Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Deletion but Not Inhibition of Extracellular PCSK9 Reduces Infarct Sizes Ex Vivo but Not In Vivo
Abstract
1. Introduction
2. Results
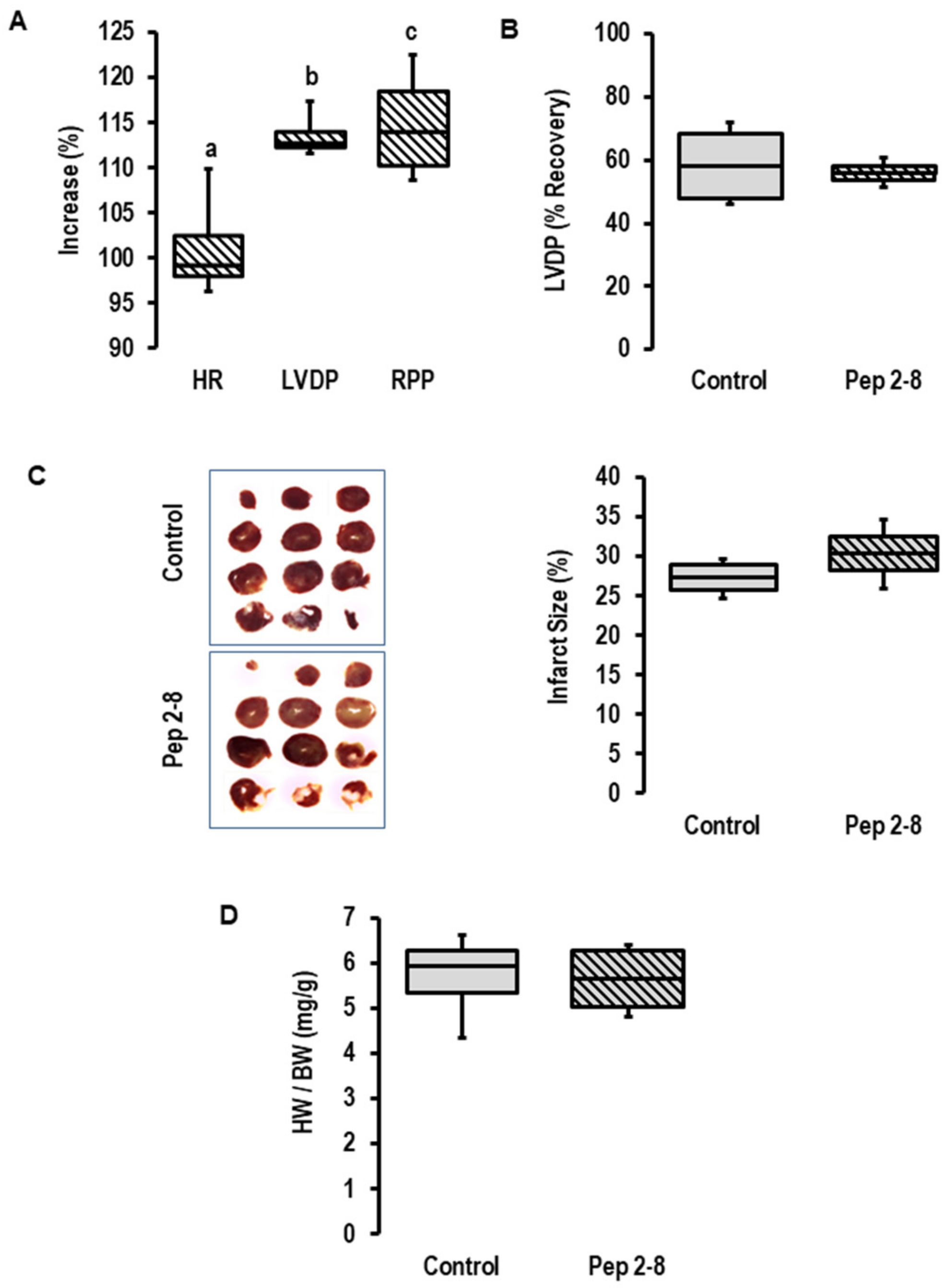
2.1. Influence of I/R on PCSK9 mRNA Expression, Release and Contribution on I/R Injury in Rats Ex Vivo
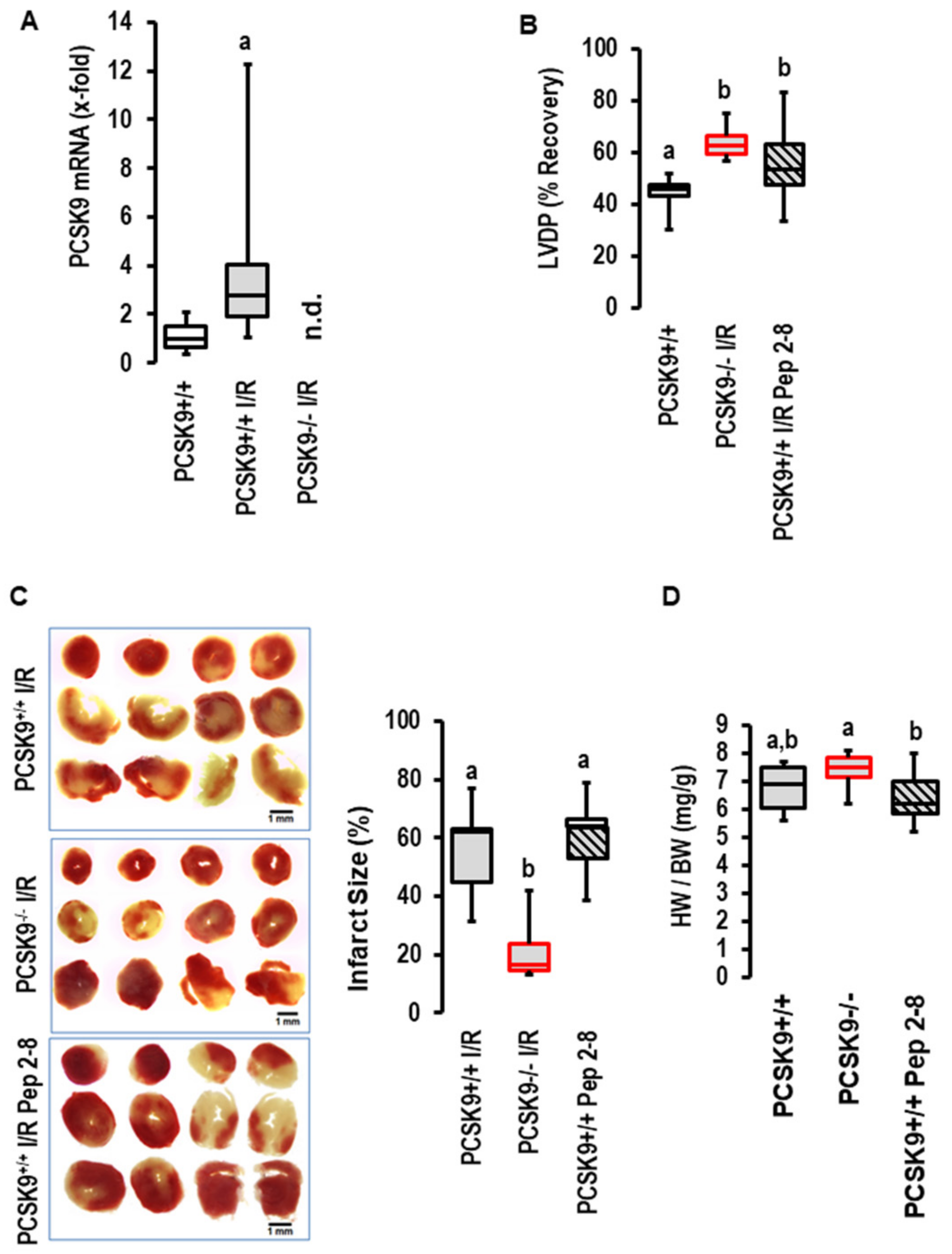
2.2. Effects of I/R on PCSK9 Expression and Contribution to I/R in Mice Ex Vivo
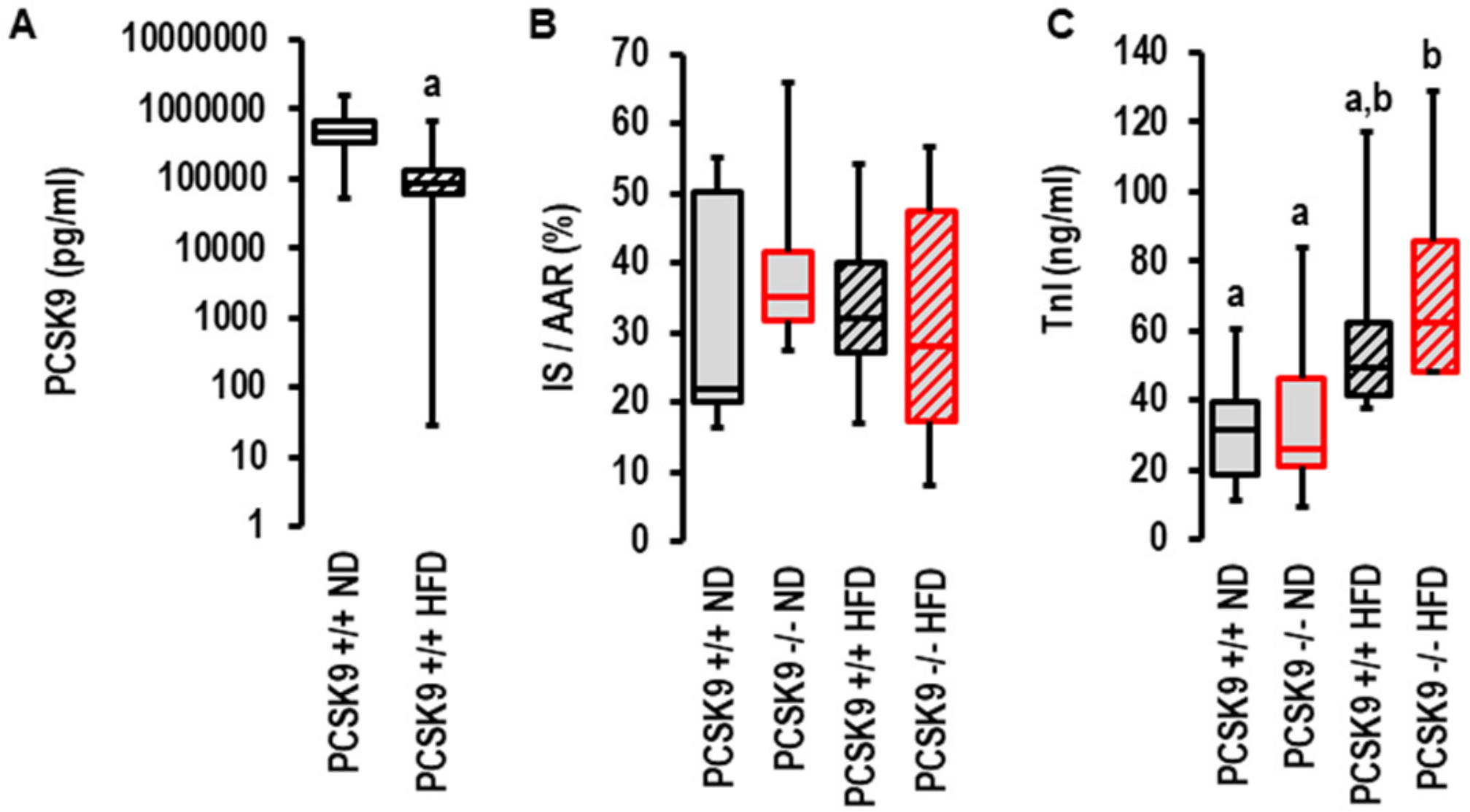
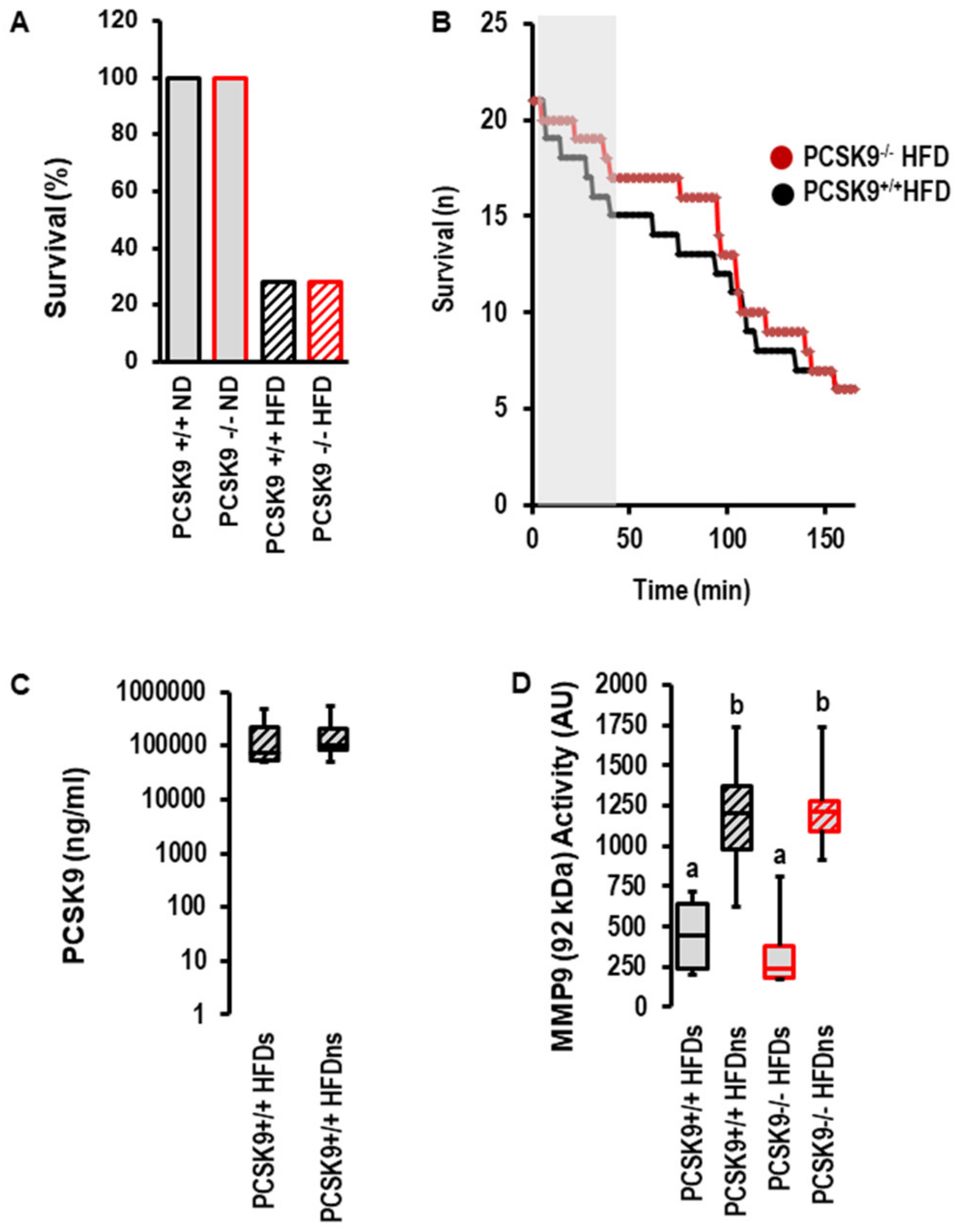
2.3. Effects of I/R on PCSK9 Plasma Concentration and Contribution of PCSK9 to I/R Injury In Vivo under Normal Diet and High-Fat Diet
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. I/R Ex Vivo Procedures
4.3. I/R Experimental Protocols
4.4. I/R In Vivo
4.5. TTC Staining
4.6. RNA Isolation and qRT-PCR
4.7. MMP9 Activity Measurement
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNamara, K.; Alzubaidi, K.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Pract. 2019, 8, 1–11. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.C.; DeBacker, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Tsoumani, M.; Vilahur, G.; Ikonomidis, I.; Badimon, L.; Varga, Z.V.; Ferdinandy, P.; Schulz, R. PCSK9 in myocardial infarction and cardioprotection: Importance of lipid metabolism and inflammation. Front. Physiol. 2020, 11, 602497. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Lagace, T.A.; McNutt, M.C.; Horton, J.D.; Deisenhofer, J. Molecular basis of LDL receptor recognition by PCSK9. Proc. Natl. Acad. Sci. USA 2008, 105, 1820–1825. [Google Scholar] [CrossRef]
- Burnap, S.A.; Sattler, K.; Pechlaner, R.; Duregotti, E.; Lu, R.; Theofilatos, K.; Takov, K.; Heusch, G.; Tsimikas, S.; Fernandez-Hernando, C.; et al. PCSK9 activity is potentiated through HDL binding. Circ. Res. 2021, 129, 1039. [Google Scholar] [CrossRef]
