Abstract
Ribosome biogenesis is tightly associated with plant growth and reproduction. Mutations in genes encoding ribosomal proteins (RPs) or ribosome biogenesis factors (RBFs) generally result in retarded growth and delayed flowering. However, the early-flowering phenotype resulting from the ribosome biogenesis defect is rarely reported. We previously identified that the AAA-ATPase MIDASIN 1 (MDN1) functions as a 60S RBF in Arabidopsis. Here, we found that its weak mutant mdn1-1 is early-flowering. Transcriptomic analysis showed that the expression of FLOWERING LOCUS C (FLC) is down-regulated, while that of some autonomous pathway genes and ABSCISIC ACID-INSENSITIVE 5 (ABI5) is up-regulated in mdn1-1. Phenotypic analysis revealed that the flowering time of mdn1-1 is severely delayed by increasing FLC expression, suggesting that the early flowering in mdn1-1 is likely associated with the downregulation of FLC. We also found that the photoperiod pathway downstream of CONSTANTS (CO) and FLOWERING LOCUS T (FT) might contribute to the early flowering in mdn1-1. Intriguingly, the abi5-4 allele completely blocks the early flowering in mdn1-1. Collectively, our results indicate that the ribosome biogenesis defect elicited by the mutation of MDN1 leads to early flowering by affecting multiple flowering regulation pathways.
1. Introduction
The eukaryotic 80S ribosome consists of two subunits, the 60S large subunit, and the 40S small subunit. The 60S subunit comprises 25S, 5.8S, and 5S rRNA and approximately 47 RPs, whereas the 40S subunit comprises 18S rRNA and approximately 33 RPs [1,2]. Ribosome biogenesis is a fundamental process. Based on the research on yeast and animal there are hundreds of RBFs involved in the pre-rRNA transcription, modification, processing, folding, and the incorporation of RPs [2]. Ribosome biogenesis begins with the transcription of the 45S rRNA precursor (pre-rRNA) by RNA polymerase I, and the 45S pre-rRNA functions as a platform for the 90S particle assembly [3,4]. MIDASIN1 (MDN1), an AAA-ATPase, is conserved in different species. MDN1 successively interacts with the nucleolus protein Ytm1/PES2 and the nucleoplasm protein NOTCHLESS (NLE) through its C-terminal metal ion-dependent adhesion site (MIDAS) domain, to trigger these proteins’ release from the pre-60S particle [5,6,7,8]. The ribosome is the main component of the protein synthesis machinery and is essential for normal cell growth, and defects in RBFs and RPs always exert pleiotropic effects on cell proliferation and growth [4,9]. In Arabidopsis, the ribosome biogenesis defect generally causes abnormal embryogenesis, seed germination, leaf morphology, retarded root elongation, and delayed flowering [4,6,7,9,10]. These findings imply ribosome-mediated regulation roles throughout the entire plant life cycle. In the face of endogenous and environmental stresses, the most urgent task for plants is to survive and to reproduce the next generation, which is tightly associated with the flowering time. Therefore, elucidating the mechanism of flowering time regulation under the ribosome biogenesis defect is helpful for us to understand ribosome-mediated regulation roles.
Flowering is one of the most studied developmental processes in the plant over the last 30 years. A large number of studies have provided a good understanding of how endogenous and environmental cues precisely control the switch from vegetative growth to reproductive growth [11,12,13]. In winter-annual accessions of Arabidopsis, FRIGIDA (FRI) activates the expression of the flowering suppressor FLOWERING LOCUS C (FLC) to a high level by enrichment of active epigenetic modification at the FLC locus and leads to late flowering [14,15,16]. The vernalization pathway triggers flowering by directly repressing FLC expression by enrichments of repressive epigenetic modification at the FLC locus [17,18]. In contrast, rapid-cycling accessions of Arabidopsis contain a loss-of-function mutation in FRI and therefore have a low FLC level and are early flowering without vernalization [16]. Loss-of-function mutants of autonomous pathway genes in rapid cycling background display a similar late-flowering phenotype as the functional FRI containing winter annuals [13,14,19]. Those autonomous pathway genes repress the FLC expression also by enrichments of repressive epigenetic modification at the FLC locus [13]. The autonomous pathway proteins include FLOWERING LOCUS D (FLD, H3K4 demethylase), FLOWERING LOCUS VE (FVE, recruits histone deacetylase complex), LUMINIDEPENDENS (LD, a homeodomain-containing protein), FLOWERING CONTROL LOCUS A (FCA), and FPA (with the RNA-binding motifs that function partly through FLD to mediate FLC locus), FLOWERING LOCUS KH DOMAIN (FLK) and FY (RNA-binding and RNA-processing proteins) [20,21,22,23,24,25,26]. Polycomb group (PcG) proteins function as transcriptional repressors of developmental gene expression and Polycomb repressive complex2 (PRC2) catalyzes repressive histone 3 Lys-27 trimethylation (H3K27me3) [27,28]. In Arabidopsis, CURLY LEAF (CLF) is one of the homologs of the Drosophila H3K27 methyltransferase Enhancer of Zeste [E(z)] and plays an important role in the developmental switch from the vegetative phase to reproduction [29]. CONSTANTS (CO) acts as a flowering activator that promotes the expression of FLOWERING LOCUS T (FT) and mediates the photoperiod pathway in long-day conditions, whereas in short-day conditions, CO protein is not being stably produced and might not function in the control of flowering [30,31,32,33,34]. CLF catalyzes H3K27 trimethylation on both the FLC and FT locus to repress their expression [35]. Consequently, CO and FLC, as two central flowering regulators, CO and FLC, antagonistically regulate flowering [31,36]. Their downstream flowering pathway integrators, such as FT, SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1), and LEAFY (LFY), can integrate signals from multiple flowering pathways and determine the flowering time depending on their expression levels [37,38,39].
Abscisic acid (ABA) has been reported to play both positive and negative roles in flowering time regulation [40]. ABA biogenesis-related mutants in the rapid-cycling background (Col-0, aba1, aba1-6, aba2-1, and aba2-4) of Arabidopsis are late flowering under long-day conditions, whereas ABA insensitive mutants (abi1-1, abi4-1, and abi5-4) are early flowering [41,42,43,44]. Endogenous ABA promotes FT expression, meanwhile, ABSCISIC ACID-INSENSITIVE 4 (ABI4) and ABI5, two positive ABA signaling regulators, can directly bind to the FLC promoter and activate its transcription [41,42].
Generally, defects in ribosome biogenesis lead to retarded cell division and elongation, and an associated late-flowering phenotype [10,45,46,47,48,49,50]. Here, we described that deficiency in the MDN1 function elicits an unexpected early-flowering phenotype by affecting multiple flowering regulation pathways.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Arabidopsis accession Col-0 was used in this study. The mdn1-1 mutant was screened in our lab as described previously [6,7]. T-DNA insertion mutant mdn1-2 (Salk_057010) was obtained from the Arabidopsis Biological Resource Center. To generate mdn1-1/+ mdn1-2/+, mdn1-1 was crossed with mdn1-2/+ and genotyped with specific primers (Table S2). The flc-3 (104 bp deletion in the first exon), flk-4 (Salk_112850), fve-4 (TGG to TGA mutation in the 6th exon), co (SAIL_24_H04), ft-10 (GABI_290E08), and clf-28 (SALK_139371) mutants were kindly provided by prof. Scott Michaels (Indiana University Bloomington). The double mutant of mdn1-1 abi5 had been described in our previous study [51]. Seedlings and plants were grown under 16 h light/8 h dark (LD, long day) or 8 h light/16 h dark (SD, short day) conditions at 21 °C.
2.2. Quantification of Flowering Time
Seedlings and plants were grown to flowering in the growth chamber under LD or SD condition. Flowering time was determined by numbering total rosette leaves at bolting and the days from germination to flowering from three biological replicates.
