TLR/WNT: A Novel Relationship in Immunomodulation of Lung Cancer
Abstract
1. Introduction
2. Toll-like Receptors (TLRs) in Lung
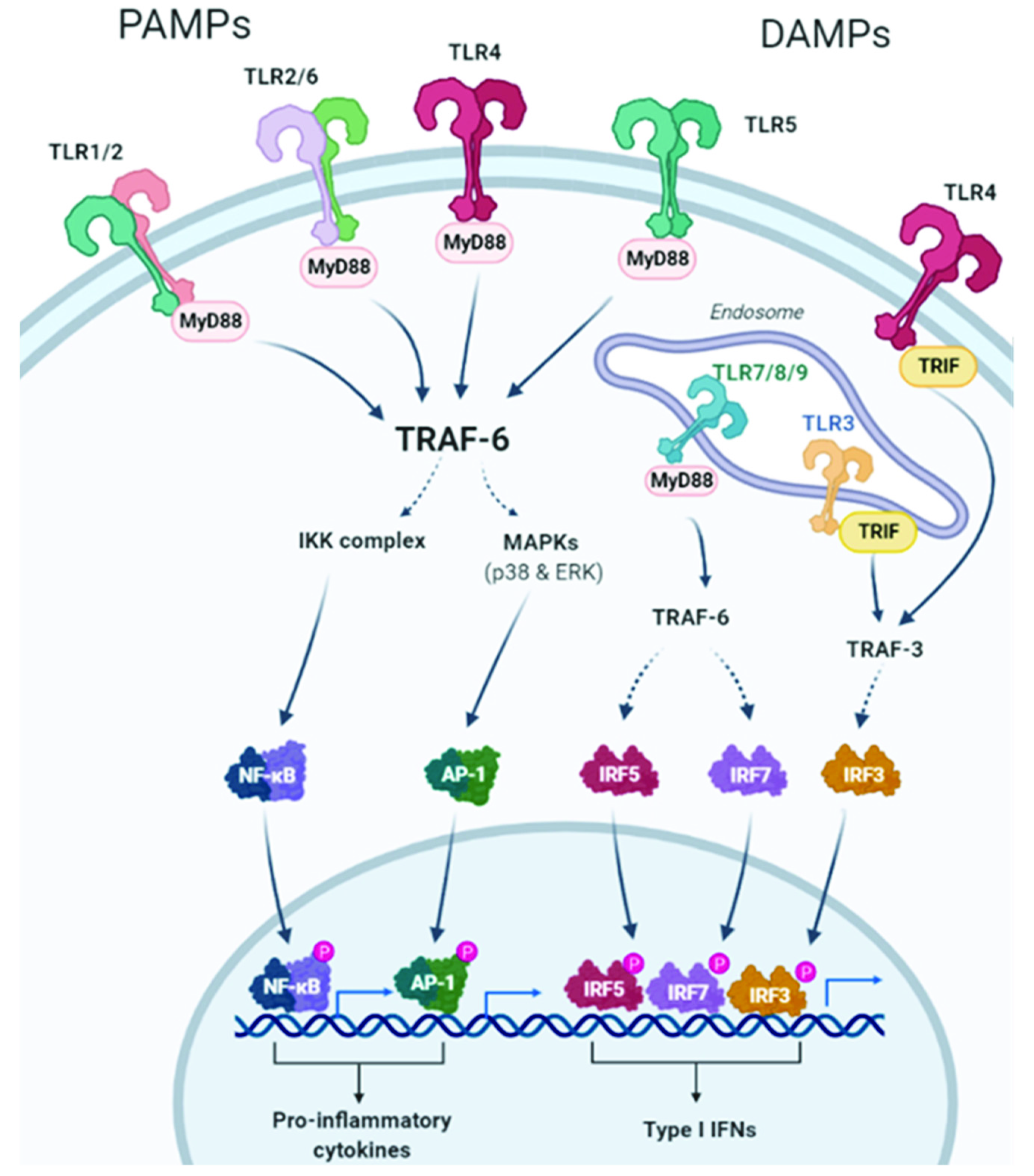
2.1. TLRs and TLR Signaling
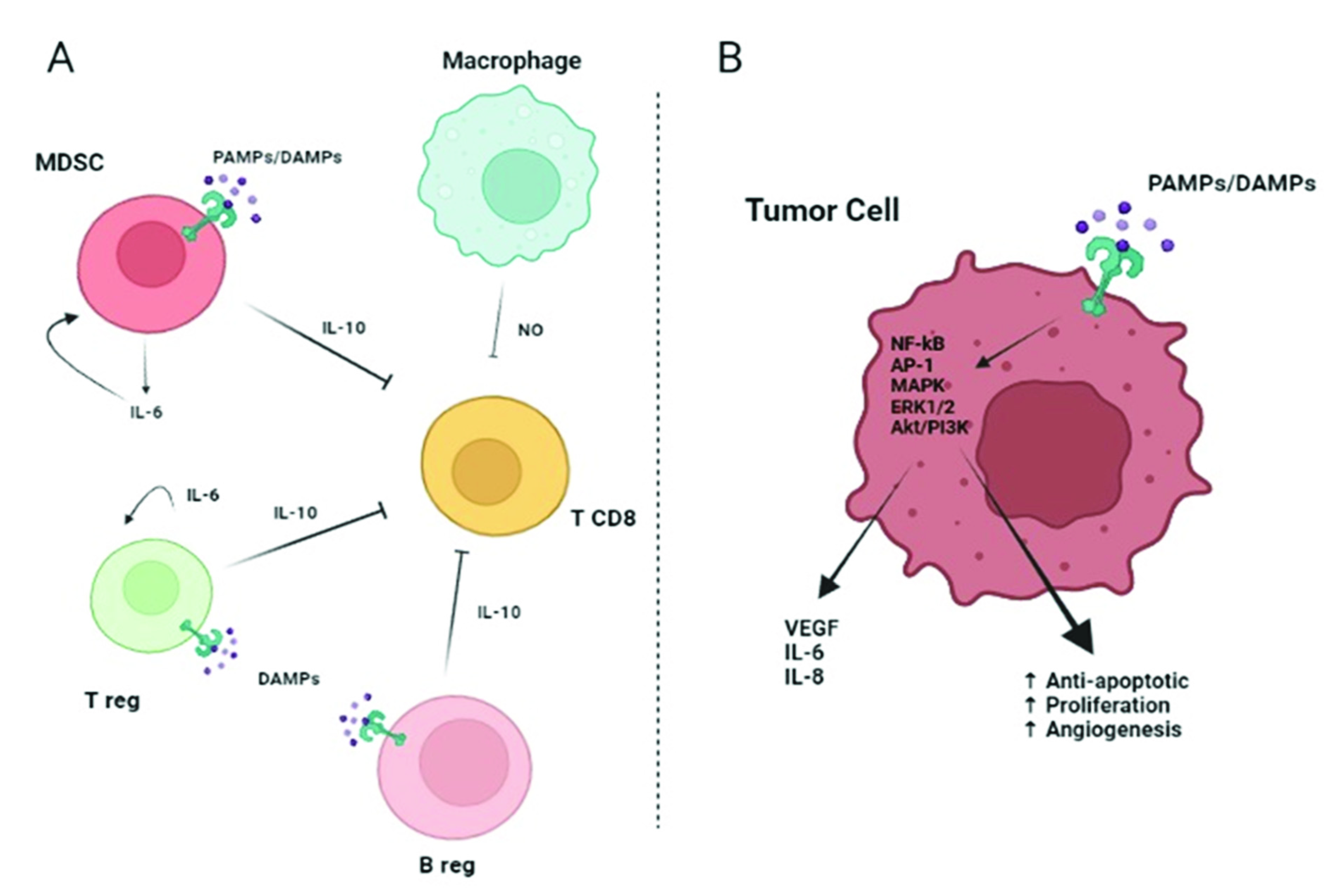
2.2. TLRs Role in Lung Cancer
3. WNT in Lung Cancer
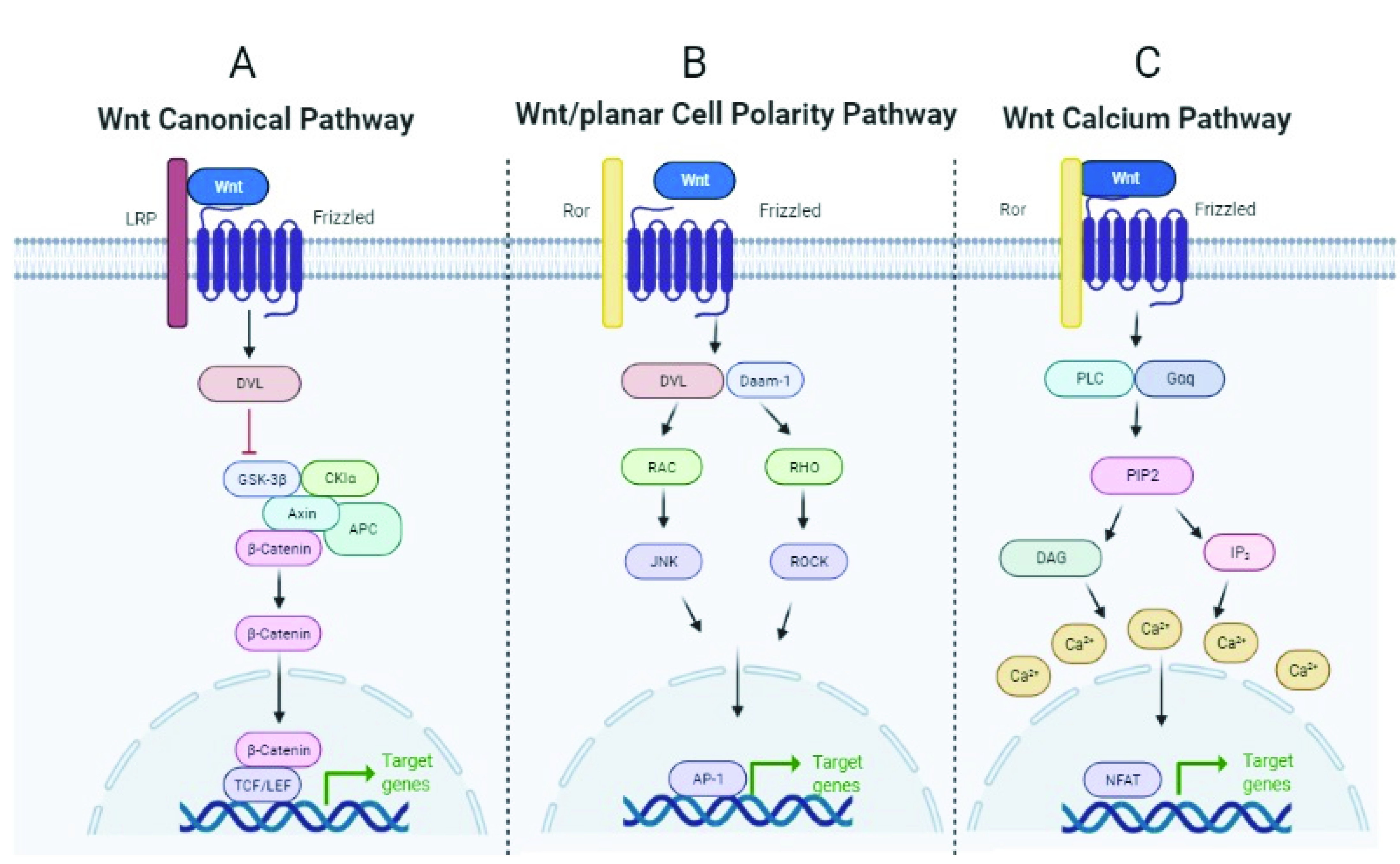
3.1. WNT Signaling Pathway
3.2. Alterations of the WNT Signaling Pathway in Lung Cancer
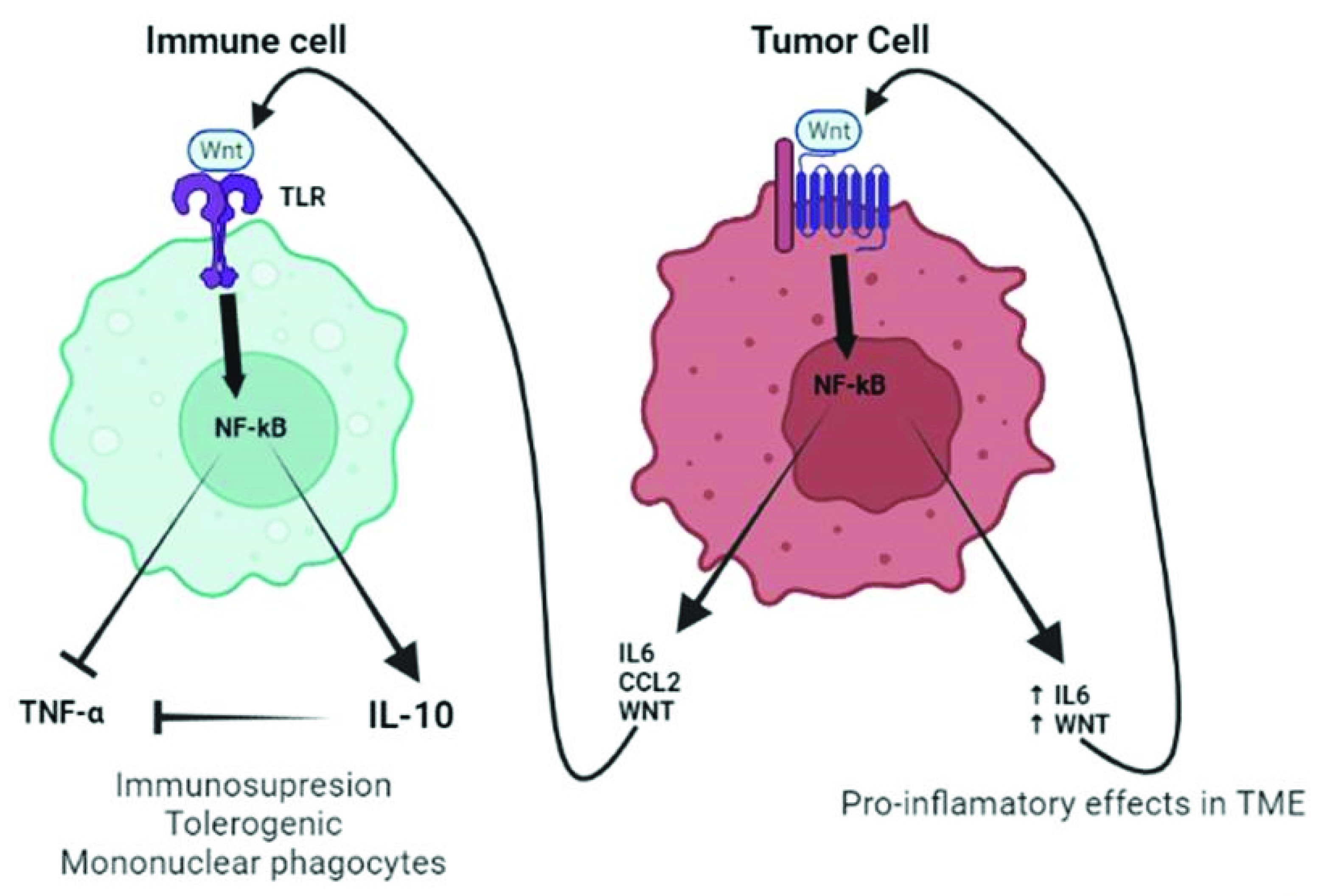
4. TLR/WNT Crosstalk
4.1. TLR/WNT Crosstalk in the Lung
4.2. TLR/WNT in Lung Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.-W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta 2015, 1856, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Travis, W.D. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod. Pathol. 2012, 25, S18–S30. [Google Scholar] [CrossRef]
- Li, X.; Jiang, S.; Tapping, R.I. Toll-like receptor signaling in cell proliferation and survival. Cytokine 2010, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Martin, T.R.; Frevert, C.W. Innate Immunity in the Lungs. Proc. Am. Thorac. Soc. 2005, 2, 403–411. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-Like Receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef]
- Menck, K.; Heinrichs, S.; Baden, C.; Bleckmann, A. The WNT/ROR Pathway in Cancer: From Signaling to Therapeutic Intervention. Cells 2021, 10, 142. [Google Scholar] [CrossRef]
- Roach, J.C.; Glusman, G.; Rowen, L.; Kaur, A.; Purcell, M.K.; Smith, K.D.; Hood, L.E.; Aderem, A. The evolution of vertebrate Toll-like receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 9577–9582. [Google Scholar] [CrossRef]
- Droemann, D.; Goldmann, T.; Tiedje, T.; Zabel, P.; Dalhoff, K.; Schaaf, B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir. Res. 2005, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Naito, S. Tissue-Specific mRNA Expression Profiles of Human Toll-Like Receptors and Related Genes. Biol. Pharm. Bull. 2005, 28, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Gerondakis, S. Coordinating TLR-activated signaling pathways in cells of the immune system. Immunol. Cell Biol. 2007, 85, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H. Endogenous TLR Ligands and Autoimmunity. Adv. Immunol. 2006, 91, 159–173. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Hasan, U.A.; Trinchieri, G.; Vlach, J. Toll-like Receptor Signaling Stimulates Cell Cycle Entry and Progression in Fibroblasts. J. Biol. Chem. 2005, 280, 20620–20627. [Google Scholar] [CrossRef]
- Jego, G.; Bataille, R.; Geffroyluseau, A.; Descamps, G.; Deceunynck, C. Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia 2006, 20, 1130–1137. [Google Scholar] [CrossRef]
- Pradere, J.-P.; Dapito, D.H.; Schwabe, R.F. The Yin and Yang of Toll-like receptors in cancer. Oncogene 2013, 33, 3485–3495. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, J.; Unkeless, J.C.; Feng, Z.H.; Xiong, H. TLR signaling by tumor and immune cells: A double-edged sword. Oncogene 2008, 27, 218–224. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Immune escape of tumors: Apoptosis resistance and tumor counterattack. J. Leukoc. Biol. 2002, 71, 907–920. [Google Scholar]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mohan, C. Toll-Like Receptor Signaling Pathways—Therapeutic Opportunities. Mediat. Inflamm. 2010, 2010, 781235. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbeck’s Arch. Surg. 2006, 391, 499–510. [Google Scholar] [CrossRef]