- Norata, G.D.; Tavori, H.; Pirillo, A.; Fazio, S.; Catapano, A.L. Biology of proprotein convertase subtilisin kexin 9: Beyond low-density lipoprotein cholesterol lowering. Cardiovasc. Res. 2016, 112, 429–442. [Google Scholar] [CrossRef]
- Schulz, R.; Schlüter, K.-D.; Laufs, U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9). Basic Res. Cardiol. 2015, 110, 4. [Google Scholar] [CrossRef]
- Schlüter, K.-D.; Wolf, A.; Schreckenberg, R. Coming back to physiology: Extrahepatic functions of proprotein convertase subtilisin/kexin type 9. Front. Physiol. 2020, 11, 598649. [Google Scholar] [CrossRef]
- Seidha, N.G.; Prat, A. The multifacted biology of PCSK9. Endocr. Rev. 2022, 43, 558–582. [Google Scholar] [CrossRef]
- Macchi, C.; Ferri, N.; Sirtori, C.R.; Corsini, A.; Banach, M.; Ruscica, M. Proprotein convertase subtilisin/kexin type 9: A view beyond the canonical cholesterol-lowering impact. Am. J. Pathol. 2021, 191, 1385–1397. [Google Scholar] [CrossRef]
- Puteri, M.U.; Azmi, N.U.; Kato, M.; Saputri, F.C. PCSK9 promotes cardiovascular diseases: Recent evidence about its association with platelet activation-induced myocardial infarction. Life 2022, 12, 190. [Google Scholar] [CrossRef]
- Schlüter, K.-D.; Wolf, A.; Weber, M.; Schreckenberg, R.; Schulz, R. Oxidized low-density lipoprotein (oxLDL) affects load-free cell shortening of cardiomyocytes in a proprotein convertase subtilisin/kexin 9 (PCSK9) dependent way. Basic Res. Cardiol. 2017, 112, 63. [Google Scholar] [CrossRef]