2.3. RNA Sequencing and RNA-seq Data Analysis
Total RNA was extracted from 7 DAG seedling aerial parts grown at 21 °C under LD conditions using the RNAiso (Takara) and treated with DNase I (Takara) to remove genomic DNA according to the manufacturer’s protocol as described previously [52]. RNA quality of all samples was detected by Agilent 2100 Bio analyzer (Agilent RNA 6000 Nano Kit) and NanoDrop. The mRNA was enriched by the Oligo dT Selection method and cleaved into short fragments. Fragmented the RNA and reversed transcription to double-strand cDNA (dscDNA) by N6 random primer. The synthesized cDNA was subjected to end-repair and then was 3′ adenylated. Adaptors were ligated to the ends of these 3′ adenylated cDNA fragments. The ligation products were purified, and many rounds of PCR amplification were performed to enrich the purified cDNA template using PCR primer. Denature the PCR product by heat and the single-strand DNA is cyclized by splint oligo and DNA ligase to construct the cDNA library. Three biological replicates of each sample were used for RNA-Seq on the BGISEQ-500 platform by Beijing Genomics Institute (BGI). The RNA-seq raw sequencing data have been submitted in the SRA database under BioProjects: PRJNA564539 (https://www.ncbi.nlm.nih.gov/sra/PRJNA564539, 7 October 2020).
The low-quality reads with adaptors and reads with unknown bases (N bases more than 5%) were filtered to get the clean reads using SOAPnuke software. All clean reads were aligned to the Arabidopsis TAIR10 reference genome using Bowtie2 (http://bowtie-bio.sourceforge.net/Bowtie2/index.shtml) and calculated gene expression level with RSEM (http://deweylab.biostat.wisc.edu/RSEM). Gene expression level was normalized by using the FPKM (Fragments Per Kilobase of transcript per Million mapped reads) method. Differentially expressed genes (DEGs) were detected using DEGseq software (Fold Change ≥ 2 and Adjusted p-value ≤ 0.001) and used Volcano Plot to show the summary of DEGs. KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway was classified according to official classification, and the significantly enriched KEGG pathways were identified using a hypergeometric test under the standard of p-value ≤ 0.01.
2.4. Analysis of Transcript Abundance
Total RNA was extracted from 5 DAG seedling aerial parts grown at 21 °C under LD conditions using the RNAiso (Takara). Using DNase I treatment to remove genomic DNA and 5 µg of total RNA was used for first-strand cDNA synthesis (Takara) according to manufacturer’s protocol. Actin-2 was used as a control gene for qRT-PCR and the relative expression of genes was calculated by ABI7500 Real-Time PCR System using the 2−△△Ct method.
3. Results
3.1. Mutation in MDN1 Leads to Early Flowering
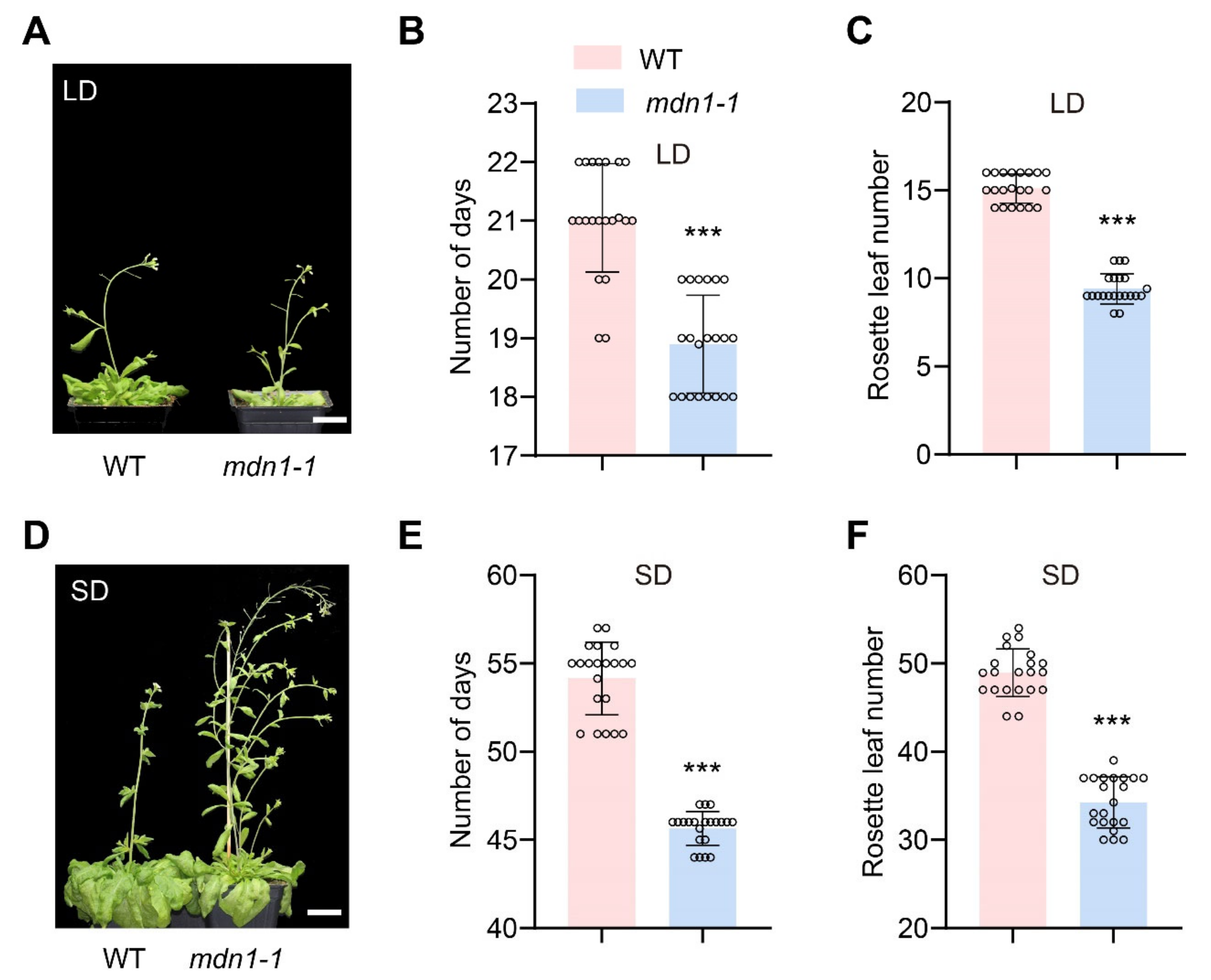
Previous studies showed a common late-flowering phenotype in Arabidopsis ribosome-deficient mutants (Table S1). To know if the mutation of MDN1 influences flowering time, we compared this phenotype between mdn1-1 and wild-type (WT) plants. Intriguingly, the mdn1-1 plants flowered about 2 and 8.5 days earlier than WT under long-day conditions (LD, 16 h of light and 8 h of darkness at 21 °C) and short-day conditions (SD, 8 h of light and 16 h of darkness in 21 °C), respectively (Figure 1A,B,D,E). The early-flowering phenotype of mdn1-1 was also testified by numbering the rosette leaves at the first flower bud bolting. mdn1-1 plants produced fewer rosette leaves under both LD (9 vs. 15) and SD (34 vs. 49) conditions than WT (Figure 1C,F). To confirm that the early-flowering phenotype was caused by the mdn1 mutation, we crossed mdn1-1 with a heterozygous T-DNA insertion mutant mdn1-2/+ (Salk_057010). We found that mdn1-1/mdn1-2 showed a similar early-flowering phenotype with mdn1-1, suggesting that mutation in MDN1 led to early flowering (Figure S1). These observations indicate a difference between mdn1-1 and other known ribosome-associated mutants in timing the reproductive growth, which led us to explore the mechanism underlying the early-flowering phenotype of mdn1-1.
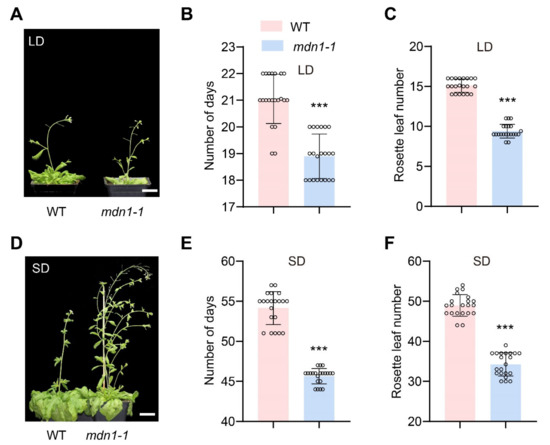
Figure 1.