- Dutta, J.; Fan, Y.; Gupta, N.; Fan, G.; Gélinas, C. Current insights into the regulation of programmed cell death by NF-κB. Oncogene 2006, 25, 6800–6816. [Google Scholar] [CrossRef]
- Patidar, A.; Selvaraj, S.; Sarode, A.; Chauhan, P.; Chattopadhyay, D.; Saha, B. DAMP-TLR-cytokine axis dictates the fate of tumor. Cytokine 2018, 104, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.-Y.; Lee, J.W.; Kim, Y.S.; Lee, S.E.; Han, H.D.; Hong, K.-J.; Kang, T.H.; Park, Y.-M. Interactions between tumor-derived proteins and Toll-like receptors. Exp. Mol. Med. 2020, 52, 1926–1935. [Google Scholar] [CrossRef]
- Choi, C.H.; Kang, T.H.; Song, J.S.; Kim, Y.S.; Chung, E.J.; Ylaya, K.; Kim, S.; Koh, S.S.; Chung, J.-Y.; Kim, J.-H.; et al. Elevated expression of pancreatic adenocarcinoma upregulated factor (PAUF) is associated with poor prognosis and chemoresistance in epithelial ovarian cancer. Sci. Rep. 2018, 8, 12161. [Google Scholar] [CrossRef]
- Srikrishna, G.; Freeze, H.H. Endogenous Damage-Associated Molecular Pattern Molecules at the Crossroads of Inflammation and Cancer. Neoplasia 2009, 11, 615–628. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, S.; Han, D.; Xu, Y.; Jiao, D.; Wu, J.; Yang, F.; Ge, Y.; Shi, S.; Li, Y.; et al. High mobility group box 1 promotes the epithelial-to-mesenchymal transition in prostate cancer PC3 cells via the RAGE/NF-κB signaling pathway. Int. J. Oncol. 2018, 53, 659–671. [Google Scholar] [CrossRef]
- Kelly, M.G.; Alvero, A.B.; Chen, R.; Silasi, D.-A.; Abrahams, V.M.; Chan, S.; Visintin, I.; Rutherford, T.; Mor, G. TLR-4 Signaling Promotes Tumor Growth and Paclitaxel Chemoresistance in Ovarian Cancer. Cancer Res. 2006, 66, 3859–3868. [Google Scholar] [CrossRef]
- Park, H.D.; Lee, Y.; Oh, Y.K.; Jung, J.G.; Park, Y.W.; Myung, K.; Kim, K.-H.; Koh, S.S.; Lim, D.-S. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene 2011, 30, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, J.; Mencin, A.; Schwabe, R.F. Toll-like receptors, wound healing, and carcinogenesis. J. Mol. Med. 2009, 87, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; De Souza, P.M.; Sriskandan, S.; Duffin, C.; Paul-Clark, M.J.; Mitchell, J.A. Pattern recognition receptors and interleukin-8 mediate effects of Gram-positive and Gram-negative bacteria on lung epithelial cell function. Br. J. Pharmacol. 2008, 154, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef]
- Angell, H.; Galon, J. From the immune contexture to the Immunoscore: The role of prognostic and predictive immune markers in cancer. Curr. Opin. Immunol. 2013, 25, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Garaude, J.; Kent, A.; van Rooijen, N.; Blander, J.M. Simultaneous Targeting of Toll- and Nod-Like Receptors Induces Effective Tumor-Specific Immune Responses. Sci. Transl. Med. 2012, 4, 120ra16. [Google Scholar] [CrossRef]
- Drobits, B.; Holcmann, M.; Amberg, N.; Swiecki, M.; Grundtner, R.; Hammer, M.; Colonna, M.; Sibilia, M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J. Clin. Investig. 2012, 122, 575–585. [Google Scholar] [CrossRef]
- Peng, G.; Guo, Z.; Kiniwa, Y.; Voo, K.S.; Peng, W.; Fu, T.; Wang, D.Y.; Li, Y.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor 8-Mediated Reversal of CD4+ Regulatory T Cell Function. Science 2005, 309, 1380–1384. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Syed, M.A.; Boddupalli, C.S.; Dhodapkar, M.V.; Homer, R.J.; Minoo, P.; Bhandari, V. Conditional overexpression of TGFβ1 promotes pulmonary inflammation, apoptosis and mortality via TGFβR2 in the developing mouse lung. Respir. Res. 2015, 16, 4. [Google Scholar] [CrossRef]