- Li, X.; Dai, F.; Wang, H.; Wei, G.; Jiang, Q.; Yin, P.; Wang, S.; Ge, J.; Yang, C.; Wu, J.; et al. PCSK9 participates in oxidized-low density lipoprotein-induced myocardial injury through mitochondrial oxidative stress and Drp1-mediated mitochondrial fission. Clin. Transl. Med. 2022, 12, e729. [Google Scholar] [CrossRef]
- Da Dalt, L.; Castiglioni, L.; Baragetti, A.; Audano, M.; Svecla, M.; Bonacina, F.; Pedretti, S.; Uboldi, P.; Benzoni, P.; Giannetti, F.; et al. PCSK9 deficiency rewires heart metabolism and drives heart failure with preserved ejection fraction. Eur. Heart J. 2021, 42, 3078–3090. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Liu, S.; Shahanawaz, J.; Theus, S.; Fan, Y.; Deng, X.; Zhou, S.; Mehta, J.L. PCSK9 expression in the ischemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc. Res. 2018, 114, 1738–1751. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, S.; Xu, R.-X.; Sun, J.; Tang, Y.; Li, J.-J. Proprotein convertase subtilisin/kexin type 9 expression is transiently up-regulated in the acute period of myocardial infarction in rat. BMC Cardiovasc. Disord. 2014, 14, 192. [Google Scholar] [CrossRef]
- Almontashiri, N.A.M.; Vilmundarson, R.O.; Ghasemzadeh, N.; Dandona, S.; Roberts, R.; Quyyumi, A.A.; Chen, H.-H.; Stewart, A.F.R. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS ONE 2014, 9, e106294. [Google Scholar] [CrossRef]
- Palee, S.; McSweeney, C.M.; Maneechote, C.; Moisescu, D.M.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, S.C.; Chattipakorn, N. PCSK9 inhibitor improves cardiac function and reduces infarct size in rats with ischemia/reperfusion injury: Benefits beyond lipid-lowering effects. J. Cell. Mol. Med. 2019, 23, 7310–7319. [Google Scholar] [CrossRef]
