The Combined Inactivation of Intestinal and Hepatic ZIP14 Exacerbates Manganese Overload in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Genotyping and Tissue Collection
2.2. Metal Content Measurement
2.3. Western Blot Analysis
2.4. Statistical Analysis
3. Results
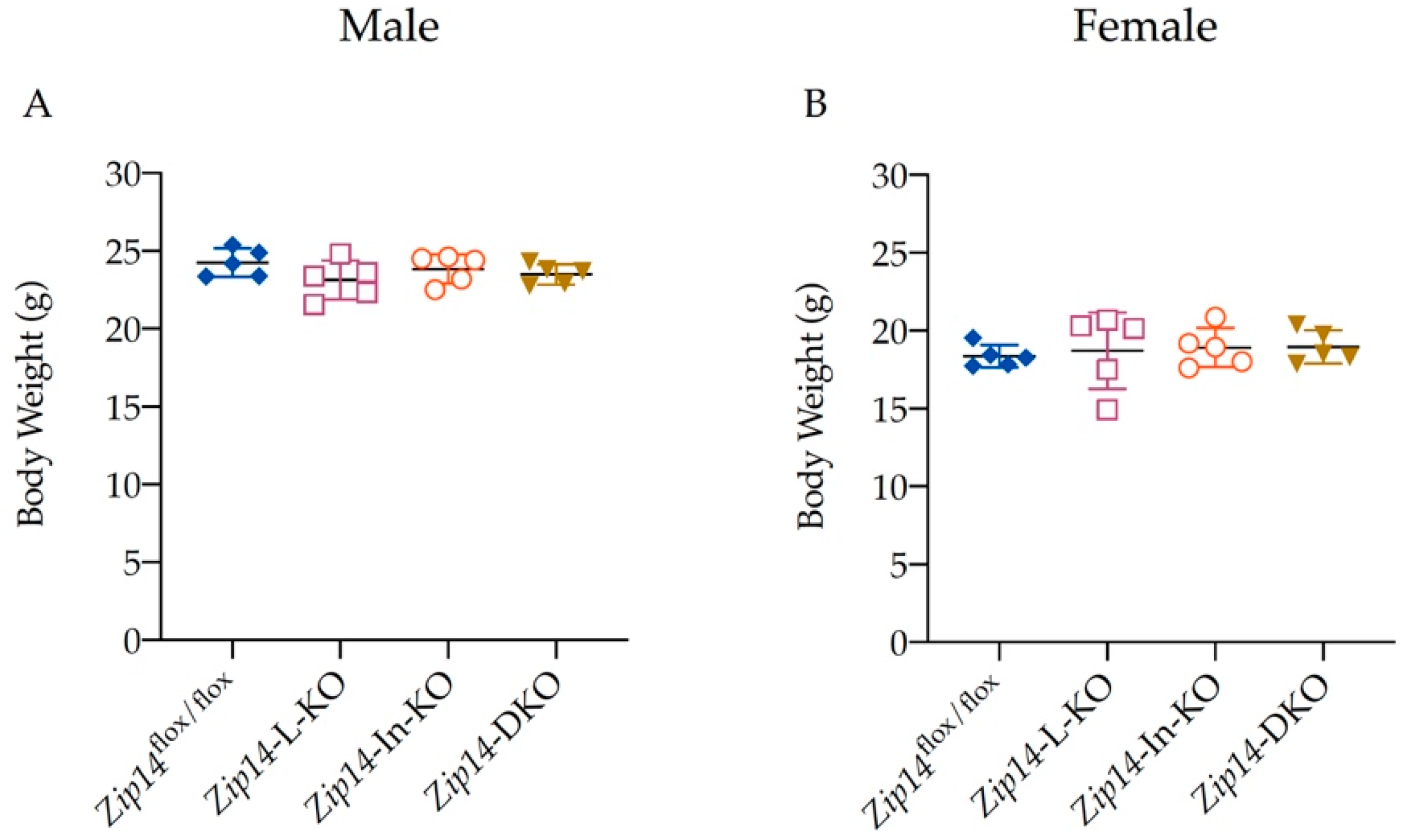
3.1. Generation of Mice with Tissue-Specific Zip14 Deletion
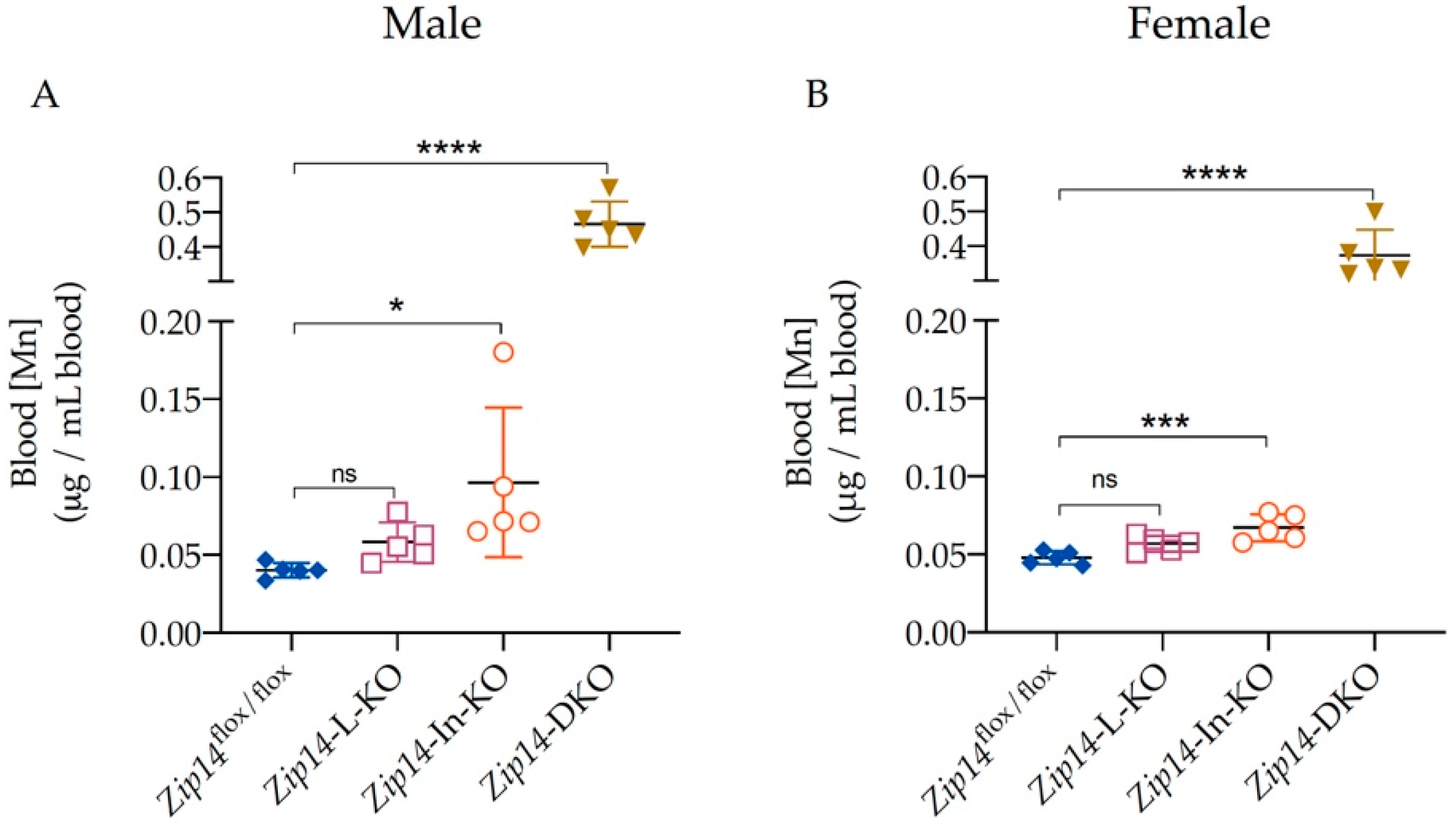
3.2. Blood Manganese Increases Significantly in Zip14-DKO Mice
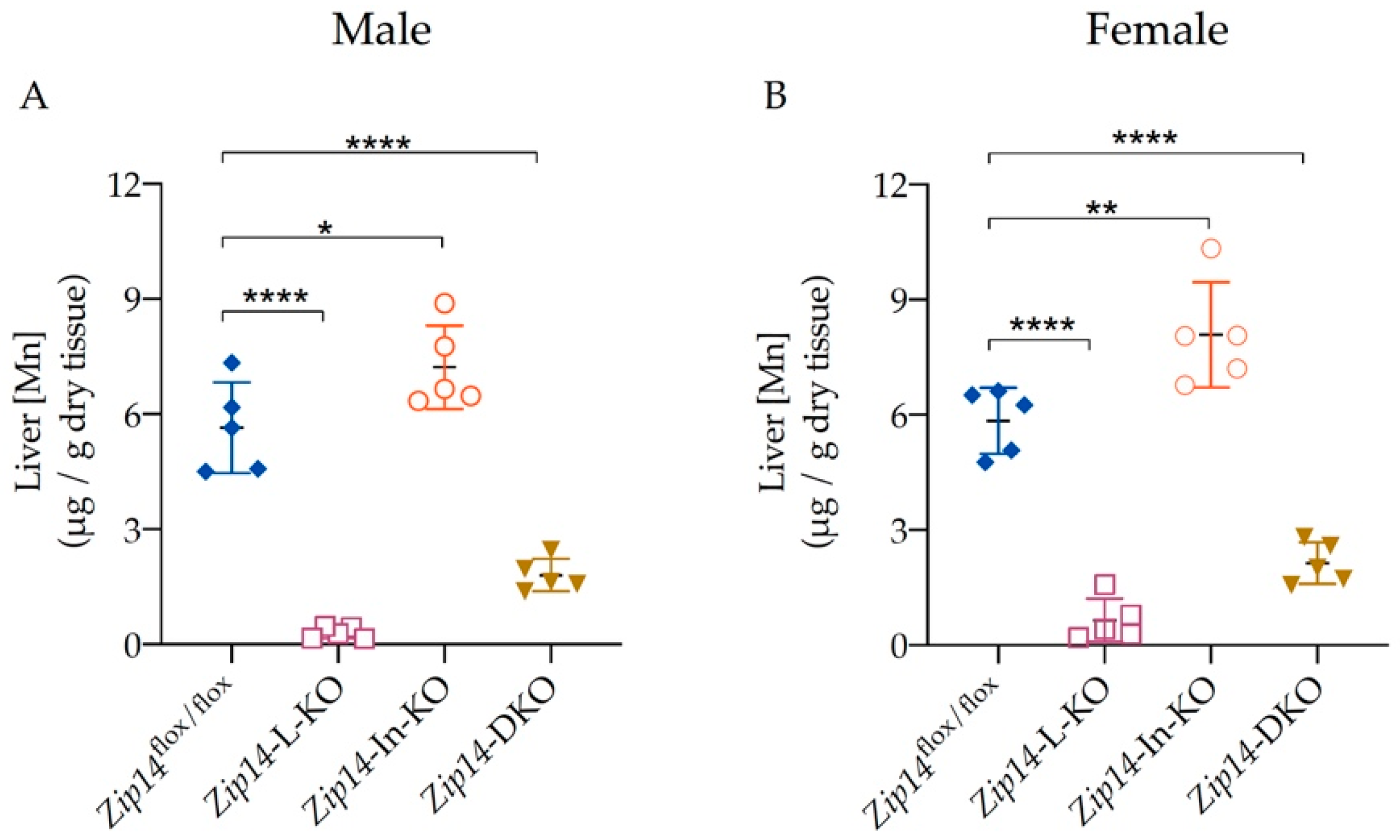
3.3. Liver Manganese Increases in Zip14-In-KO Mice, but Decreases Significantly in Both Zip14-L-KO and Zip14-DKO Mice
3.4. ZIP14 Deletion in Both the Liver and Intestine Significantly Increases Brain Manganese Accumulation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Asp. Med. 2005, 26, 353–362. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. Met. Ions Life Sci. 2013, 13, 199–227. [Google Scholar] [CrossRef]
- Garcia-Aranda, J.A.; Wapnir, R.A.; Lifshitz, F. In vivo intestinal absorption of manganese in the rat. J. Nutr. 1983, 113, 2601–2607. [Google Scholar] [CrossRef]
- Lutz, T.A.; Schroff, A.; Scharrer, E. Effects of calcium and sugars on intestinal manganese absorption. Biol. Trace Elem. Res. 1993, 39, 221–227. [Google Scholar] [CrossRef]
- Miller, W.J. Dynamics of absorption rates, endogenous excretion, tissue turnover, and homeostatic control mechanisms of zinc, cadmium, manganese, and nickel in ruminants. Fed. Proc. 1973, 32, 1915–1920. [Google Scholar]
