A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications
Abstract
:1. Introduction
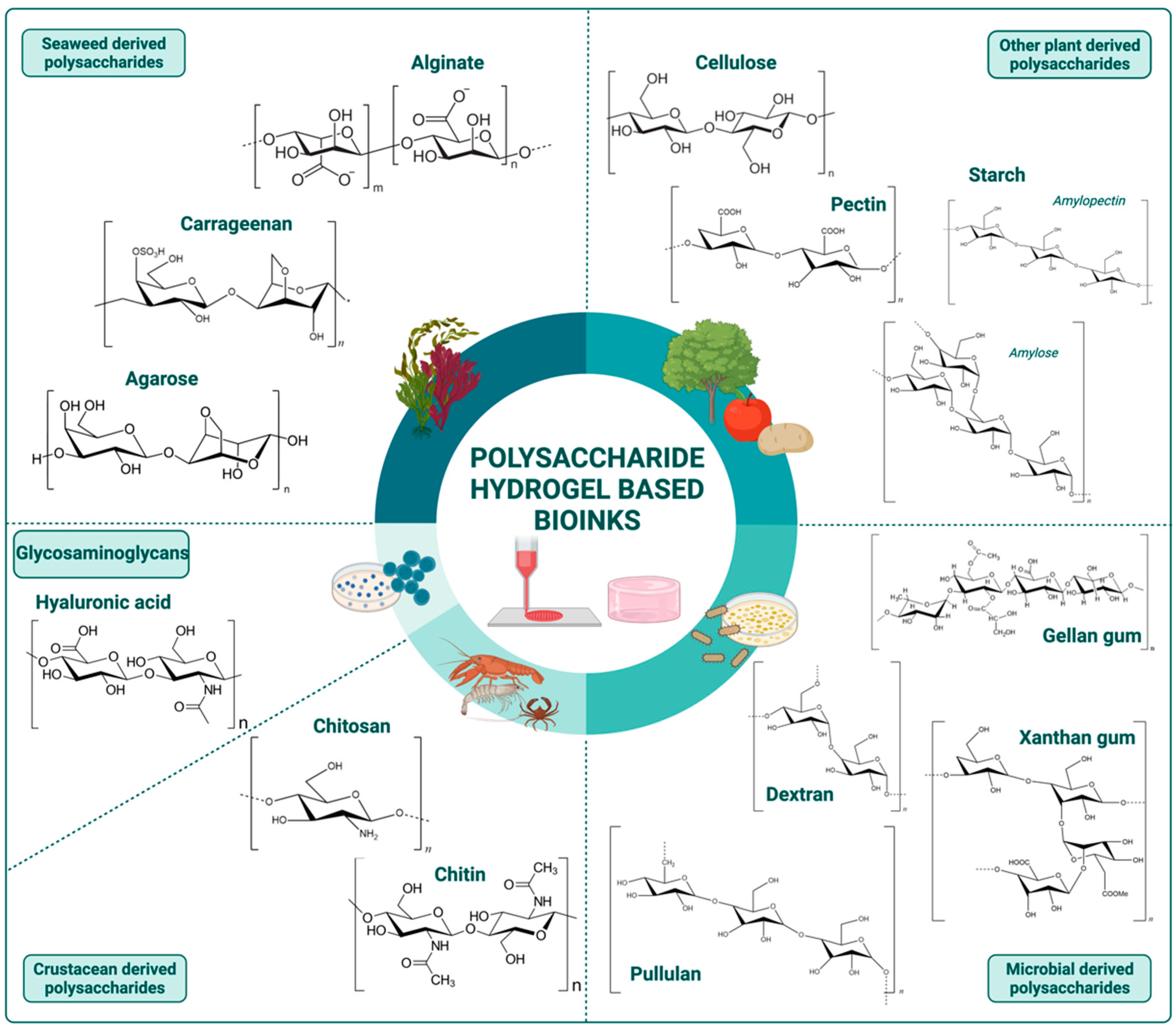
2. Polysaccharide-Based Hydrogel Bioinks
2.1. Seaweed Derived Polysaccharides
2.1.1. Alginate
2.1.2. Carrageenan
2.1.3. Agarose
2.2. Other Plants Derived Polysaccharides
2.2.1. Cellulose
2.2.2. Pectin
2.2.3. Starch
2.3. Microbial Derived Polysaccharides
2.3.1. Dextran
2.3.2. Xanthan Gum
2.3.3. Gellan Gum
2.3.4. Pullulan
2.4. Crustacean Derived Polysaccharides
2.5. Glycosaminoglycans
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hong, N.; Yang, G.-H.; Lee, J.; Kim, G. 3D Bioprinting and Its in Vivo Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef]
- Burke, M.; Carter, B.M.; Perriman, A.W. Bioprinting: Uncovering the Utility Layer-by-Layer. J. 3D Print. Med. 2017, 1, 165–179. [Google Scholar] [CrossRef]
- Chen, X.; Naghieh, S. Extrusion Bioprinting of Scaffolds. In Extrusion Bio-Printing of Scaffolds for Tissue Engineering Applications; Springer: Saskatoon, SK, Canada, 2019; pp. 9–10. ISBN 978-3-030-03459-7. [Google Scholar]
- Kumar, P.; Ebbens, S.; Zhao, X. Inkjet Printing of Mammalian Cells—Theory and Applications. Bioprinting 2021, 23, e00157. [Google Scholar] [CrossRef]
- Zennifer, A.; Subramanian, A.; Sethuraman, S. Design Considerations of Bioinks for Laser Bioprinting Technique towards Tissue Regenerative Applications. Bioprinting 2022, 27, e00205. [Google Scholar] [CrossRef]
- Zennifer, A.; Manivannan, S.; Sethuraman, S.; Kumbar, S.G.; Sundaramurthi, D. 3D Bioprinting and Photocrosslinking: Emerging Strategies & Future Perspectives. Biomater. Adv. 2022, 134, 112576. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Peng, W.; Ozbolat, V. Application Areas of 3D Bioprinting. Drug Discov. Today 2016, 21, 1257–1271. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Koçak, E.; Yıldız, A.; Acartürk, F. Three Dimensional Bioprinting Technology: Applications in Pharmaceutical and Biomedical Area. Colloids Surfaces B Biointerfaces 2021, 197, 111396. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Thorat, N.D.; Pricl, S.; Patil, R.M.; Rohiwal, S.; Townley, H. Bioink: A 3D-Bioprinting Tool for Anticancer Drug Discovery and Cancer Management. Drug Discov. Today 2021, 26, 1574–1590. [Google Scholar] [CrossRef]
- Williams, D.; Thayer, P.; Martinez, H.; Gatenholm, E.; Khademhosseini, A. A Perspective on the Physical, Mechanical and Biological Specifications of Bioinks and the Development of Functional Tissues in 3D Bioprinting. Bioprinting 2018, 9, 19–36. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Chen, Q.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Progress in Organ 3D Bioprinting. Int. J. Bioprinting 2018, 4, 128. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The Bioink: A Comprehensive Review on Bioprintable Materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Suntornnond, R.; An, J.; Chua, C.K. Roles of Support Materials in 3D Bioprinting. Int. J. Bioprinting 2017, 3, 83–86. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-Free Vascular Tissue Engineering Using Bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-Based 3D Bioprinting: A Comprehensive Review on Cell-Laden Hydrogels, Bioink Formulations, and Future Perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Bi, X.; Liang, A. In Situ-Forming Cross-linking Hydrogel Systems: Chemistry and Biomedical Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, A.L.E.-S.B., Ed.; InTech: Rijeka, Croatia, 2016; pp. 131–158. ISBN 978-953-51-2510-5. [Google Scholar]