Mutation in MDN1 leads to early flowering. (A)Wild-type (WT) and mdn1-1 plants grown in long days (LD). (B,C) The number of days and rosette leaf number at flowering grown in LD. Twenty plants (grown in LD) were scored. (D) WT and mdn1-1 plants grown in short days (SD). (E,F) The number of days and rosette leaf number at flowering grown in SD. Twenty plants (grown in SD) were scored. (*** p < 0.001, Student’s t-test). Scale bars, 2 cm.
3.2. Multiple Flowering Pathways Are Influenced in mdn1-1
To gain insight into the global transcription responses of the MDN1 mutation, we performed transcriptome profiles analysis of the aerial part from 7 DAG seedlings (under LD conditions). A total of 2049 differentially expressed genes (DEGs, |log2(fold-change)| ≥ 1 and p-value < 0.001) were identified in mdn1-1. Among these genes, 922 were up-regulated, and 1127 were down-regulated (Figure S1).
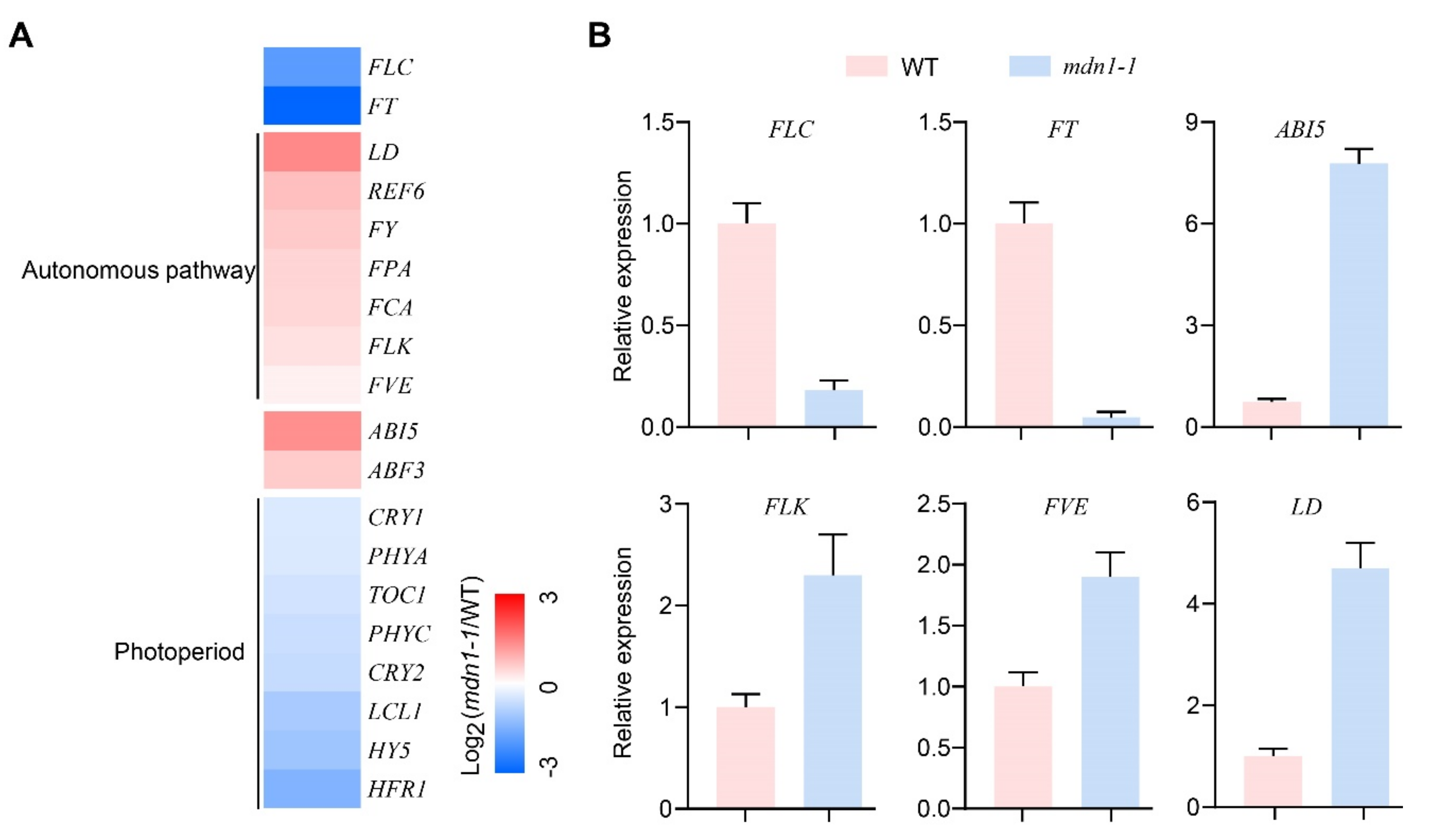
To gain more information on flowering time regulation, gene expression of flowering time regulators was analyzed (Figure 2). Importantly, autonomous pathway genes (LD, REF6, FY, FPA, FCA, FLK, and FVE) were up-regulated, and FLC was down-regulated (Figure 2A). The expression of ABI5 and ABSCISIC ACID RESPONSIVE ELEMENTS-BINDING FACTOR 3 (ABF3) was up-regulated (Figure 2A). The expression of photoperiod pathway-related genes, including FT, CRYPTOCHROMEs (CRY1 and CRY2), and PHYTOCHROMEs (PHYA and PHYC), was down-regulated (Figure 2A). In addition, the expression of TIMING OF CAB OF EXPRESSION 1 (TOC1), LHY/CCA1-LIKE 1 (LCL1), ELONGATED HYPOCOTYL 5 (HY5), and LONG HYPOCOTYL IN FAR-RED 1 (HFR1) was also down-regulated (Figure 2A). Decreased FLC and FT expression and increased ABI5, FLK, FVE, and LD expression in the mdn1-1 mutant were confirmed by qRT-PCR (Figure 2B). The above observations suggest that the MDN1 mutation produces a global influence on the expression of flowering time regulator genes.
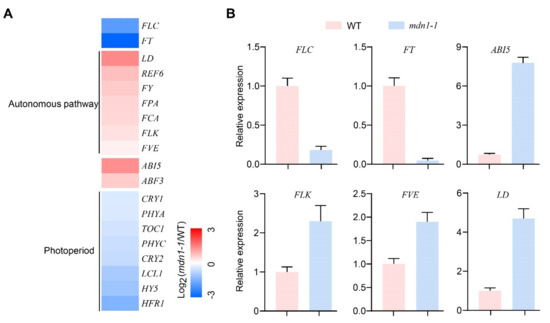
Figure 2.
Gene expression profiling of WT and mdn1-1 seedlings. (A) The expression analysis of genes involved in flowering time regulation uses the log2-transformed fold-change values. Red, blue, and white indicate an increase, decrease, and no difference in expression levels, respectively. (B) Quantitative RT-PCR (qRT-PCR) results showing the expression of the indicated genes in the WT and mdn1-1 seedlings.