- Minana, G.; Nunez, J.; Bayes-Genis, A.; Revuelta-Lopez, E.; Rios-Navarro, C.; Nunez, E.; Chorro, F.J.; Lopez-Lereu, M.P.; Monmeneu, J.V.; Lupon, J.; et al. Role of PCSK9 in the course of ejection fraction changes after ST-segment elevation myocardial infarction: A pilot study. Heart Fail. 2020, 7, 118–123. [Google Scholar] [CrossRef]
- Andreadou, I.; Daiber, A.; Baxter, G.F.; Brizzi, M.F.; di Lisa, F.; Kaludercic, N.; Lazou, A.; Varga, Z.V.; Zuurbier, C.J.; Schulz, R.; et al. Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: Role of cardiac redox signaling. Free Rad. Biol. Med. 2021, 166, 33. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Lazou, A.; Görbe, A.; Giricz, Z.; Schulz, R.; Ferdinandy, P. Effect of hypercholesterolaemia on myocardial function, ischaemia-reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2017, 174, 1555. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, C.; Li, L.; Zeng, Z.; Ma, X.; Gui, C. Neuregulin-1 protects cardiac electrical conduction through downregulating matrix metalloproteinase-9 and upregulating connexin 43 in a rat myocardial infarction model. Pharmazie 2019, 74, 231–234. [Google Scholar]
- Wu, X.; Huang, W.; Luo, G.; Alian, L.A. Hypoxia induces connexin 43 dysregulation by modulating matrix metalloproteinases via AMPK signaling. Mol. Cell. Biochem. 2013, 384, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Li, H.-M.; Yan, Z.-X.; Li, M.-C.; Wei, J.-P.; Zheng, W.-X.; Liu, S.-Q.; Deng, Y.-T.; Xie, H.-F.; Li, C.-G. Renin-angiotensin system activation and imbalance of matrix metalloproteinase-9/tissue inhibitor of matrix metalloproteinase-1 in cold-induced stroke. Life Sci. 2019, 231, 116563. [Google Scholar]