- Silva, N.H.C.S.; Vilela, C.; Marrucho, I.M.; Freire, C.S.R.; Pascoal Neto, C.; Silvestre, A.J.D. Protein-Based Materials: From Sources to Innovative Sustainable Materials for Biomedical Applications. J. Mater. Chem. B 2014, 2, 3715. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2021, 27, 94. [Google Scholar] [CrossRef]
- Stanton, M.M.; Samitier, J.; Sánchez, S. Bioprinting of 3D Hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef]
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V.G. 3D Printing of Biopolymer Nanocomposites for Tissue Engineering: Nanomaterials, Processing and Structure-Function Relation. Eur. Polym. J. 2019, 121, 109340. [Google Scholar] [CrossRef]
- Diekjürgen, D.; Grainger, D.W. Polysaccharide Matrices Used in 3D in Vitro Cell Culture Systems. Biomaterials 2017, 141, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.R.; Patel, S.; Singh, D. Natural Polymer-Based Hydrogels as Scaffolds for Tissue Engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–260. ISBN 9780323428651. [Google Scholar]
- Mohan, T.; Maver, T.; Štiglic, A.D.; Stana-Kleinschek, K.; Kargl, R. 3D Bioprinting of Polysaccharides and Their Derivatives: From Characterization to Application. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.S.B.T.-F.B.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 105–141. ISBN 9780081021958. [Google Scholar]
- Axpe, E.; Oyen, M. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Smith, D.K. Multicomponent Polysaccharide Alginate-Based Bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic Acid as a Bioink for Extrusion-Based 3D Printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Xu, C. Nanocellulose-Based Inks for 3D Bioprinting: Key Aspects in Research Development and Challenging Perspectives in Applications—A Mini Review. Bioengineering 2020, 7, 40. [Google Scholar] [CrossRef]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Decante, G.; Costa, J.B.; Silva-Correia, J.; Collins, M.N.; Reis, R.L.; Oliveira, J.M. Engineering Bioinks for 3D Bioprinting. Biofabrication 2021, 13, 032001. [Google Scholar] [CrossRef]
- Ganpisetti, R.; Lalatsa, A. Cellulose Bio–Ink on 3D Printing Applications. J. Young Pharm. 2021, 13, 1–6. [Google Scholar] [CrossRef]
- Khoeini, R.; Nosrati, H.; Akbarzadeh, A.; Eftekhari, A.; Kavetskyy, T.; Khalilov, R.; Ahmadian, E.; Nasibova, A.; Datta, P.; Roshangar, L.; et al. Natural and Synthetic Bioinks for 3D Bioprinting. Adv. NanoBiomed Res. 2021, 1, 2000097. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent Trends in Natural Polysaccharide Based Bioinks for Multiscale 3D Printing in Tissue Regeneration: A Review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Khonakdar, H.A.; Arjmand, M.; Jafari, S.H.; Bagher, Z.; Moghaddam, Z.S.; Chimerad, M.; Sisakht, M.M.; Shojaei, S. Review of Bioprinting in Regenerative Medicine: Naturally Derived Bioinks and Stem Cells. ACS Appl. Bio Mater. 2021, 4, 4049–4070. [Google Scholar] [CrossRef]
- Pedroza-González, S.C.; Rodriguez-Salvador, M.; Pérez Benítez, B.E.; Alvarez, M.M.; Trujillo-de Santiago, G. Bioinks for 3D Bioprinting: A Scientometric Analysis of Two Decades of Progress. Int. J. Bioprinting 2021, 7, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Nanocellulosic Materials as Bioinks for 3D Bioprinting. Biomater. Sci. 2017, 5, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Saddique, A.; Cheong, I.W. Recent Advances in Three-Dimensional Bioprinted Nanocellulose-Based Hydrogel Scaffolds for Biomedical Applications. Korean J. Chem. Eng. 2021, 38, 2171–2194. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Jessop, Z.M.; Jovic, T.; Hawkins, K.; Whitaker, I.S. Candidate Bioinks for Extrusion 3D Bioprinting—A Systematic Review of the Literature. Front. Bioeng. Biotechnol. 2021, 9, 383. [Google Scholar] [CrossRef]