3.3. Downregulation of FLC Is Associated with Early Flowering in mdn1-1
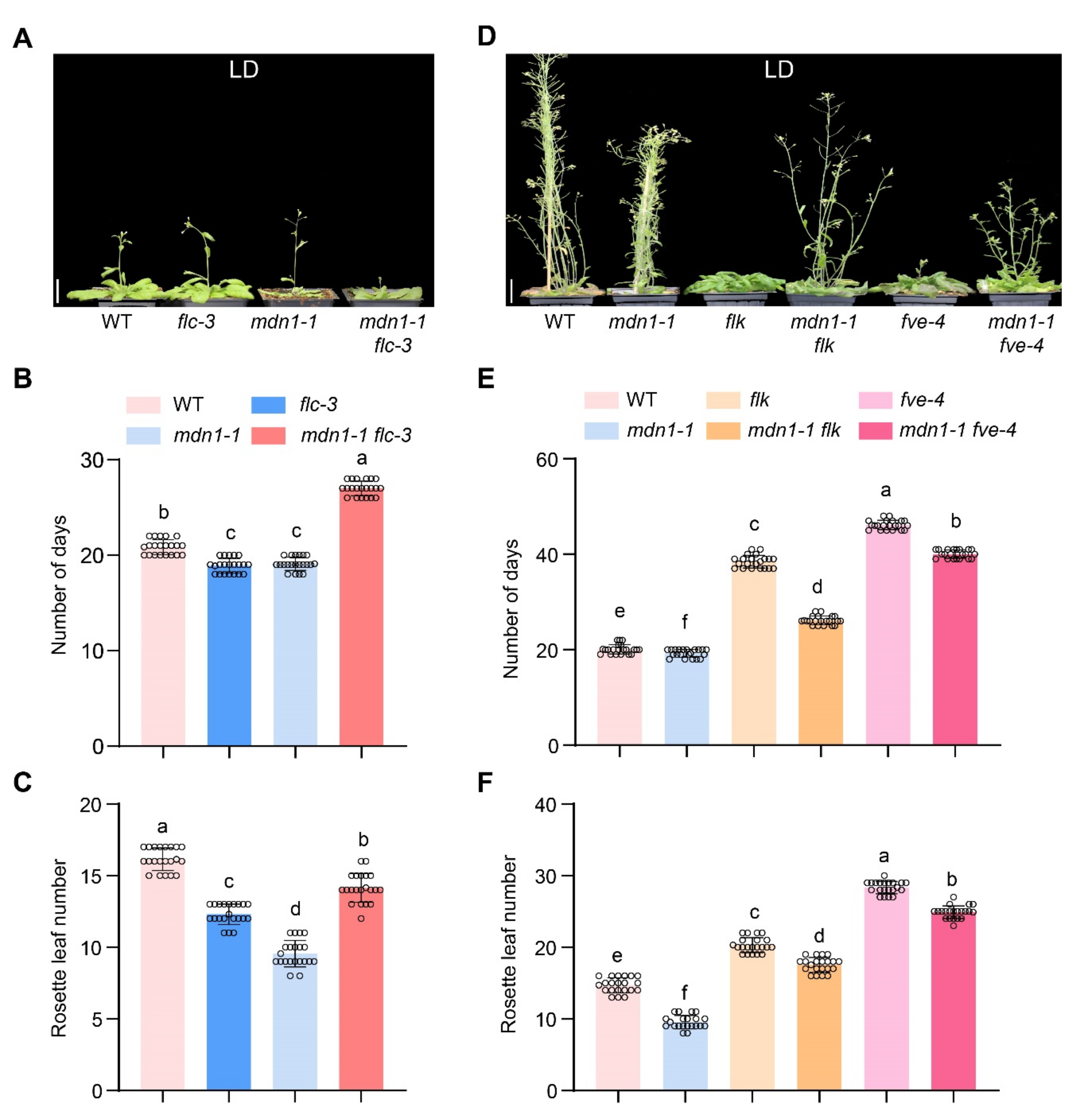
FLC is a repressor of flowering, and down-regulation of the FLC mRNA level results in early flowering under both LD and SD conditions in some Arabidopsis mutants [14,53]. To test the involvement of FLC in mdn1-1 early flowering, the flc-3 allele was introduced into the mdn1-1 background. Under LD conditions, the flowering time of flc-3 was similar to mdn1-1, which showed about 2 d earlier than WT (Figure 3A,B). In addition, the number of rosette leaves of flc-3 was about four less than WT and three more than that of mdn1-1 (Figure 3C). Unexpectedly, the flowering time of the mdn1-1 flc-3 double mutant was later than both single mutants (Figure 3A–C). Meanwhile, the number of rosette leaves of mdn1-1 flc-3 was more than that of flc-3 and mdn1-1 (Figure 3C). To test the effect of improving FLC expression on mdn1-1 flowering time, two weak autonomous pathway mutants, flk-4 and fve-4, were employed. The expression of FLC was up-regulated in both mdn1-1 flk-4 and mdn1-1 fve-4 double mutants (Figure S3). Phenotypic analysis showed that both flk-4 and fve-4 could significantly delay the flowering time in mdn1-1 (Figure 3D–F), suggesting that the early-flowering phenotype of mdn1-1 might be associated with the downregulated FLC.
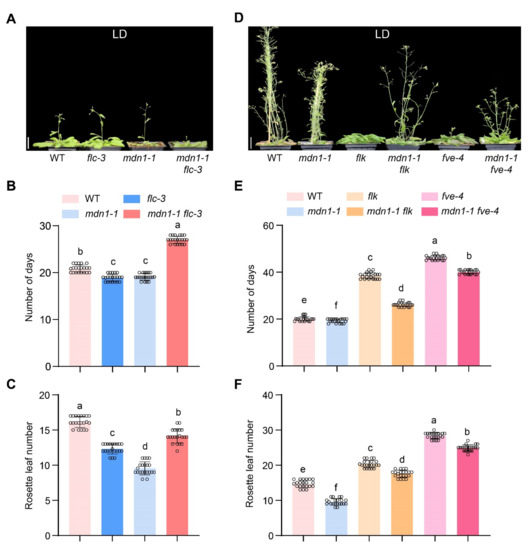
Figure 3.
The flowering time of FLC-related mutants in mdn1-1 background. (A) flc-3, mdn1-1 and mdn1-1 flc-3 plants grown in LD. (B,C) The number of days and rosette leaf number at the flowering in LD. (D) mdn1-1, flk, mdn1-1 flk, fve-4, and mdn1-1 fve-4 plants grown in LD. (E,F) The number of days and rosette leaf number at the flowering in SD. Twenty plants per line were scored. One-way ANOVA was employed, and the lowercase letters denote distinct groups (p < 0.01). Scale bars, 2 cm.
3.4. Photoperiod Pathway Contributes to mdn1-1 Early Flowering
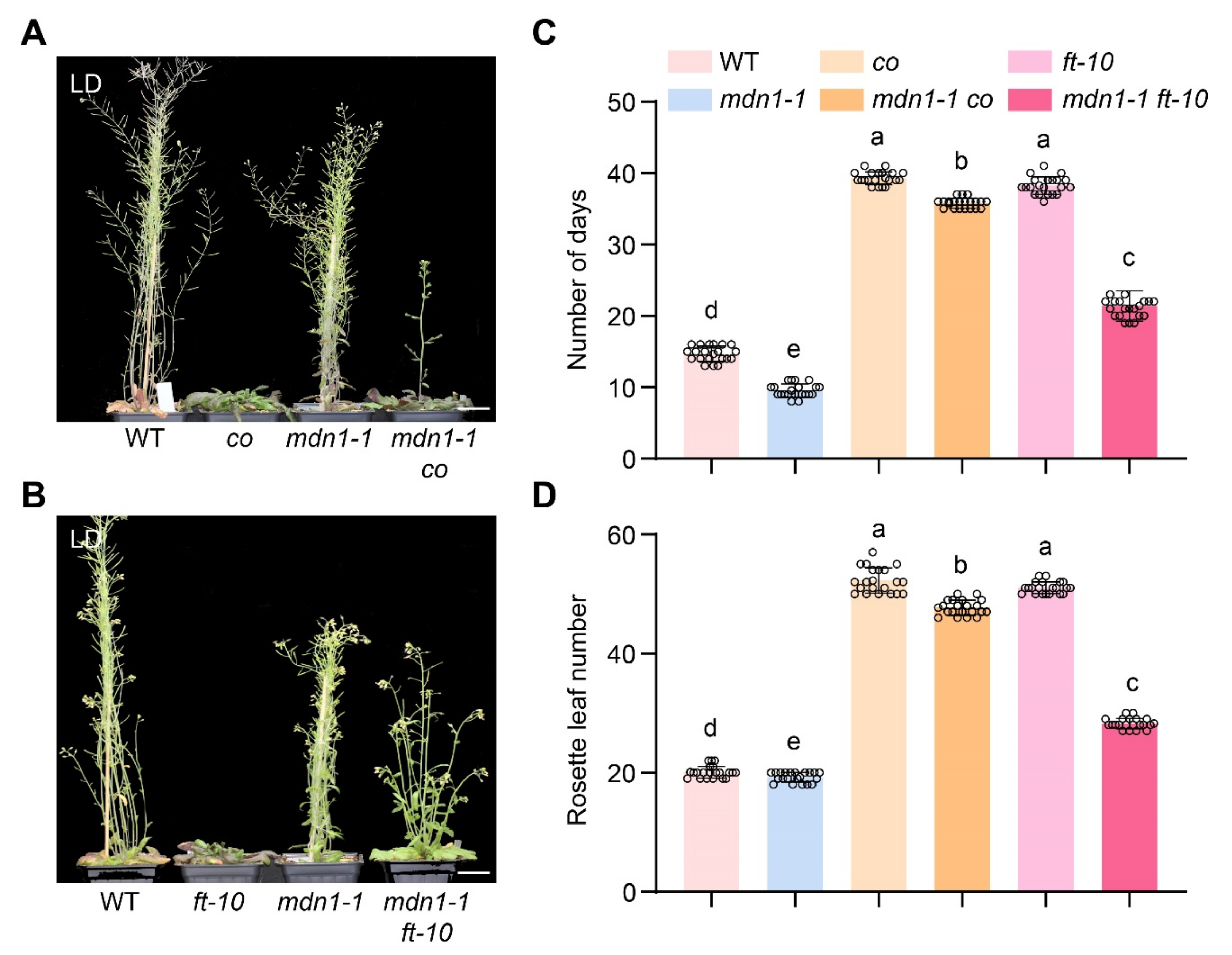