- Arora, S.; Ahmad, S.; Irshad, R.; Goyal, Y.; Rafat, S.; Siddiqui, N.; Dev, K.; Husain, M.; Ali, S.; Mohan, A.; et al. TLRs in pulmonary diseases. Life Sci. 2019, 233, 116671. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Q.; Wang, L.; Chen, W.; Li, N.; Cao, X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol. Immunol. 2007, 44, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef] [PubMed]
- Cherfils-Vicini, J.; Platonova, S.; Gillard, M.; Laurans, L.; Validire, P.; Caliandro, R.; Magdeleinat, P.; Mami-Chouaib, F.; Dieu-Nosjean, M.-C.; Fridman, W.H.; et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J. Clin. Investig. 2010, 120, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Droemann, D.; Albrecht, D.; Gerdes, J.; Ulmer, A.J.; Branscheid, D.; Vollmer, E.; Dalhoff, K.; Zabel, P.; Goldmann, T. Human lung cancer cells express functionally active Toll-like receptor 9. Respir. Res. 2005, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Karin, M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011, 70, i104–i108. [Google Scholar] [CrossRef]
- Tye, H.; Kennedy, C.L.; Najdovska, M.; McLeod, L.; McCormack, W.; Hughes, N.; Dev, A.; Sievert, W.; Ooi, C.-H.; Ishikawa, T.-O.; et al. STAT3-Driven Upregulation of TLR2 Promotes Gastric Tumorigenesis Independent of Tumor Inflammation. Cancer Cell 2012, 22, 466–478. [Google Scholar] [CrossRef]
- Kim, S.; Takahashi, H.; Lin, W.W.; Descargues, P.; Grivennikov, S.; Kim, Y.; Luo, J.-L.; Karin, M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009, 457, 102–106. [Google Scholar] [CrossRef]
- Jia, D.; Yang, W.; Li, L.; Liu, H.; Tan, Y.; Ooi, S.; Chi, L.; Filion, L.G.; Figeys, D.; Wang, L. β-Catenin and NF-κB co-activation triggered by TLR3 stimulation facilitates stem cell-like phenotypes in breast cancer. Cell Death Differ. 2015, 22, 298–310. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.-Y.; Pradère, J.-P.; Jang, M.-K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef]
- Fukata, M.; Hernandez, Y.; Conduah, D.; Cohen, J.; Chen, A.; Breglio, K.; Goo, T.; Hsu, D.; Xu, R.; Abreu, M.T. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm. Bowel Dis. 2009, 15, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Saccheri, F.; Venereau, E.; Pusterla, T.; Bianchi, M.E.; Rescigno, M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010, 29, 2242–2252. [Google Scholar] [CrossRef] [PubMed]
- Ochi, A.; Graffeo, C.S.; Zambirinis, C.P.; Rehman, A.; Hackman, M.; Fallon, N.; Barilla, R.; Henning, J.R.; Jamal, M.; Rao, R.; et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J. Clin. Investig. 2012, 122, 4118–4129. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Bolli, E.; Tarone, L.; Cavallo, F.; Conti, L. Toll-Like Receptor 2 at the Crossroad between Cancer Cells, the Immune System, and the Microbiota. Int. J. Mol. Sci. 2020, 21, E9418. [Google Scholar] [CrossRef]
- Chin, A.I.; Miyahira, A.K.; Covarrubias, A.; Teague, J.; Guo, B.; Dempsey, P.W.; Cheng, G. Toll-like Receptor 3–Mediated Suppression of TRAMP Prostate Cancer Shows the Critical Role of Type I Interferons in Tumor Immune Surveillance. Cancer Res. 2010, 70, 2595–2603. [Google Scholar] [CrossRef]