- Parimala Chelvi Ratnamani, M.; Zhang, X.; Wang, H. A Comprehensive Assessment on the Pivotal Role of Hydrogels in Scaffold-Based Bioprinting. Gels 2022, 8, 239. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Hosseini Rad, S.; Naderi, G.; Saeb, M.R.; et al. Chitosan-Based Inks for 3D Printing and Bioprinting. Green Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Zhou, K.; Sun, Y.; Yang, J.; Mao, H.; Gu, Z. Hydrogels for 3D Embedded Bioprinting: A Focused Review on Bioinks and Support Baths. J. Mater. Chem. B 2022, 10, 1897–1907. [Google Scholar] [CrossRef]
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D Printing of Nano-Cellulosic Biomaterials for Medical Applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34. [Google Scholar] [CrossRef]
- Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Choudhury, N.R. 3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review. Polymers 2019, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Kandasubramanian, B. Review of Alginate-Based Hydrogel Bioprinting for Application in Tissue Engineering. Biofabrication 2019, 11, 42001. [Google Scholar] [CrossRef]
- Badhe, R.V.; Godse, A.; Ahinkar, A. Biomaterials in 3D Printing: A Special Emphasis on Nanocellulose. Indian J. Pharm. Educ. Res. 2020, 54, 526–540. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef]
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Mokhtari, H.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Valiani, A.; Poursamar, S.A.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications. Materials 2020, 13, 3980. [Google Scholar] [CrossRef]
- Venugopal, V. Sulfated and Non-Sulfated Polysaccharides from Seaweeds and Their Uses: An Overview. EC Nutr. 2019, 14, 126–141. [Google Scholar]
- Arokiarajan, M.S.; Thirunavukkarasu, R.; Joseph, J.; Ekaterina, O.; Aruni, W. Advance Research in Biomedical Applications on Marine Sulfated Polysaccharide. Int. J. Biol. Macromol. 2022, 194, 870–881. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Qin, X.; Wa, Q. 3D Printing of Concentrated Alginate/Gelatin Scaffolds with Homogeneous Nano Apatite Coating for Bone Tissue Engineering. Mater. Des. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-Alginate as Bioink for Three-Dimensional (3D) Cell Printing Based Cartilage Tissue Engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Somasekharan, L.T.; Raju, R.; Kumar, S.; Geevarghese, R.; Nair, R.P.; Kasoju, N.; Bhatt, A. Biofabrication of Skin Tissue Constructs Using Alginate, Gelatin and Diethylaminoethyl Cellulose Bioink. Int. J. Biol. Macromol. 2021, 189, 398–409. [Google Scholar] [CrossRef]
- Schmid, R.; Schmidt, S.K.; Detsch, R.; Horder, H.; Blunk, T.; Schrüfer, S.; Schubert, D.W.; Fischer, L.; Thievessen, I.; Heltmann-Meyer, S.; et al. A New Printable Alginate/Hyaluronic Acid/Gelatin Hydrogel Suitable for Biofabrication of In Vitro and In Vivo Metastatic Melanoma Models. Adv. Funct. Mater. 2022, 32, 2107993. [Google Scholar] [CrossRef]
- Lewicki, J.; Bergman, J.; Kerins, C.; Hermanson, O. Optimization of 3D Bioprinting of Human Neuroblastoma Cells Using Sodium Alginate Hydrogel. Bioprinting 2019, 16, e00053. [Google Scholar] [CrossRef]