- Lu, H.; Howatt, D.A.; Balakrishnan, A.; Graham, M.J.; Mullick, A.E.; Daugerhty, A. Hypercholesterolemia induced by a PCSK9 Gain-of-Function mutation augments angiotensin II-induced abdominal aortic aneurysms in C57BL/6 mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Kutsche, H.S.; Schreckenberg, R.; Weber, M.; Li, L.; Rohrbach, S.; Schulz, R.; Schlüter, K.-D. Autocrine effects of PCSK9 on cardiomyocytes. Basic Res. Cardiol. 2020, 115, 65. [Google Scholar] [CrossRef]
- Roubtsova, A.; Chamberland, A.; Marcinkiewicz, J.; Essalmani, R.; Fazel, A.; Bergeron, J.J.; Seidah, N.G.; Prat, A. PCSK9 deficiency unmasks a sex- and tissue-specific subcellular distribution of the LDL and the VLDL receptors in mice. J. Lipid Res. 2015, 56, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Liu, S.; Brickell, A.N.; Zhang, J.; Wu, Z.; Zhou, S.; Ding, Z. PCSK9 regulates pyroptosis via mtDNA damage in chronic myocardial ischemia. Basic Res. Cardiol. 2020, 115, 66. [Google Scholar] [CrossRef]
- Ruscica, M.; Ricci, C.; Macchi, C.; Magni, P.; Cristofani, R.; Liu, J.; Corsini, A.; Ferri, N. Suppressor of cytokine signaling-3 (SOCS-3) induces proprotein convertase subtilisin kexin type 9 (PCSK9) expression in hepatic HepG2 cell line. J. Biol. Chem. 2016, 291, 3508–3519. [Google Scholar] [CrossRef]
- Huang, G.; Lu, X.; Zhou, H.; Li, R.; Huang, Q.; Xiong, X.; Luo, Z.; Li, W. PCSK9 inhibition protects against myocardial ischemia-reperfusion injury via suppressing autophagy. Microvasc. Res. 2022, 142, 104371. [Google Scholar] [CrossRef]
- Kathiresan, S.; Myocardial Infarction Genetic Consortium. A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N. Engl. J. Med. 2009, 358, 2299–2300. [Google Scholar] [CrossRef]
- Laugsand, L.E.; Asvold, B.O.; Vatten, L.J.; Janszyk, I.; Platou, G.C.; Michelsen, A.E.; Damas, J.K.; Aukrust, P.; Ueland, T. Circulating PCSK9 and risk of myocardial infarction: The HUNT study in Norway. JACC Basic Transl. Sci. 2016, 1, 568–575. [Google Scholar] [CrossRef][Green Version]
