in health and disease. Temperature 2017, 4, 258–291. [Google Scholar] [CrossRef] [Green Version]
- Hukshorn, C.J.; Saris, W.H.; Westerterp-Plantenga, M.S.; Farid, A.R.; Smith, F.J.; Campfield, L.A. Weekly subcutaneous pegylated recombinant native human leptin (peg-ob) administration in obese men. J. Clin. Endocrinol. Metab. 2000, 85, 4003–4009. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Greenberg, A.S.; Fujioka, K.; Dixon, R.M.; Kushner, R.; Hunt, T.; Lubina, J.A.; Patane, J.; Self, B.; Hunt, P.; et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. Jama 1999, 282, 1568–1575. [Google Scholar] [CrossRef]
- Lee, J.; Liu, J.; Feng, X.; Salazar Hernandez, M.A.; Mucka, P.; Ibi, D.; Choi, J.W.; Ozcan, U. Withaferin a is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 2016, 22, 1023–1032. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevaskis, J.L.; Coffey, T.; Cole, R.; Lei, C.; Wittmer, C.; Walsh, B.; Weyer, C.; Koda, J.; Baron, A.D.; Parkes, D.G.; et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: Magnitude and mechanisms. Endocrinology 2008, 149, 5679–5687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, C.S.; Lecoultre, V.; Ravussin, E. Novel strategy for the use of leptin for obesity therapy. Expert Opin. Biol. Ther. 2011, 11, 1677–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarta, C.; Sanchez-Garrido, M.A.; Tschop, M.H.; Clemmensen, C. Renaissance of leptin for obesity therapy. Diabetologia 2016, 59, 920–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, H.Y.; Qian, K.; Morris-Natschke, S.L.; Hsu, C.S.; Lee, K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef]
- Berglund, E.D.; Vianna, C.R.; Donato, J., Jr.; Kim, M.H.; Chuang, J.C.; Lee, C.E.; Lauzon, D.A.; Lin, P.; Brule, L.J.; Scott, M.M.; et al. Direct leptin action on pomc neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Investig. 2012, 122, 1000–1009. [Google Scholar] [CrossRef] [Green Version]
- Pocai, A.; Morgan, K.; Buettner, C.; Gutierrez-Juarez, R.; Obici, S.; Rossetti, L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 2005, 54, 3182–3189. [Google Scholar] [CrossRef] [Green Version]
- Van den Hoek, A.M.; Teusink, B.; Voshol, P.J.; Havekes, L.M.; Romijn, J.A.; Pijl, H. Leptin deficiency per se dictates body composition and insulin action in ob/ob mice. J. Neuroendocr. 2008, 20, 120–127. [Google Scholar] [CrossRef]
- Simonds, S.E.; Pryor, J.T.; Ravussin, E.; Greenway, F.L.; Dileone, R.; Allen, A.M.; Bassi, J.; Elmquist, J.K.; Keogh, J.M.; Henning, E.; et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 2014, 159, 1404–1416. [Google Scholar] [CrossRef] [Green Version]
- Enriori, P.J.; Sinnayah, P.; Simonds, S.E.; Garcia Rudaz, C.; Cowley, M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011, 31, 12189–12197. [Google Scholar] [CrossRef] [Green Version]
- Münzberg, H.; Flier, J.S.; Bjørbæk, C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004, 145, 4880–4889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorbaek, C.; Elmquist, J.K.; Frantz, J.D.; Shoelson, S.E.; Flier, J.S. Identification of socs-3 as a potential mediator of central leptin resistance. Mol. Cell 1998, 1, 619–625. [Google Scholar] [CrossRef]
- Briancon, N.; McNay, D.E.; Maratos-Flier, E.; Flier, J.S. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1b reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes 2010, 59, 3074–3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, H.; Hanada, R.; Hanada, T.; Aki, D.; Mashima, R.; Nishinakamura, H.; Torisu, T.; Chien, K.R.; Yasukawa, H.; Yoshimura, A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004, 10, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Flier, J.S. Attenuation of leptin and insulin signaling by socs proteins. Trends Endocrinol. Metab. 2006, 17, 365–371. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; Ramos-Lobo, A.M.; Donato, J., Jr. Socs3 as a future target to treat metabolic disorders. Hormones 2019, 18, 127–136. [Google Scholar] [CrossRef]