- Roth, J.A. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol. Res. 2006, 39, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, H.A.; Balassa, J.J.; Tipton, I.H. Essential trace metals in man: Manganese. A study in homeostasis. J. Chronic Dis. 1966, 19, 545–571. [Google Scholar] [CrossRef]
- Butterworth, R.F. Parkinsonism in cirrhosis: Pathogenesis and current therapeutic options. Metab. Brain Dis. 2013, 28, 261–267. [Google Scholar] [CrossRef]
- Burnett, W.T., Jr.; Bigelow, R.R.; Kimball, A.W.; Sheppard, C.W. Radio-manganese studies on the mouse, rat and pancreatic fistula dog. Am. J. Physiol. 1952, 168, 620–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotzias, G.C.; Papavasiliou, P.S. Primordial Homeostasis in a Mammal as Shown by the Control of Manganese. Nature 1964, 201, 828–829. [Google Scholar] [CrossRef]
- Bertinchamps, A.J.; Miller, S.T.; Cotzias, G.C. Interdependence of routes excreting manganese. Am. J. Physiol. 1966, 211, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Tenas, J.J.; Sparkman, B.K.; Shawki, A.; Illing, A.C.; Mitchell, C.J.; Zhao, N.; Liuzzi, J.P.; Cousins, R.J.; Knutson, M.D.; Mackenzie, B. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 2011, 301, C862–C871. [Google Scholar] [CrossRef] [Green Version]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012, 4, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat. Commun. 2016, 7, 11601. [Google Scholar] [CrossRef] [Green Version]
- Marti-Sanchez, L.; Ortigoza-Escobar, J.D.; Darling, A.; Villaronga, M.; Baide, H.; Molero-Luis, M.; Batllori, M.; Vanegas, M.I.; Muchart, J.; Aquino, L.; et al. Hypermanganesemia due to mutations in SLC39A14: Further insights into Mn deposition in the central nervous system. Orphanet J. Rare Dis. 2018, 13, 28. [Google Scholar] [CrossRef]