- Khan, S.U.; Yedlapati, S.H.; Lone, A.N.; Hao, Q.; Guyatt, G.; Delvaux, N.; Bekkering, G.E.; Vandvik, P.O.; Riaz, I.B.; Li, S.; et al. PCSK9 inhibitors and ezetimibe with or without statin therapy for cardiovascular risk reduction: A systematic review and network meta-analysis. BMJ 2022, 377, e069116. [Google Scholar] [CrossRef]
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction. JAMA 2022, 327, 1721–1781. [Google Scholar] [CrossRef]
- Zhang, Y.; Eigenbrot, C.; Zhou, L.; Shia, S.; Li, W.; Quan, C.; Tom, J.; Moran, P.; di Lello, P.; Skelton, N.J.; et al. Identification of a small peptide that inhibits PCSK9 binding to the low density lipoprotein receptor. J. Biol. Chem. 2014, 289, 942–955. [Google Scholar] [CrossRef]
- Schreckenberg, R.; Weber, P.; Cabrera-Fuentes, H.A.; Steinert, I.; Preissner, K.T.; Bencsik, P.; Sarközy, M.; Csonka, C.; Ferdinandy, P.; Schulz, R.; et al. Mechanism and consequences of the shift in cardiac arginine metabolism following ischaemia and reperfusion in rats. Thromb. Haemost. 2015, 113, 482–493. [Google Scholar]
- Heidorn, M.; Frodermann, T.; Böning, A.; Schreckenberg, R.; Schlüter, K.-D. Citrulline improves early post-ischemic recovery of rat hearts in vitro by shifting arginine metabolism from polyamine to nitric oxide formation. Clin. Med. Insights Cardiol. 2018, 12, 1–5. [Google Scholar] [CrossRef]
- Schreckenberg, R.; Maier, T.; Schlüter, K.-D. Post-conditioning restores pre-ischaemic receptor coupling in rat isolated hearts. Br. J. Pharmacol. 2009, 156, 901–908. [Google Scholar] [CrossRef][Green Version]
- Hirschhäuser, C.; Lissoni, A.; Görge, P.M.; Lampe, P.D.; Heger, J.; Schlüter, K.-D.; Leybaert, L.; Schulz, R.; Boengler, K. Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischaemic preconditioning. Basic Res. Cardiol. 2021, 116, 21. [Google Scholar] [CrossRef]