- Pedroso, J.A.; Buonfiglio, D.C.; Cardinali, L.I.; Furigo, I.C.; Ramos-Lobo, A.M.; Tirapegui, J.; Elias, C.F.; Donato, J., Jr. Inactivation of socs3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol. Metab. 2014, 3, 608–618. [Google Scholar] [CrossRef]
- Zampieri, T.T.; Ramos-Lobo, A.M.; Furigo, I.C.; Pedroso, J.A.; Buonfiglio, D.C.; Donato, J., Jr. Socs3 deficiency in leptin receptor-expressing cells mitigates the development of pregnancy-induced metabolic changes. Mol. Metab. 2015, 4, 237–245. [Google Scholar] [CrossRef]
- Bohlen, T.M.; Silveira, M.A.; Zampieri, T.T.; Frazao, R.; Donato, J., Jr. Fatness rather than leptin sensitivity determines the timing of puberty in female mice. Mol. Cell Endocrinol. 2016, 423, 11–21. [Google Scholar] [CrossRef]
- Pedroso, J.A.; Silveira, M.A.; Lima, L.B.; Furigo, I.C.; Zampieri, T.T.; Ramos-Lobo, A.M.; Buonfiglio, D.C.; Teixeira, P.D.; Frazao, R.; Donato, J., Jr. Changes in leptin signaling by socs3 modulate fasting-induced hyperphagia and weight regain in mice. Endocrinology 2016, 157, 3901–3914. [Google Scholar] [CrossRef]
- Zampieri, T.T.; da Silva, T.E.; de Paula Romeu, D.; da Silva Torrao, A.; Donato, J., Jr. Socs3 expression within leptin receptor-expressing cells regulates food intake and leptin sensitivity but does not affect weight gain in pregnant mice consuming a high-fat diet. Physiol. Behav. 2016, 157, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lobo, A.M.; Furigo, I.C.; Teixeira, P.D.S.; Zampieri, T.T.; Wasinski, F.; Buonfiglio, D.C.; Donato, J., Jr. Maternal metabolic adaptations are necessary for normal offspring growth and brain development. Physiol. Rep. 2018, 6, e13643. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Galaz, C.; Fernandez-Agullo, T.; Perez, C.; Peralta, S.; Arribas, C.; Andres, A.; Carrascosa, J.M.; Ros, M. Long-term food restriction prevents ageing-associated central leptin resistance in wistar rats. Diabetologia 2002, 45, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, S.; Carrascosa, J.M.; Gallardo, N.; Ros, M.; Arribas, C. Ageing increases socs-3 expression in rat hypothalamus: Effects of food restriction. Biochem. Biophys. Res. Commun. 2002, 296, 425–428. [Google Scholar] [CrossRef]
- Qian, H.; Azain, M.J.; Hartzell, D.L.; Baile, C.A. Increased leptin resistance as rats grow to maturity. Proc. Soc. Exp. Biol. Med. 1998, 219, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Scarpace, P.J.; Tumer, N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol. Behav. 2001, 74, 721–727. [Google Scholar] [CrossRef]
- Zhang, Y.; Matheny, M.; Tümer, N.; Scarpace, P.J. Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance. Neurobiol. Aging 2004, 25, 1349–1360. [Google Scholar] [CrossRef]
- Radhika, H.M.; Xiaohui, M.; Xiaoman, Y.; Gil, A.; Nir, B. Central resistance to the inhibitory effects of leptin on stimulated insulin secretion with aging. Neurobiol. Aging 2006, 27, 1308–1314. [Google Scholar]
- Morrison, C.D.; White, C.L.; Wang, Z.; Lee, S.Y.; Lawrence, D.S.; Cefalu, W.T.; Zhang, Z.Y.; Gettys, T.W. Increased hypothalamic protein tyrosine phosphatase 1b contributes to leptin resistance with age. Endocrinology 2007, 148, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Scarpace, P.J.; Matheny, M.; Moore, R.L.; Tümer, N. Impaired leptin responsiveness in aged rats. Diabetes 2000, 49, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Scarpace, P.J.; Matheny, M.; Tümer, N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 2001, 104, 1111–1117. [Google Scholar] [CrossRef]