- Chew, V.; Tow, C.; Huang, C.; Bard-Chapeau, E.; Copeland, N.G.; Jenkins, N.A.; Weber, A.; Lim, K.H.; Toh, H.C.; Heikenwalder, M.; et al. Toll-Like Receptor 3 Expressing Tumor Parenchyma and Infiltrating Natural Killer Cells in Hepatocellular Carcinoma Patients. JNCI J. Natl. Cancer Inst. 2012, 104, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Sanchez, A.; Shi, Z.; Zhang, T.; Liu, M.; Zhang, D. Activation of Toll-like Receptor 5 on Breast Cancer Cells by Flagellin Suppresses Cell Proliferation and Tumor Growth. Cancer Res. 2011, 71, 2466–2475. [Google Scholar] [CrossRef]
- Rhee, S.H.; Im, E.; Pothoulakis, C. Toll-Like Receptor 5 Engagement Modulates Tumor Development and Growth in a Mouse Xenograft Model of Human Colon Cancer. Gastroenterology 2008, 135, 518–528. [Google Scholar] [CrossRef]
- He, X.; Semenov, M.; Tamai, K.; Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development 2004, 131, 1663–1677. [Google Scholar] [CrossRef]
- Macdonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Gajos-Michniewicz, A.; Czyz, M. WNT Signaling in Melanoma. Int. J. Mol. Sci. 2020, 21, 4852. [Google Scholar] [CrossRef]
- Kotton, D.N.; Morrisey, E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014, 20, 822–832. [Google Scholar] [CrossRef]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef]
- Koni, M.; Pinnarò, V.; Brizzi, M.F. The Wnt Signalling Pathway: A Tailored Target in Cancer. Int. J. Mol. Sci. 2020, 21, 7697. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, I.S.; Goel, A.; Warrier, S.; Kumar, A.P.; Sethi, G.; Garg, M. The multidimensional role of the Wnt/β-catenin signaling pathway in human malignancies. J. Cell. Physiol. 2022, 237, 199–238. [Google Scholar] [CrossRef]
- Radtke, F.; Clevers, H. Self-Renewal and Cancer of the Gut: Two Sides of a Coin. Science 2005, 307, 1904–1909. [Google Scholar] [CrossRef]
- Aros, C.J.; Pantoja, C.J.; Gomperts, B.N. Wnt signaling in lung development, regeneration, and disease progression. Commun. Biol. 2021, 4, 601. [Google Scholar] [CrossRef]
- Giles, R.H.; van Es, J.H.; Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 2003, 1653, 1–24. [Google Scholar] [CrossRef]
- Akiri, G.; Cherian, M.M.; Vijayakumar, S.; Liu, G.; Bafico, A.; Aaronson, S.A. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene 2009, 28, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Rapp, J.; Kiss, E.; Meggyes, M.; Szabo-Meleg, E.; Feller, D.; Smuk, G.; Laszlo, T.; Sarosi, V.; Molnar, T.F.; Kvell, K.; et al. Increased Wnt5a in squamous cell lung carcinoma inhibits endothelial cell motility. BMC Cancer 2016, 16, 915. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, N.; Liu, D.; Huang, C.-L.; Ueno, M.; Zhang, X.; Yokomise, H. Wnt3 gene expression promotes tumor progression in non-small cell lung cancer. Lung Cancer 2012, 76, 228–234. [Google Scholar] [CrossRef]
- Stewart, D.J. Wnt Signaling Pathway in Non-Small Cell Lung Cancer. JNCI J. Natl. Cancer Inst. 2014, 106, djt356. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ma, R.; Xu, Y.; Li, N.; Li, Z.; Yue, J.; Li, H.; Guo, Y.; Qi, D. Wnt2 promotes non-small cell lung cancer progression by activating WNT/β-catenin pathway. Am. J. Cancer Res. 2015, 5, 1032–1046. [Google Scholar]
- Jin, J.; Zhan, P.; Qian, H.; Wang, X.; Katoh, M.; Phan, K.; Chung, J.-H.; Lv, T.; Song, Y.; Written on behalf of the AME Lung Cancer Collaborative Group. Prognostic value of wingless-type proteins in non-small cell lung cancer patients: A meta-analysis. Transl. Lung Cancer Res. 2016, 5, 436–442. [Google Scholar] [CrossRef]