- Boengler, K.; Bencsik, P.; Paloczi, J.; Kiss, K.; Papisz, M.; Pipis, J.; Ferdinandy, P.; Schlüter, K.-D.; Schul, R. Lack of contribution of p66shc and ist mitochondrial translocation to ischemia-reperfusion injury and cardioprotection by ischemic preconditioning. Front. Physiol. 2017, 8, 733. [Google Scholar] [CrossRef]
- Schreckenberg, R.; Klein, J.; Kutsche, H.S.; Schulz, R.; Gömöri, K.; Bencsik, P.; Benczik, B.; Agg, B.; Saghy, E.; Ferdinandy, P.; et al. Ischaemic post-conditioning in rats: Responder and non-responder differ in transcriptome of mitochondrial proteins. J. Cell. Mol. Med. 2019, 24, 5528–5541. [Google Scholar] [CrossRef]
- Bencsik, P.; Sasi, V.; Kiss, K.; Kupai, K.; Molosscary, M.; Maurovich-Horvat, P.; Csont, T.; Ungi, I.; Merkely, B.; Ferdinandy, P. Serum lipids and cardiac function correlate with nitrotyrosine and MMP activity in coronary artery disease patients. Eur. J. Clin. Investig. 2015, 45, 692–701. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-(delta delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Dry matter | % | 88 |
| Crude protein | % | 22.04 |
| Crude fat | % | 3.35 |
| Crude fiber | % | 6.34 |
| Crude ash | % | 9.31 |
| Lyisine | % | 0.99 |
| Methionine | % | 0.4 |
| Methionine + cystine | % | 0.78 |
| Calcium | % | 0.81 |
| Phosphorous | % | 0.66 |
| Sodium | % | 0.17 |
| Vitamin A | IU/kg | 12,250 |
| Vitamin D3 | IU/kg | 1800 |
| Vitamin E | mg/kg | 61 |
| Gene | Forward | Reverse |
|---|---|---|
| B2M | GCTATCCAGAAAACCCCTCAA | CATGTCTCGATCCCAGTAGACGGT |
| GAPDH | ACGGCACAGTCAAGGCCGAG | CACCCTTCAAGTGGGCCCCG |
| HPRT | CCA GCG TCG TGA TTA GCG AT | CAA GTC TTT CAG TCC TGT CC |