- Nagaishi, V.S.; Cardinali, L.I.; Zampieri, T.T.; Furigo, I.C.; Metzger, M.; Donato, J., Jr. Possible crosstalk between leptin and prolactin during pregnancy. Neuroscience 2014, 259, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.M.; Lachey, J.L.; Sternson, S.M.; Lee, C.E.; Elias, C.F.; Friedman, J.M.; Elmquist, J.K. Leptin targets in the mouse brain. J. Comp. Neurol. 2009, 514, 518–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akselrod, S.; Gordon, D.; Ubel, F.A.; Shannon, D.C.; Berger, A.C.; Cohen, R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef]
- Parati, G.; Saul, J.P.; Di Rienzo, M.; Mancia, G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 1995, 25, 1276–1286. [Google Scholar] [CrossRef]
- Saul, J. Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. Physiology 1990, 5, 32–37. [Google Scholar] [CrossRef]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, I.B.; Gomes, D.A.; Alenina, N.; Bader, M.; Dos Santos, R.A.; Barreto-Chaves, M.L.M. Cardioprotective effect of thyroid hormone is mediated by at2 receptor and involves nitric oxide production via akt activation in mice. Hear. Vessel. 2018, 33, 671–681. [Google Scholar] [CrossRef]
- Garfield, A.S.; Shah, B.P.; Madara, J.C.; Burke, L.K.; Patterson, C.M.; Flak, J.; Neve, R.L.; Evans, M.L.; Lowell, B.B.; Myers, M.G., Jr.; et al. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab. 2014, 20, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- Faber, C.L.; Matsen, M.E.; Velasco, K.R.; Damian, V.; Phan, B.A.; Adam, D.; Therattil, A.; Schwartz, M.W.; Morton, G.J. Distinct neuronal projections from the hypothalamic ventromedial nucleus mediate glycemic and behavioral effects. Diabetes 2018, 67, 2518–2529. [Google Scholar] [CrossRef] [Green Version]
- Flak, J.N.; Goforth, P.B.; Dell’Orco, J.; Sabatini, P.V.; Li, C.; Bozadjieva, N.; Sorensen, M.; Valenta, A.; Rupp, A.; Affinati, A.H.; et al. Ventromedial hypothalamic nucleus neuronal subset regulates blood glucose independently of insulin. J. Clin. Investig. 2020, 130, 2943–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verberne, A.J.; Sabetghadam, A.; Korim, W.S. Neural pathways that control the glucose counterregulatory response. Front. Neurosci. 2014, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flak, J.N.; Patterson, C.M.; Garfield, A.S.; D’Agostino, G.; Goforth, P.B.; Sutton, A.K.; Malec, P.A.; Wong, J.M.; Germani, M.; Jones, J.C.; et al. Leptin-inhibited pbn neurons enhance responses to hypoglycemia in negative energy balance. Nat. Neurosci. 2014, 17, 1744–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroso, J.A.B.; de Mendonca, P.O.R.; Fortes, M.A.S.; Tomaz, I.; Pecorali, V.L.; Auricino, T.B.; Costa, I.C.; Lima, L.B.; Furigo, I.C.; Bueno, D.N.; et al. Socs3 expression in sf1 cells regulates adrenal differentiation and exercise performance. J. Endocrinol. 2017, 235, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Furigo, I.C.; de Souza, G.O.; Teixeira, P.D.S.; Guadagnini, D.; Frazao, R.; List, E.O.; Kopchick, J.J.; Prada, P.O.; Donato, J., Jr. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J. 2019, 33, 11909–11924. [Google Scholar] [CrossRef] [Green Version]
- Li, R.L.; Sherbet, D.P.; Elsbernd, B.L.; Goldstein, J.L.; Brown, M.S.; Zhao, T.J. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J. Biol. Chem. 2012, 287, 17942–17950. [Google Scholar] [CrossRef] [Green Version]