Plants sense upcoming environmental changes by detecting photoperiod information and precisely control the timing of flowering at a suitable condition. To investigate the role of photoperiod pathway regulators in mdn1-1 flowering time, we also introduced co and ft-10 mutations into the mdn1-1 mutant background. Under LD condition, the mdn1-1 co double mutant showed about a 5-d acceleration of flowering than co and about 28-d repression than mdn1-1 (Figure 4A,C). Correspondingly, the number of rosette leaves of mdn1-1 co was about 4 less than that of co and about 26 more than that of mdn1-1 (Figure 4A,D). The mdn1-1 ft-10 double mutant showed about a 23-d acceleration of flowering than ft-10 and about 9-d repression than mdn1-1 (Figure 4B,C). Meanwhile, the number of rosette leaves of mdn1-1 ft-10 was about 17 less than that of ft-10 and was about 12 more than that of mdn1-1 (Figure 4A,D). mdn1-1 co and mdn1-1 ft-10 plants flowered earlier than co and ft-10, respectively, and both later than the mdn1-1 plants (Figure 4B,C), indicating that the early flowering in mdn1-1 is partially dependent on the photoperiod pathway.
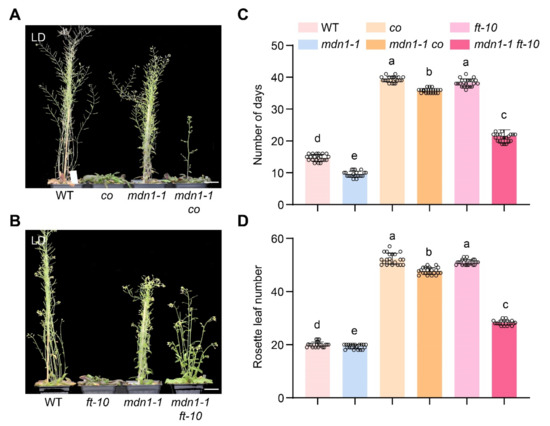
Figure 4.
co and ft-10 inhibit the early flowering of mdn1-1. (A) co, mdn1-1 and mdn1-1 co plants grown in LD. (B) ft-10, mdn1-1 and mdn1-1 ft-10 grown in LD. (C,D) The number of days and rosette leaf number at the flowering in LD. Twenty plants per line were scored. One-way ANOVA was employed, and the lowercase letters denote distinct groups (p < 0.01). Scale bars, 2 cm.
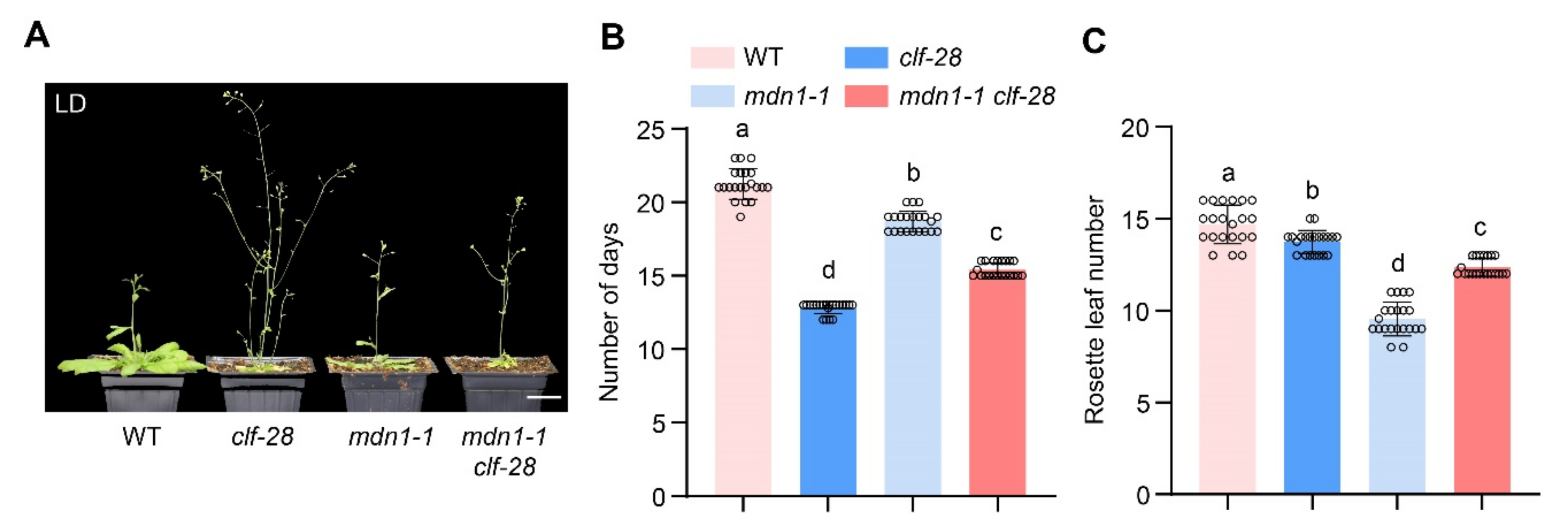
In the clf-28 plant, the expression of FLC and FT is de-repressed, while the overexpression of FT bypasses the FLC-dependent pathway, which results in an early-flowering phenotype in the clf-28 plant [35]. We introduced clf-28 mutation into the mdn1-1 mutant background. mdn1-1 clf-28 double mutant flowered earlier than mdn1-1, but later than clf-28 under LD conditions. This observation further supports the above hypothesis (Figure 5).
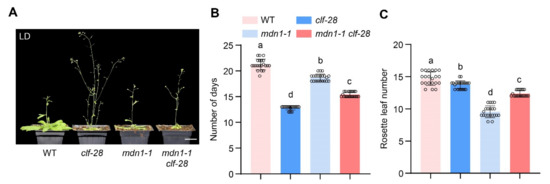
Figure 5.
clf-28 accelerates the early flowering of mdn1-1. (A) clf-28, mdn1-1 and mdn1-1 clf-28 grown in LD. (B,C) The number of days and rosette leaf number at the flowering in LD. Twenty plants per line were scored. One-way ANOVA was employed, and the lowercase letters denote distinct groups (p < 0.01). Scale bars, 2 cm.