| PCSK9 | CACCATGGGCACCGTCAGCTCCAG | AAACTGGAGCTCCTGGGAGGCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreckenberg, R.; Wolf, A.; Szabados, T.; Gömöri, K.; Szabó, I.A.; Ágoston, G.; Brenner, G.; Bencsik, P.; Ferdinandy, P.; Schulz, R.; et al. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Deletion but Not Inhibition of Extracellular PCSK9 Reduces Infarct Sizes Ex Vivo but Not In Vivo. Int. J. Mol. Sci. 2022, 23, 6512. https://doi.org/10.3390/ijms23126512
Schreckenberg R, Wolf A, Szabados T, Gömöri K, Szabó IA, Ágoston G, Brenner G, Bencsik P, Ferdinandy P, Schulz R, et al. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Deletion but Not Inhibition of Extracellular PCSK9 Reduces Infarct Sizes Ex Vivo but Not In Vivo. International Journal of Molecular Sciences. 2022; 23(12):6512. https://doi.org/10.3390/ijms23126512
Chicago/Turabian StyleSchreckenberg, Rolf, Annemarie Wolf, Tamara Szabados, Kamilla Gömöri, István Adorján Szabó, Gergely Ágoston, Gábor Brenner, Péter Bencsik, Péter Ferdinandy, Rainer Schulz, and et al. 2022. "Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Deletion but Not Inhibition of Extracellular PCSK9 Reduces Infarct Sizes Ex Vivo but Not In Vivo" International Journal of Molecular Sciences 23, no. 12: 6512. https://doi.org/10.3390/ijms23126512
APA StyleSchreckenberg, R., Wolf, A., Szabados, T., Gömöri, K., Szabó, I. A., Ágoston, G., Brenner, G., Bencsik, P., Ferdinandy, P., Schulz, R., & Schlüter, K.-D. (2022). Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Deletion but Not Inhibition of Extracellular PCSK9 Reduces Infarct Sizes Ex Vivo but Not In Vivo. International Journal of Molecular Sciences, 23(12), 6512. https://doi.org/10.3390/ijms23126512