- Li, C.; Song, G.; Zhang, S.; Wang, E.; Cui, Z. Wnt3a Increases the Metastatic Potential of Non-Small Cell Lung Cancer Cells in Vitro in Part via Its Upregulation of Notch3. Oncol. Rep. 2015, 33, 1207–1214. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, M.; Xia, W.; Xu, Y.; Mao, Q.; Wang, J.; Dong, G.; Xu, L.; Yang, X.; Yin, R. Glypican-5 suppresses Epithelial-Mesenchymal Transition of the lung adenocarcinoma by competitively binding to Wnt3a. Oncotarget 2016, 7, 79736–79746. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, G.; Zhang, B.; Xu, G.; Xiong, W.; Yang, H. Wnt-5a regulates proliferation in lung cancer cells. Oncol. Rep. 2010, 23, 177–181. [Google Scholar] [CrossRef]
- Huang, C.-L.; Liu, D.; Nakano, J.; Ishikawa, S.; Kontani, K.; Yokomise, H.; Ueno, M. Wnt5a Expression Is Associated With the Tumor Proliferation and the Stromal Vascular Endothelial Growth Factor—An Expression in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 8765–8773. [Google Scholar] [CrossRef]
- Vesel, M.; Rapp, J.; Feller, D.; Kiss, E.; Jaromi, L.; Meggyes, M.; Miskei, G.; Duga, B.; Smuk, G.; Laszlo, T.; et al. ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respir. Res. 2017, 18, 52. [Google Scholar] [CrossRef]
- Winn, R.A.; Marek, L.; Han, S.-Y.; Rodriguez, K.; Rodriguez, N.; Hammond, M.; Van Scoyk, M.; Acosta, H.; Mirus, J.; Barry, N.; et al. Restoration of Wnt-7a Expression Reverses Non-small Cell Lung Cancer Cellular Transformation through Frizzled-9-mediated Growth Inhibition and Promotion of Cell Differentiation. J. Biol. Chem. 2005, 280, 19625–19634. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem Cell Signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Sánchez-Rivera, F.J.; Cetinbas, N.M.; Wu, K.; Joshi, N.; Helenius, K.; Park, Y.; Azimi, R.; Kerper, N.R.; Wesselhoeft, R.A.; et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 2017, 545, 355–359. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kosaka, N.; Araya, J.; Kuwano, K.; Ochiya, T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol. Med. 2015, 21, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Shamskhou, E.A.; Orcholski, M.E.; Nathan, A.; Reddy, S.; Honda, H.; Mani, V.; Zeng, Y.; Ozen, M.O.; Wang, L.; et al. Loss of Endothelium-Derived Wnt5a Is Associated With Reduced Pericyte Recruitment and Small Vessel Loss in Pulmonary Arterial Hypertension. Circulation 2019, 139, 1710–1724. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Yamamoto, H.; Kishida, S.; Kishida, M.; Awada, C.; Takao, T.; Kikuchi, A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017, 108, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Liu, Y.; Khoor, A.; Wang, X.; Thompson, E.A.; Leitges, M.; Justilien, V.; Weems, C.; Murray, N.R.; Fields, A.P. Protein Kinase Cι and Wnt/β-Catenin Signaling: Alternative Pathways to Kras/Trp53-Driven Lung Adenocarcinoma. Cancer Cell 2019, 36, 156–167. [Google Scholar] [CrossRef]
- Pacheco-Pinedo, E.C.; Durham, A.C.; Stewart, K.M.; Goss, A.M.; Lu, M.M.; DeMayo, F.J.; Morrisey, E.E. Wnt/β-catenin signaling accelerates mouse lung tumorigenesis by imposing an embryonic distal progenitor phenotype on lung epithelium. J. Clin. Investig. 2011, 121, 1935–1945. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Chiang, A.C.; Zhang, X.H.-F.; Kim, J.Y.; Kris, M.G.; Ladanyi, M.; Gerald, W.L.; Massagué, J. WNT/TCF Signaling through LEF1 and HOXB9 Mediates Lung Adenocarcinoma Metastasis. Cell 2009, 138, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tu, K.; Xia, L.; Luo, K.; Luo, W.; Tang, J.; Lu, K.; Hu, X.; He, Y.; Qiao, W.; et al. The Open Chromatin Landscape of Non–Small Cell Lung Carcinoma. Cancer Res. 2019, 79, 4840–4854. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Zhang, Y.; Bao, W.; Kong, X. Low-Density Lipoprotein Receptor-Related Protein 6 (LRP6) rs10845498 Polymorphism Is Associated with a Decreased Risk of Non-Small Cell Lung Cancer. Int. J. Med. Sci. 2014, 11, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Rao, M.; Humphries, A.E.; Hong, J.A.; Liu, F.; Yang, M.; Caragacianu, D.; Schrump, D.S. Tobacco Smoke Induces Polycomb-Mediated Repression of Dickkopf-1 in Lung Cancer Cells. Cancer Res. 2009, 69, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Hojjat-Farsangi, M.; Moshfegh, A.; Daneshmanesh, A.H.; Khan, A.S.; Mikaelsson, E.; Österborg, A.; Mellstedt, H. The receptor tyrosine kinase ROR1—An oncofetal antigen for targeted cancer therapy. Semin. Cancer Biol. 2014, 29, 21–31. [Google Scholar] [CrossRef]
- Khaledian, B.; Taguchi, A.; Shin-Ya, K.; Kondo-Ida, L.; Kagaya, N.; Suzuki, M.; Kajino, T.; Yamaguchi, T.; Shimada, Y.; Takahashi, T. Inhibition of heat shock protein 90 destabilizes receptor tyrosine kinase ROR1 in lung adenocarcinoma. Cancer Sci. 2021, 112, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef]
- Oderup, C.; LaJevic, M.; Butcher, E.C. Canonical and Noncanonical Wnt Proteins Program Dendritic Cell Responses for Tolerance. J. Immunol. 2013, 190, 6126–6134. [Google Scholar] [CrossRef]
- van Loosdregt, J.; Fleskens, V.; Tiemessen, M.M.; Mokry, M.; van Boxtel, R.; Meerding, J.; Pals, C.E.G.M.; Kurek, D.; Baert, M.R.M.; Delemarre, E.M.; et al. Canonical Wnt Signaling Negatively Modulates Regulatory T Cell Function. Immunity 2013, 39, 298–310. [Google Scholar] [CrossRef]
- Katoh, M. Transcriptional mechanisms of WNT5A based on NF-κB, Hedgehog, TGFβ, and Notch signaling cascades. Int. J. Mol. Med. 2009, 23, 763–769. [Google Scholar] [CrossRef]
- Trinath, J.; Holla, S.; Mahadik, K.; Prakhar, P.; Singh, V.; Balaji, K.N. The WNT Signaling Pathway Contributes to Dectin-1-Dependent Inhibition of Toll-Like Receptor-Induced Inflammatory Signature. Mol. Cell. Biol. 2014, 34, 4301–4314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehmeti, M.; Bergenfelz, C.; Källberg, E.; Millrud, C.R.; Björk, P.; Ivars, F.; Johansson-Lindbom, B.; Kjellström, S.; André, I.; Leandersson, K. Wnt5a is a TLR2/4-ligand that induces tolerance in human myeloid cells. Commun. Biol. 2019, 2, 176. [Google Scholar] [CrossRef]
- Blumenthal, A.; Ehlers, S.; Lauber, J.; Buer, J.; Lange, C.; Goldmann, T.; Heine, H.; Brandt, E.; Reiling, N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 2006, 108, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Schaale, K.; Farhat, K.; Endermann, T.; Ulmer, A.J.; Ehlers, S.; Reiling, N. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J. 2010, 24, 4599–4612. [Google Scholar] [CrossRef] [PubMed]
- Schaale, K.; Brandenburg, J.; Kispert, A.; Leitges, M.; Ehlers, S.; Reiling, N. Wnt6 Is Expressed in Granulomatous Lesions of Mycobacterium tuberculosis—Infected Mice and Is Involved in Macrophage Differentiation and Proliferation. J. Immunol. 2013, 191, 5182–5195. [Google Scholar] [CrossRef]
- Zhao, C.; Bu, X.; Wang, W.; Ma, T.; Ma, H. GEC-derived SFRP5 Inhibits Wnt5a-Induced Macrophage Chemotaxis and Activation. PLoS ONE 2014, 9, e85058. [Google Scholar] [CrossRef]
- Pereira, C.; Schaer, D.J.; Bachli, E.B.; Kurrer, M.O.; Schoedon, G. Wnt5A/CaMKII Signaling Contributes to the Inflammatory Response of Macrophages and Is a Target for the Antiinflammatory Action of Activated Protein C and Interleukin-10. Arter. Thromb. Vasc. Biol. 2008, 28, 504–510. [Google Scholar] [CrossRef]