- Zeglam, A.; Abugrara, A.; Kabuka, M. Autosomal-recessive iron deficiency anemia, dystonia and hypermanganesemia caused by new variant mutation of the manganese transporter gene SLC39A14. Acta. Neurol. Belg. 2019, 119, 379–384. [Google Scholar] [CrossRef]
- Rodan, L.H.; Hauptman, M.; D’Gama, A.M.; Qualls, A.E.; Cao, S.; Tuschl, K.; Al-Jasmi, F.; Hertecant, J.; Hayflick, S.J.; Wessling-Resnick, M.; et al. Novel founder intronic variant in SLC39A14 in two families causing Manganism and potential treatment strategies. Mol. Genet. Metab. 2018, 124, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Shamim, U.; Joshi, A.; Mathur, A.; Uppili, B.; Sairam, S.; Ambawat, S.; Dixit, R.; Faruq, M. A novel mutation in SLC39A14 causing hypermanganesemia associated with infantile onset dystonia. J. Gene Med. 2018, 20, e3012. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Kim, M.H.; Kim, J.; Colon-Perez, L.M.; Banan, G.; Mareci, T.H.; Febo, M.; Cousins, R.J. Metal Transporter Zip14 (Slc39a14) Deletion in Mice Increases Manganese Deposition and Produces Neurotoxic Signatures and Diminished Motor Activity. J. Neurosci. 2017, 37, 5996–6006. [Google Scholar] [CrossRef] [Green Version]
- Jenkitkasemwong, S.; Akinyode, A.; Paulus, E.; Weiskirchen, R.; Hojyo, S.; Fukada, T.; Giraldo, G.; Schrier, J.; Garcia, A.; Janus, C.; et al. SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E1769–E1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, Y.; Gao, H.; Wang, J.; Qiang, Y.; Imam, M.U.; Li, Y.; Wang, J.; Zhang, R.; Zhang, H.; Yu, Y.; et al. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell. Discov. 2017, 3, 17025. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.F.; Wu, Y.; Morgan, S.E.; Zhao, N. The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 2019, 294, 9147–9160. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Thorn, T.L.; Ruggiero, C.H.; Pompilus, M.; Febo, M.; Cousins, R.J. Intestine-specific deletion of metal transporter Zip14 (Slc39a14) causes brain manganese overload and locomotor defects of manganism. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G673–G681. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, G.; Zhao, N. Restriction of Manganese Intake Prevents the Onset of Brain Manganese Overload in Zip14(-/-) Mice. Int. J. Mol. Sci. 2021, 22, 6773. [Google Scholar] [CrossRef]
- Reed, D.R.; Lawler, M.P.; Tordoff, M.G. Reduced body weight is a common effect of gene knockout in mice. BMC Genet. 2008, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Hojyo, S.; Fukada, T.; Shimoda, S.; Ohashi, W.; Bin, B.H.; Koseki, H.; Hirano, T. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS ONE 2011, 6, e18059. [Google Scholar] [CrossRef] [Green Version]
- Clegg, M.S.; Lonnerdal, B.; Hurley, L.S.; Keen, C.L. Analysis of whole blood manganese by flameless atomic absorption spectrophotometry and its use as an indicator of manganese status in animals. Anal. Biochem. 1986, 157, 12–18. [Google Scholar] [CrossRef]