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar]
- Vianna, C.R.; Donato, J., Jr.; Rossi, J.; Scott, M.; Economides, K.; Gautron, L.; Pierpont, S.; Elias, C.F.; Elmquist, J.K. Cannabinoid receptor 1 in the vagus nerve is dispensable for body weight homeostasis but required for normal gastrointestinal motility. J. Neurosci. 2012, 32, 10331–10337. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, J.R.; Miller, J.W.; Keogh, J.M.; Henning, E.; Satterwhite, J.H.; Cameron, G.S.; Astruc, B.; Mayer, J.P.; Brage, S.; See, T.C.; et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 2009, 360, 44–52. [Google Scholar] [CrossRef]
- Mizuno, A.; Murakami, T.; Otani, S.; Kuwajima, M.; Shima, K. Leptin affects pancreatic endocrine functions through the sympathetic nervous system. Endocrinology 1998, 139, 3863–3870. [Google Scholar] [CrossRef]
- Carlyle, M.; Jones, O.B.; Kuo, J.J.; Hall, J.E. Chronic cardiovascular and renal actions of leptin: Role of adrenergic activity. Hypertension 2002, 39, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.M.; Kleopoulos, S.P.; Bergen, H.T.; Roberts, J.L.; Priest, C.A.; Mobbs, C.V. Hypothalamic pro-opiomelanocortin mrna is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 1998, 47, 294–297. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, J.R. Melanocortin signalling and the regulation of blood pressure in human obesity. J. Neuroendocr. 2011, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Copperi, F.; Kim, J.D.; Diano, S. Role of the melanocortin system in the central regulation of cardiovascular functions. Front. Physiol. 2021, 12, 725709. [Google Scholar] [CrossRef] [PubMed]
- Krebs, D.L.; Hilton, D.J. Socs proteins: Negative regulators of cytokine signaling. Stem Cells 2001, 19, 378–387. [Google Scholar] [CrossRef]
- Da Silva, A.A.; Kuo, J.J.; Hall, J.E. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension 2004, 43, 1312–1317. [Google Scholar] [CrossRef] [Green Version]
- Do Carmo, J.M.; da Silva, A.A.; Freeman, J.N.; Wang, Z.; Moak, S.P.; Hankins, M.W.; Drummond, H.A.; Hall, J.E. Neuronal suppressor of cytokine signaling 3: Role in modulating chronic metabolic and cardiovascular effects of leptin. Hypertension 2018, 71, 1248–1257. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; Ferno, J.; Gonzalez, F.; Dieguez, C.; Leis, R.; Nogueiras, R.; Lopez, M. Hypothalamic-autonomic control of energy homeostasis. Endocrine 2015, 50, 276–291. [Google Scholar] [CrossRef]
- Matsui, H.; Motooka, M.; Koike, H.; Inoue, M.; Iwasaki, T.; Suzuki, T.; Kurabayashi, M.; Yokoyama, T. Ischemia/reperfusion in rat heart induces leptin and leptin receptor gene expression. Life Sci. 2007, 80, 672–680. [Google Scholar] [CrossRef]
- McGaffin, K.R.; Sun, C.K.; Rager, J.J.; Romano, L.C.; Zou, B.; Mathier, M.A.; O’Doherty, R.M.; McTiernan, C.F.; O’Donnell, C.P. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc. Res. 2008, 77, 54–63. [Google Scholar] [CrossRef] [Green Version]
- McGaffin, K.R.; Moravec, C.S.; McTiernan, C.F. Leptin signaling in the failing and mechanically unloaded human heart. Circ. Hear. Fail. 2009, 2, 676–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGaffin, K.R.; Witham, W.G.; Yester, K.A.; Romano, L.C.; O’Doherty, R.M.; McTiernan, C.F.; O’Donnell, C.P. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc. Res. 2011, 89, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.E.; Smith, G.; Hall, J.E.; Stec, D.E. Cardiomyocyte-specific deletion of leptin receptors causes lethal heart failure in cre-recombinase-mediated cardiotoxicity. Am. J. Physiol. Integr. Comp. Physiol. 