3.5. ABI5 Contributes to Early Flowering in mdn1-1
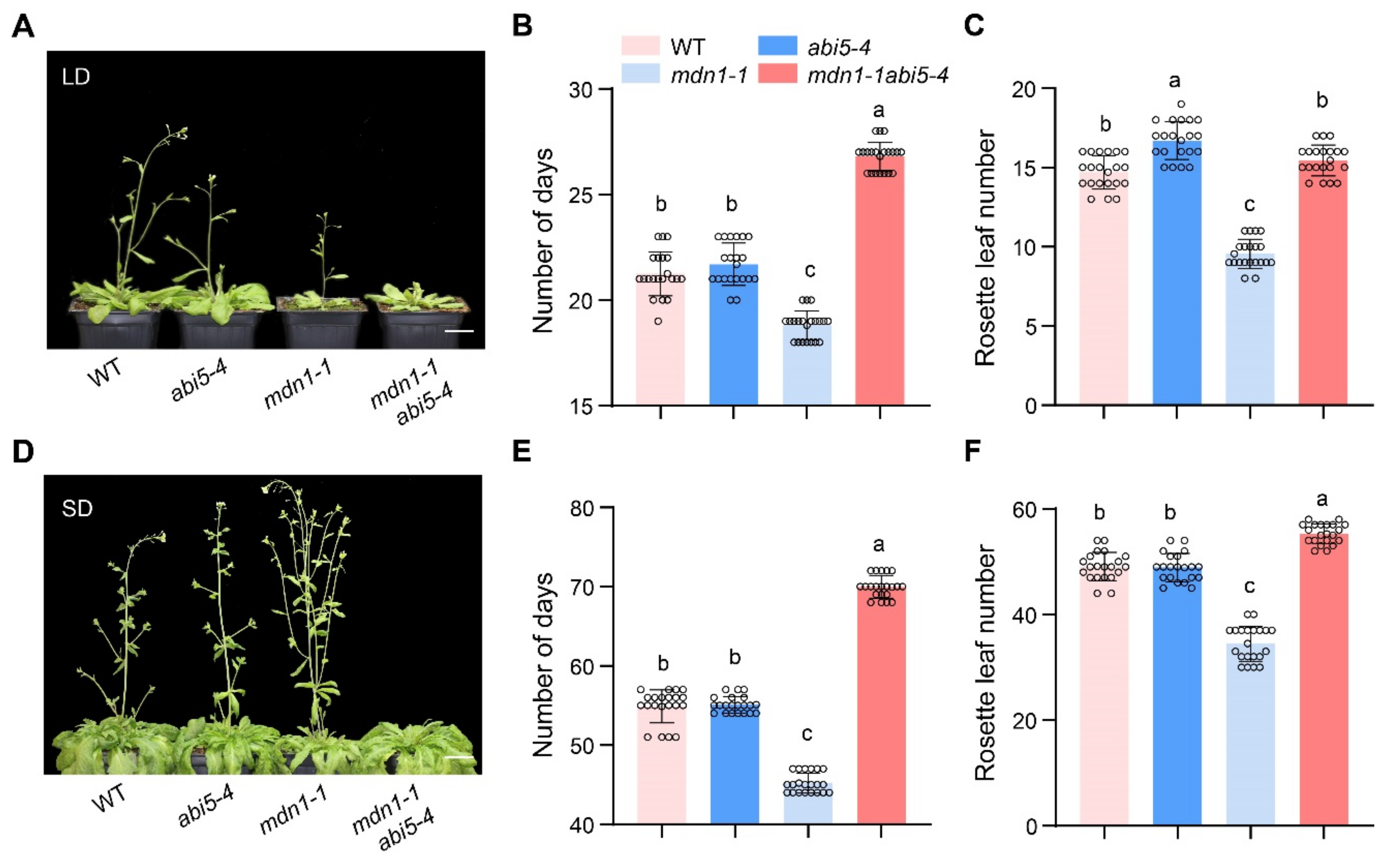
Since ABA signaling regulates plant flowering and ABI5 was overexpressed in mdn1-1, we wonder the role of ABI5 in the alteration of mdn1-1 flowering time. To address this issue, we employed the double mutant of mdn1-1 abi5-4 which had been described in our previous study [51]. Under both LD and SD condition, abi5-4 showed a similar flowering time compared with WT in our experimental conditions. Intriguingly, the early-flowering phenotype of mdn1-1 was completely blocked by the abi5-4 allele, and the mdn1-1 abi5-4 double mutant even bolted later than both WT and abi5-4. The flowering time of mdn1-1 abi5-4 double mutant showed about 8 and 25 d later than that of mdn1-1 under LD and SD conditions, respectively (Figure 6A–C,E). In addition, the number of rosette leaves of this double mutant was about 6 and 21 more than that of mdn1-1 under LD and SD conditions, respectively (Figure 6D,F). These results suggest that ABI5 positively regulates flowering in mdn1-1.
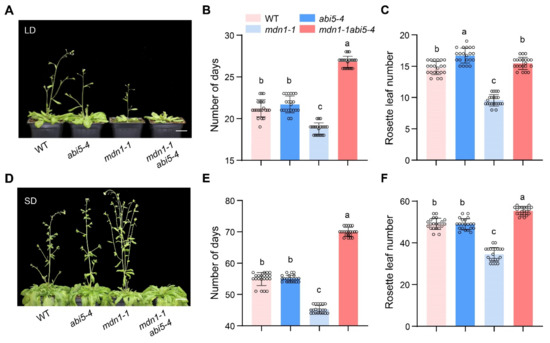
Figure 6.
abi5-4 inhibits the early flowering of mdn1-1. (A) abi5-4, mdn1-1 and mdn1-1 abi5-4 grown in LD. (B,C) The number of days and rosette leaf number at the flowering in LD. (D) abi5-4, mdn1-1 and mdn1-1 abi5-4 grown in SD. (E,F) The number of days and rosette leaf number at the flowering in SD. Twenty plants per line were scored. One-way ANOVA was employed, and the lowercase letters denote distinct groups (p < 0.01). Scale bars, 2 cm.
4. Discussion
Understanding the mechanism by which plants coordinate their vegetative and reproductive growth at fitness time is an important paradigm in plant biology. However, our understanding of how plants coordinate the vegetative and reproductive growth in response to ribosome deficiency is limiting. Ribosome biogenesis is a complex process that requires the coordinated function of RNA polymerases, hundreds of RBFs, and RPs to facilitate rRNA maturation and RP incorporation, and any dysfunction would significantly influence the plant development [1,9,54]. Emerging studies highlighted the existence of a signaling pathway in ribosome deficiency-induced plant abnormal development, but the detailed mechanism is lacking due to the multiple phenotype outputs [9,54]. The flowering time may serve as a model for us to explore how plant adaptation responds to ribosome deficiency.
In Arabidopsis, the phenotypes of mutants with defects in RBFs and RPs have been widely described [4,10,54]. Mutants related to Arabidopsis ribosome biogenesis from previous studies showed a common late-flowering phenotype (Table S1). Most of these previous studies focused on rRNA modification and processing defects and ribosomal protein mutations. Generally, ribosome biogenesis defects triggered by these mutations can be compensated by activating alternative rRNA processing pathways or replacing them by other ribosomal protein members in plants (Table S1) [4,10]. For example, two orthologues of LSG1, LSG1-1, and LSG1-2, have been identified in Arabidopsis, and only LSG1-2 physically interacts with the ribosome and functions in the final step of pre-40S maturation [55]. The homozygous T-DNA insertion lines of LSG1-1 and LSG1-2 are viable with normal flowering time, whereas flowering is drastically delayed in lsg1-1+/– lsg1-2–/– (Table S1) [55].