- Weigand, E.; Kirchgessner, M.; Helbig, U. True absorption and endogenous fecal excretion of manganese in relation to its dietary supply in growing rats. Biol. Trace Elem. Res. 1986, 10, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Britton, A.A.; Cotzias, G.C. Dependence of manganese turnover on intake. Am. J. Physiol. 1966, 211, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Dorman, D.C.; Struve, M.F.; James, R.A.; McManus, B.E.; Marshall, M.W.; Wong, B.A. Influence of dietary manganese on the pharmacokinetics of inhaled manganese sulfate in male CD rats. Toxicol. Sci. 2001, 60, 242–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.D.; Wolf, T.L.; Greger, J.L. Varying levels of manganese and iron affect absorption and gut endogenous losses of manganese by rats. J. Nutr. 1992, 122, 1300–1308. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fung, C.K.; Zhao, N. The Combined Inactivation of Intestinal and Hepatic ZIP14 Exacerbates Manganese Overload in Mice. Int. J. Mol. Sci. 2022, 23, 6495. https://doi.org/10.3390/ijms23126495
Fung CK, Zhao N. The Combined Inactivation of Intestinal and Hepatic ZIP14 Exacerbates Manganese Overload in Mice. International Journal of Molecular Sciences. 2022; 23(12):6495. https://doi.org/10.3390/ijms23126495
Chicago/Turabian StyleFung, Caitlin K., and Ningning Zhao. 2022. "The Combined Inactivation of Intestinal and Hepatic ZIP14 Exacerbates Manganese Overload in Mice" International Journal of Molecular Sciences 23, no. 12: 6495. https://doi.org/10.3390/ijms23126495
APA StyleFung, C. K., & Zhao, N. (2022). The Combined Inactivation of Intestinal and Hepatic ZIP14 Exacerbates Manganese Overload in Mice. International Journal of Molecular Sciences, 23(12), 6495. https://doi.org/10.3390/ijms23126495