- Li, D.; Beisswenger, C.; Herr, C.; Hellberg, J.; Han, G.; Zakharkina, T.; Voss, M.; Wiewrodt, R.; Bohle, R.M.; Menger, M.D.; et al. Myeloid cell RelA/p65 promotes lung cancer proliferation through Wnt/β-catenin signaling in murine and human tumor cells. Oncogene 2014, 33, 1239–1248. [Google Scholar] [CrossRef][Green Version]
- Kerdidani, D.; Chouvardas, P.; Arjo, A.R.; Giopanou, I.; Ntaliarda, G.; Guo, Y.A.; Tsikitis, M.; Kazamias, G.; Potaris, K.; Stathopoulos, G.T.; et al. Wnt1 silences chemokine genes in dendritic cells and induces adaptive immune resistance in lung adenocarcinoma. Nat. Commun. 2019, 10, 1405. [Google Scholar] [CrossRef]
- Kumawat, K.; Gosens, R. WNT-5A: Signaling and functions in health and disease. Cell. Mol. Life Sci. 2016, 73, 567–587. [Google Scholar] [CrossRef]
- Du, Q.; Geller, D.A. Cross-Regulation Between Wnt and NF-κB Signaling Pathways. Forum Immunopathol. Dis. Ther. 2010, 1, 155–181. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco Smoke Carcinogens and Lung Cancer. JNCI J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef] [PubMed]
- Whang, Y.M.; Jo, U.; Sung, J.S.; Ju, H.J.; Kim, H.K.; Park, K.H.; Lee, J.W.; Koh, I.S.; Kim, Y.H. Wnt5a Is Associated with Cigarette Smoke-Related Lung Carcinogenesis via Protein Kinase C. PLoS ONE 2013, 8, e53012. [Google Scholar] [CrossRef]
- Haw, T.J.; Starkey, M.R.; Pavlidis, S.; Fricker, M.; Arthurs, A.L.; Nair, P.M.; Liu, G.; Hanish, I.; Kim, R.Y.; Foster, P.S.; et al. Toll-like receptor 2 and 4 have Opposing Roles in the Pathogenesis of Cigarette Smoke-induced Chronic Obstructive Pulmonary Disease. Am. J. Physiol. bLung Cell. Mol. Physiol. 2018, 314, L298–L317. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Sun, B.; Zhao, X.; Zhao, X.; Gu, Q.; Dong, X.; Zheng, Y.; Sun, J.; Cheng, R.; Qi, H.; et al. Overexpression of Wnt5a Promotes Angiogenesis in NSCLC. BioMed Res. Int. 2014, 2014, 832562. [Google Scholar] [CrossRef]
- Baarsma, H.; Skronska-Wasek, W.; Mutze, K.; Ciolek, F.; Wagner, D.E.; John-Schuster, G.; Heinzelmann, K.; Günther, A.; Bracke, K.R.; Dagouassat, M.; et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 2017, 214, 143–163. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, P.; Jiang, G.; Cohn, L.; Lee, P.J. Toll-like receptor 4 deficiency causes pulmonary emphysema. J. Clin. Investig. 2006, 116, 3050–3059. [Google Scholar] [CrossRef]
- Teng, F.; Slavik, V.; Duffy, K.E.; Mateo, L.S.; Goldschmidt, R. Toll-like receptor 3 is involved in airway epithelial cell response to nontypeable Haemophilus influenzae. Cell. Immunol. 2010, 260, 98–104. [Google Scholar] [CrossRef]
- Carobene, L.; Spina, D.; Disanto, M.G.; Micheletto, C.; Mazzei, M.A.; Paladini, P.; Ghiribelli, C.; Bargagli, E.; Rottoli, P. Lung cancer and interstitial lung diseases: The lack of prognostic impact of lung cancer in IPF. Intern. Emerg. Med. 2022, 17, 457–464. [Google Scholar] [CrossRef]
- Königshoff, M.; Balsara, N.; Pfaff, E.-M.; Kramer, M.; Chrobak, I.; Seeger, W.; Eickelberg, O. Functional Wnt Signaling Is Increased in Idiopathic Pulmonary Fibrosis. PLoS ONE 2008, 3, e2142. [Google Scholar] [CrossRef]