However, the MDN1 mutation led to an early-flowering phenotype (Figure 1). This contradiction might be associated with MDN1 functions in essential ribosome biogenesis and transport checkpoints (pre-ribosome transport from the nucleolus to the nucleoplasm and from nucleoplasm to cytoplasm) [3,6]. At the same time, MDN1 itself might have a specific role in regulating the reproductive growth transition. To clarify the mechanism of the early flowering in mdn1-1, global gene expression profiles of the aerial part of 7 DAG seedlings were employed. The expression of FLC was down-regulated in mdn1-1 (Figure 2), which contributed to the early-flowering phenotype to some extent (Figure 3). FLC expression can be suppressed by the autonomous pathway complex, which mainly contains two types of factors, RNA processing mediated by FPA, FY, FLK, and FCA, and histone modification mediated by FLD, FVE, REF6, and LD. Thus, the up-regulation of LD, REF6, FY, FPA, FCA, FLK, and FVE in mdn1-1 (Figure 2) might partly contribute to FLC down-regulation. The expression of FLC is indeed increased in mdn1-1 flk and mdn1-1 fve-4 double mutants, which further supports this view (Figure S3). However, knockout of the FLC function in mdn1-1 led to a severely delayed flowering phenotype (see mdn1-1 flc-3 double mutant in Figure 3), suggesting that FLC is also required for timely flowering in mdn1-1 (Figure 3A–C). Since FLC is a MADS-box transcription factor, it is likely that there are other factors in mdn1-1 promoting flowering by forming a complex with FLC, but the FLC mutation makes the complex ineffective. This hypothesis needs to be further explored. Additionally, the mdn1-1 flk and mdn1-1 fve-4 double mutants flowered earlier flk and fve-4, suggesting that in addition to the autonomous-FLC pathway, other flowering pathways might be also involved in mdn1-1 early flowering (Figure 3D–F and Figure S3). Transcriptomic analysis showed that the expression of several photoperiod pathway genes was changed, implying that photoperiod-pathway genes might play a role in mdn1-1 early flowering (Figure 2). Furthermore, the flowering time of both co and ft-10 mutants could be accelerated by introducing the mdn1-1 allele, suggesting that other flowering integrators downstream of CO and FT might play a role in mdn1-1 early flowering (Figure 4). In addition, the mdn1-1 clf-28 double mutant bolted earlier than mdn1-1, further suggesting that the photoperiod pathway played a significant role in mdn1-1 early flowering (Figure 5).
The double mutant mdn1-1 abi5 exhibited a late-flowering phenotype than the mdn1-1 single mutant, suggesting that the ABI5 played a positive role in mdn1-1 early flowering (Figure 6). Nevertheless, the molecular mechanisms by which ABI5 responds to the MDN1 mutation and regulates flowering in mdn1-1 remain to be illustrated.
In this study, we found that the mutation of MDN1 led to early flowering, which is distinctive from previously reported RBF mutants. Transcriptome and genetic analysis suggest that multiple flowering regulation pathways might devote to the early flowering in mdn1-1. Intriguingly, we found a positive regulator, ABI5, in response to ribosome biogenesis defect for growth stage switching.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23126509/s1.
Author Contributions
K.L., P.L. and X.W. designed the study. K.L., P.W., T.D., L.H., G.L., C.Z. and S.Z. performed the research and analyzed the data. K.L., P.L. and X.W. contributed to writing the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation (31500257, 31861143009, 32100266); Shandong Natural Science Foundation (ZR2021QC022), Shandong Taishan Scholar Project (ts20190964); Shandong provincial crop elite variety development project (2016LZGC025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Scott Michaels and Xuhong Yu (Indiana University Bloomington) for their help with the quantification and confirmation of flowering time, and for their constructive suggestion for this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABA | Abscisic acid |
| ABI5 | ABSCISIC ACID-INSENSITIVE 5 |
| CCA1 | CIRCADIAN CLOCK ASSOCIATED 1 |
| CDF1 | CYCLING DOF FACTOR 1 |
| CO | CONSTANTS |
| CRYs | CRYPTOCHROMEs |
| DAG | days after germination |
| FCA | FLOWERING CONTROL LOCUS A |
| FLC | FLOWERING LOCUS C |
| FLD | FLOWERING LOCUS D |
| FLK | FLOWERING LOCUS KH DOMAIN |
| FRI | FRIGIDA |
| FT | FLOWERING LOCUS T |
| HFR1 | LONG HYPOCOTYL IN FAR-RED 1 |
| LCL1 | LHY/CCA1-LIKE 1 |
| LD | LUMINIDEPENDENS |
| MDN1 | MIDASIN 1 |
| PHYA | PHYTOCHROME A |
| RBFs | ribosome biogenesis factors |
| RPs | ribosomal proteins |
| TOC1 | TIMING OF CAB OF EXPRESSION 1 |
References
- Fromont-Racine, M.; Senger, B.; Saveanu, C.; Fasiolo, F. Ribosome assembly in eukaryotes. Gene 2003, 313, 17–42. [Google Scholar] [CrossRef]
- Woolford, J.L., Jr.; Baserga, S.J. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 2013, 195, 643–681. [Google Scholar] [CrossRef] [PubMed]
- Tschochner, H.; Hurt, E. Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol. 2003, 13, 255–263. [Google Scholar] [CrossRef]
- Saez-Vasquez, J.; Delseny, M. Ribosome Biogenesis in Plants: From Functional 45S Ribosomal DNA Organization to Ribosome Assembly Factors. Plant Cell 2019, 31, 1945–1967. [Google Scholar] [CrossRef]
- Barrio-Garcia, C.; Thoms, M.; Flemming, D.; Kater, L.; Berninghausen, O.; Bassler, J.; Beckmann, R.; Hurt, E. Architecture of the Rix1-Rea1 checkpoint machinery during pre-60S-ribosome remodeling. Nat. Struct. Mol. Biol. 2016, 23, 37–44. [Google Scholar] [CrossRef]
- Li, P.C.; Li, K.; Wang, J.; Zhao, C.Z.; Zhao, S.Z.; Hou, L.; Xia, H.; Ma, C.L.; Wang, X.J. The AAA-ATPase MIDASIN 1 Functions in Ribosome Biogenesis and Is Essential for Embryo and Root Development. Plant Physiol. 2019, 180, 289–304. [Google Scholar] [CrossRef]
- Li, P.C.; Yu, S.W.; Li, K.; Huang, J.G.; Wang, X.J.; Zheng, C.C. The Mutation of Glu at Amino Acid 3838 of AtMDN1 Provokes Pleiotropic Developmental Phenotypes in Arabidopsis. Sci. Rep. 2016, 6, 36446. [Google Scholar] [CrossRef]
- Ulbrich, C.; Diepholz, M.; Bassler, J.; Kressler, D.; Pertschy, B.; Galani, K.; Bottcher, B.; Hurt, E. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell 2009, 138, 911–922. [Google Scholar] [CrossRef]
- Byrne, M.E. A role for the ribosome in development. Trends Plant Sci. 2009, 14, 512–519. [Google Scholar] [CrossRef]
- Weis, B.L.; Kovacevic, J.; Missbach, S.; Schleiff, E. Plant-Specific Features of Ribosome Biogenesis. Trends Plant Sci. 2015, 20, 729–740. [Google Scholar] [CrossRef]
- He, Y. Control of the transition to flowering by chromatin modifications. Mol. Plant 2009, 2, 554–564. [Google Scholar] [CrossRef]
- Whittaker, C.; Dean, C. The FLC Locus: A Platform for Discoveries in Epigenetics and Adaptation. Annu. Rev. Cell Dev. Biol. 2017, 33, 555–575. [Google Scholar] [CrossRef]
- Cheng, J.Z.; Zhou, Y.P.; Lv, T.X.; Xie, C.P.; Tian, C.E. Research progress on the autonomous flowering time pathway in Arabidopsis. Physiol. Mol. Biol. Plants 2017, 23, 477–485. [Google Scholar] [CrossRef]
- Michaels, S.D.; Amasino, R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef]
- Sheldon, C.C.; Burn, J.E.; Perez, P.P.; Metzger, J.; Edwards, J.A.; Peacock, W.J.; Dennis, E.S. The FLF MADS box gene: A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 1999, 11, 445–458. [Google Scholar] [CrossRef]
- Johanson, U.; West, J.; Lister, C.; Michaels, S.; Amasino, R.; Dean, C. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 2000, 290, 344–347. [Google Scholar] [CrossRef]
- Bastow, R.; Mylne, J.S.; Lister, C.; Lippman, Z.; Martienssen, R.A.; Dean, C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 2004, 427, 164–167. [Google Scholar] [CrossRef]