2012, 303, R1241–R1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leifheit-Nestler, M.; Wagner, N.M.; Gogiraju, R.; Didie, M.; Konstantinides, S.; Hasenfuss, G.; Schafer, K. Importance of leptin signaling and signal transducer and activator of transcription-3 activation in mediating the cardiac hypertrophy associated with obesity. J. Transl. Med. 2013, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wang, Y.; Zhou, X.; Huang, B.; Wang, M.; Li, X.; Meng, G.; Yuan, S.; Xia, H.; Jiang, H. Leptin injection into the left stellate ganglion augments ischemia-related ventricular arrhythmias via sympathetic nerve activation. Hear. Rhythm 2018, 15, 597–606. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Steculorum, S.M.; Ruud, J.; Karakasilioti, I.; Backes, H.; Engstrom Ruud, L.; Timper, K.; Hess, M.E.; Tsaousidou, E.; Mauer, J.; Vogt, M.C.; et al. Agrp neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 2016, 165, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, G.H.; Li, W.; Garcia, A.V.; Figueiredo, M.S.; Bjorbaek, C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. Cell Rep. 2014, 7, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Dhillon, H.; Yin, H.; Yoshimura, A.; Lowell, B.B.; Maratos-Flier, E.; Flier, J.S. Selective inactivation of socs3 in sf1 neurons improves glucose homeostasis without affecting body weight. Endocrinology 2008, 149, 5654–5661. [Google Scholar] [CrossRef]
- Tong, Q.; Ye, C.; McCrimmon, R.J.; Dhillon, H.; Choi, B.; Kramer, M.D.; Yu, J.; Yang, Z.; Christiansen, L.M.; Lee, C.E.; et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007, 5, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.P.; Silva, L.E.; Katayama, P.L.; Silva, C.A.; Salgado, H.C.; Fazan, R. Correlation between rr, inter-systolic and inter-diastolic intervals and their differences for the analysis of spontaneous heart rate variability. Physiol. Meas. 2016, 37, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. Heart rate variability—A historical perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroso, J.A.B.; Silva, I.B.d.; Zampieri, T.T.; Totola, L.T.; Moreira, T.S.; Taniguti, A.P.T.; Diniz, G.P.; Barreto-Chaves, M.L.M.; Donato, J., Jr. SOCS3 Ablation in Leptin Receptor-Expressing Cells Causes Autonomic and Cardiac Dysfunctions in Middle-Aged Mice despite Improving Energy and Glucose Metabolism. Int. J. Mol. Sci. 2022, 23, 6484. https://doi.org/10.3390/ijms23126484
Pedroso JAB, Silva IBd, Zampieri TT, Totola LT, Moreira TS, Taniguti APT, Diniz GP, Barreto-Chaves MLM, Donato J Jr. SOCS3 Ablation in Leptin Receptor-Expressing Cells Causes Autonomic and Cardiac Dysfunctions in Middle-Aged Mice despite Improving Energy and Glucose Metabolism. International Journal of Molecular Sciences. 2022; 23(12):6484. https://doi.org/10.3390/ijms23126484
Chicago/Turabian StylePedroso, João A. B., Ivson B. da Silva, Thais T. Zampieri, Leonardo T. Totola, Thiago S. Moreira, Ana P. T. Taniguti, Gabriela P. Diniz, Maria Luiza M. Barreto-Chaves, and Jose Donato, Jr. 2022. "SOCS3 Ablation in Leptin Receptor-Expressing Cells Causes Autonomic and Cardiac Dysfunctions in Middle-Aged Mice despite Improving Energy and Glucose Metabolism" International Journal of Molecular Sciences 23, no. 12: 6484. https://doi.org/10.3390/ijms23126484
APA StylePedroso, J. A. B., Silva, I. B. d., Zampieri, T. T., Totola, L. T., Moreira, T. S., Taniguti, A. P. T., Diniz, G. P., Barreto-Chaves, M. L. M., & Donato, J., Jr. (2022). SOCS3 Ablation in Leptin Receptor-Expressing Cells Causes Autonomic and Cardiac Dysfunctions in Middle-Aged Mice despite Improving Energy and Glucose Metabolism. International Journal of Molecular Sciences, 23(12), 6484. https://doi.org/10.3390/ijms23126484