- Martin-Medina, A.; Lehmann, M.; Burgy, O.; Hermann, S.; Baarsma, H.; Wagner, D.E.; De Santis, M.M.; Ciolek, F.; Hofer, T.P.; Frankenberger, M.; et al. Increased Extracellular Vesicles Mediate WNT5A Signaling in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Aumiller, V.; Balsara, N.; Wilhelm, J.; Günther, A.; Königshoff, M. WNT/β-Catenin Signaling Induces IL-1β Expression by Alveolar Epithelial Cells in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2013, 49, 96–104. [Google Scholar] [CrossRef] [PubMed]

| Receptor | Tumor Type | Tumor Role | Mechanism | Pathways or Molecules Involved | Ref |
|---|---|---|---|---|---|
| TLRs | Intestine, liver, skin | Protumoral | Inflammation | NF-κB, IL-1β, TNFα and IL-6 | [46] |
| TLR2 | Gastric | Protumoral | Cell survival and proliferation in gastric tumor epithelium | PI3K/Akt, ERK1/2, JNK MAPKs, NF-kb | [47] |
| TLR2/6 | Lewis lung carcinoma | Protumoral | Inflammation, macrophage activation, metastasis | Myd88, TRIF, ECM-protein versican, IL-6, TNF-α | [48] |
| TLR2/4 | Lung | Protumoral | ECM remodeling, | EGFR | [38] |
| TLR3 | Breast | Protumoral | Tumor growth | β- Catenin, NF-κB | [49] |
| TLR4 | Liver | Protumoral | Inflammation | NF-κB | [50] |
| TLR4 | Lung | Protumoral | immunosuppression, antiapoptosis | VEGF, TGF-β, IL-8 | [42] |
| TLR4 | Colon | Protumoral | Inflammation, tumor burden | Cox-2 | [51] |
| TLR4 | Skin | Protumoral | Inflammation | Myd88 | [52] |
| TLR7 | Pancreatic | Protumoral | Stromal inflammation | NF-kB, MAPKs | [53] |
| TLR7, TLR8 | Lung | Protumoral | Inflammation, tumor cell growth and survival, chemoresistance | NF-kB, MAPK, IRF, Bcl-2, IL-6, IL-8, CSF-2, IL-1α, IL-12, NOS-2 | [44] |
| TLR9 | Lung | Protumoral | Monocyte recruitment, antiapoptosis | MCP1 | [45] |
| TLR2 | Lung | Antitumoral | Monocytic myeloid-derived suppressor cell | JNK, TNF-α, IL-12p40, IL-12p70 | [54] |
| TLR3 | Prostate, liver | Antitumoral | Treatment with agonist stops tumor growth | Type I interferons | [55] |
| TLR3 | Liver | Antitumoral | Induction of tumor parenchyma (hepatocytes) cell death; induction of intratumor expression of chemokines that attract NK cells or T cells to the tumor microenvironment; and activation of tumor-infiltrating NK cells that promote cytotoxic activity. | IFN-γ | [56] |
| TLR5 | Breast, colon | Antitumoral | Immune cell activation | Dendritic cells | [37,57,58] |
| TLR7/8 | Melanoma | Antitumoral | Immune cell activation | Dendritic cells and Natural Killer | [38,39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Medina, A.; Cerón-Pisa, N.; Martinez-Font, E.; Shafiek, H.; Obrador-Hevia, A.; Sauleda, J.; Iglesias, A. TLR/WNT: A Novel Relationship in Immunomodulation of Lung Cancer. Int. J. Mol. Sci. 2022, 23, 6539. https://doi.org/10.3390/ijms23126539
Martín-Medina A, Cerón-Pisa N, Martinez-Font E, Shafiek H, Obrador-Hevia A, Sauleda J, Iglesias A. TLR/WNT: A Novel Relationship in Immunomodulation of Lung Cancer. International Journal of Molecular Sciences. 2022; 23(12):6539. https://doi.org/10.3390/ijms23126539
Chicago/Turabian StyleMartín-Medina, Aina, Noemi Cerón-Pisa, Esther Martinez-Font, Hanaa Shafiek, Antònia Obrador-Hevia, Jaume Sauleda, and Amanda Iglesias. 2022. "TLR/WNT: A Novel Relationship in Immunomodulation of Lung Cancer" International Journal of Molecular Sciences 23, no. 12: 6539. https://doi.org/10.3390/ijms23126539
APA StyleMartín-Medina, A., Cerón-Pisa, N., Martinez-Font, E., Shafiek, H., Obrador-Hevia, A., Sauleda, J., & Iglesias, A. (2022). TLR/WNT: A Novel Relationship in Immunomodulation of Lung Cancer. International Journal of Molecular Sciences, 23(12), 6539. https://doi.org/10.3390/ijms23126539