- Sung, S.; Amasino, R.M. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 2004, 427, 159–164. [Google Scholar] [CrossRef]
- Koornneef, M.; Hanhart, C.J.; van der Veen, J.H. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 1991, 229, 57–66. [Google Scholar] [CrossRef]
- Ausín, I.; Alonso-Blanco, C.; Jarillo, J.A.; Ruiz-García, L.; Martínez-Zapater, J.M. Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat. Genet. 2004, 36, 162–166. [Google Scholar] [CrossRef]
- Gu, X.; Jiang, D.; Yang, W.; Jacob, Y.; Michaels, S.D.; He, Y. Arabidopsis homologs of retinoblastoma-associated protein 46/48 associate with a histone deacetylase to act redundantly in chromatin silencing. PLoS Genet. 2011, 7, e1002366. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, W.; He, Y.; Amasino, R.M. Arabidopsis relatives of the human lysine-specific Demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell 2007, 19, 2975–2987. [Google Scholar] [CrossRef]
- Kim, H.J.; Hyun, Y.; Park, J.Y.; Park, M.J.; Park, M.K.; Kim, M.D.; Kim, H.J.; Lee, M.H.; Moon, J.; Lee, I.; et al. A genetic link between cold responses and flowering time through FVE in Arabidopsis thaliana. Nat. Genet. 2004, 36, 167–171. [Google Scholar] [CrossRef]
- Liu, F.; Quesada, V.; Crevillen, P.; Baurle, I.; Swiezewski, S.; Dean, C. The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol. Cell 2007, 28, 398–407. [Google Scholar] [CrossRef]
- Mockler, T.C.; Yu, X.; Shalitin, D.; Parikh, D.; Michael, T.P.; Liou, J.; Huang, J.; Smith, Z.; Alonso, J.M.; Ecker, J.R.; et al. Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 12759–12764. [Google Scholar] [CrossRef]
- He, Y.; Michaels, S.D.; Amasino, R.M. Regulation of flowering time by histone acetylation in Arabidopsis. Science 2003, 302, 1751–1754. [Google Scholar] [CrossRef]
- Förderer, A.; Zhou, Y.; Turck, F. The age of multiplexity: Recruitment and interactions of Polycomb complexes in plants. Curr. Opin. Plant Biol. 2016, 29, 169–178. [Google Scholar] [CrossRef]
- Pu, L.; Sung, Z.R. PcG and trxG in plants—Friends or foes. Trends Genet. 2015, 31, 252–262. [Google Scholar] [CrossRef]
- Chanvivattana, Y.; Bishopp, A.; Schubert, D.; Stock, C.; Moon, Y.H.; Sung, Z.R.; Goodrich, J. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 2004, 131, 5263–5276. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yu, X.; Michaels, S.D. Regulation of CONSTANS and FLOWERING LOCUS T expression in response to changing light quality. Plant Physiol. 2008, 148, 269–279. [Google Scholar] [CrossRef]
- Samach, A.; Onouchi, H.; Gold, S.E.; Ditta, G.S.; Schwarz-Sommer, Z.; Yanofsky, M.F.; Coupland, G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 2000, 288, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez, P.; Wheatley, K.; Robson, F.; Onouchi, H.; Valverde, F.; Coupland, G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 2001, 410, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Hepworth, S.R.; Valverde, F.; Ravenscroft, D.; Mouradov, A.; Coupland, G. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 2002, 21, 4327–4337. [Google Scholar] [CrossRef] [PubMed]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, Y.; Wang, Y.; He, Y. Repression of FLOWERING LOCUS C and FLOWERING LOCUS T by the Arabidopsis Polycomb repressive complex 2 components. PLoS ONE 2008, 3, e3404. [Google Scholar] [CrossRef]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Parcy, F. Flowering: A time for integration. Int. J. Dev. Biol. 2005, 49, 585–593. [Google Scholar] [CrossRef]
- Simpson, G.G.; Dean, C. Arabidopsis, the Rosetta stone of flowering time? Science 2002, 296, 285–289. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Wu, M.F.; Yang, L.; Wu, G.; Poethig, R.S.; Wagner, D. The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 2009, 17, 268–278. [Google Scholar] [CrossRef]
- Conti, L. Hormonal control of the floral transition: Can one catch them all? Dev. Biol. 2017, 430, 288–301. [Google Scholar] [CrossRef]
- Shu, K.; Chen, Q.; Wu, Y.; Liu, R.; Zhang, H.; Wang, S.; Tang, S.; Yang, W.; Xie, Q. ABSCISIC ACID-INSENSITIVE 4 negatively regulates flowering through directly promoting Arabidopsis FLOWERING LOCUS C transcription. J. Exp. Bot. 2016, 67, 195–205. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Lu, Y.; Chen, X.; Wu, Y. The inhibitory effect of ABA on floral transition is mediated by ABI5 in Arabidopsis. J. Exp. Bot. 2013, 64, 675–684. [Google Scholar] [CrossRef]
- Riboni, M.; Robustelli Test, A.; Galbiati, M.; Tonelli, C.; Conti, L. ABA-dependent control of GIGANTEA signalling enables drought escape via up-regulation of FLOWERING LOCUS T in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6309–6322. [Google Scholar] [CrossRef]
- Riboni, M.; Galbiati, M.; Tonelli, C.; Conti, L. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS. Plant Physiol. 2013, 162, 1706–1719. [Google Scholar] [CrossRef]
- Weijers, D.; Franke-van Dijk, M.; Vencken, R.J.; Quint, A.; Hooykaas, P.; Offringa, R. An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 2001, 128, 4289–4299. [Google Scholar] [CrossRef]
- Nishimura, T.; Wada, T.; Yamamoto, K.T.; Okada, K. The Arabidopsis STV1 protein, responsible for translation reinitiation, is required for auxin-mediated gynoecium patterning. Plant Cell 2005, 17, 2940–2953. [Google Scholar] [CrossRef]
- Degenhardt, R.F.; Bonham-Smith, P.C. Arabidopsis ribosomal proteins RPL23aA and RPL23aB are differentially targeted to the nucleolus and are disparately required for normal development. Plant Physiol. 2008, 147, 128–142. [Google Scholar] [CrossRef]
- Imai, A.; Komura, M.; Kawano, E.; Kuwashiro, Y.; Takahashi, T. A semi-dominant mutation in the ribosomal protein L10 gene suppresses the dwarf phenotype of the acl5 mutant in Arabidopsis thaliana. Plant J. 2008, 56, 881–890. [Google Scholar] [CrossRef]
- Lange, H.; Sement, F.M.; Gagliardi, D. MTR4, a putative RNA helicase and exosome co-factor, is required for proper rRNA biogenesis and development in Arabidopsis thaliana. Plant J. 2011, 68, 51–63. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xie, B.; Hong, Z.; Yang, Q. Arabidopsis NUCLEOSTEMIN-LIKE 1 (NSN1) regulates cell cycling potentially by cooperating with nucleosome assembly protein AtNAP1;1. BMC Plant Biol. 2018, 18, 99. [Google Scholar] [CrossRef]
- Li, P.; Ma, J.; Zhou, X.; Li, G.; Zhao, C.; Xia, H.; Fan, S.; Wang, X. Arabidopsis MDN1 is involved in the establishment of a normal seed proteome and seed germination. Front. Plant Sci. 2019, 10, 1118. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Liu, C.; Li, C.; Qiu, J.; Zhao, C.; Xia, H.; Ma, C.; Wang, X.; Li, P. Expression of AtLEC2 and AtIPTs promotes embryogenic callus formation and shoot regeneration in tobacco. BMC Plant Biol. 2019, 19, 314. [Google Scholar] [CrossRef]
- Xiong, F.; Ren, J.J.; Yu, Q.; Wang, Y.Y.; Lu, C.C.; Kong, L.J.; Otegui, M.S.; Wang, X.L. AtU2AF65b functions in abscisic acid mediated flowering via regulating the precursor messenger RNA splicing of ABI5 and FLC in Arabidopsis. New Phytol. 2019, 223, 277–292. [Google Scholar] [CrossRef]
- Palm, D.; Streit, D.; Shanmugam, T.; Weis, B.L.; Ruprecht, M.; Simm, S.; Schleiff, E. Plant-specific ribosome biogenesis factors in Arabidopsis thaliana with essential function in rRNA processing. Nucleic Acids Res. 2019, 47, 1880–1895. [Google Scholar] [CrossRef]
- Weis, B.L.; Missbach, S.; Marzi, J.; Bohnsack, M.T.; Schleiff, E. The 60S associated ribosome biogenesis factor LSG1-2 is required for 40S maturation in Arabidopsis thaliana. Plant J. 2014, 80, 1043–1056. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).