- Sarker, M.D.; Naghieh, S.; McInnes, A.D.; Ning, L.; Schreyer, D.J.; Chen, X. Bio-Fabrication of Peptide-Modified Alginate Scaffolds: Printability, Mechanical Stability and Neurite Outgrowth Assessments. Bioprinting 2019, 14, e00045. [Google Scholar] [CrossRef]
- Li, H.; Li, N.; Zhang, H.; Zhang, Y.; Suo, H.; Wang, L.; Xu, M. Three-Dimensional Bioprinting of Perfusable Hierarchical Microchannels with Alginate and Silk Fibroin Double Cross-Linked Network. 3D Print. Addit. Manuf. 2020, 7, 78–84. [Google Scholar] [CrossRef]
- Li, L.; Qin, S.; Peng, J.; Chen, A.; Nie, Y.; Liu, T.; Song, K. Engineering Gelatin-Based Alginate/Carbon Nanotubes Blend Bioink for Direct 3D Printing of Vessel Constructs. Int. J. Biol. Macromol. 2020, 145, 262–271. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Hu, Q.; Shen, Z.; Rana, D.; Ramalingam, M. Designing Vascular Supportive Albumen-Rich Composite Bioink for Organ 3D Printing. J. Mech. Behav. Biomed. Mater. 2020, 104, 103642. [Google Scholar] [CrossRef]
- Delkash, Y.; Gouin, M.; Rimbeault, T.; Mohabatpour, F.; Papagerakis, P.; Maw, S.; Chen, X. Bioprinting and In Vitro Characterization of an Eggwhite-Based Cell-Laden Patch for Endothelialized Tissue Engineering Applications. J. Funct. Biomater. 2021, 12, 45. [Google Scholar] [CrossRef]
- Dogan, L.; Scheuring, R.; Wagner, N.; Ueda, Y.; Schmidt, S.; Wörsdörfer, P.; Groll, J.; Ergün, S. Human IPSC-Derived Mesodermal Progenitor Cells Preserve Their Vasculogenesis Potential after Extrusion and Form Hierarchically Organized Blood Vessels. Biofabrication 2021, 13, 045028. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Seok, J.M.; Bin Bae, S.; Park, S.A.; Park, W.H. Silk Fibroin Enhances Cytocompatibilty and Dimensional Stability of Alginate Hydrogels for Light-Based Three-Dimensional Bioprinting. Biomacromolecules 2021, 22, 1921–1931. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable Kappa-Carrageenan Hydrogels for Tissue Engineering Applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef]
- Wilson, S.A.; Cross, L.M.; Peak, C.W.; Gaharwar, A.K. Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2017, 9, 43449–43458. [Google Scholar] [CrossRef]
- Chimene, D.; Peak, C.W.; Gentry, J.L.; Carrow, J.K.; Cross, L.M.; Mondragon, E.; Cardoso, G.B.; Kaunas, R.; Gaharwar, A.K. Nanoengineered Ionic–Covalent Entanglement (NICE) Bioinks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 9957–9968. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Liu, S.; Li, L. Three-Dimensional Bioprinting of Oppositely Charged Hydrogels with Super Strong Interface Bonding. ACS Appl. Mater. Interfaces 2018, 10, 11164–11174. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Li, L. A Strategy for Strong Interface Bonding by 3D Bioprinting of Oppositely Charged κ-Carrageenan and Gelatin Hydrogels. Carbohydr. Polym. 2018, 198, 261–269. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; del Pilar Ortega Arevalo, M.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-Based 3D Printing of Photo-Crosslinkable Gelatin and κ-Carrageenan Hydrogel Blends for Adipose Tissue Regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.W.; Jung, W.-K.; Oh, J.; Nam, S.Y. Enhanced Rheological Behaviors of Alginate Hydrogels with Carrageenan for Extrusion-Based Bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3D Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Senior, J.J.; Cooke, M.E.; Grover, L.M.; Smith, A.M. Fabrication of Complex Hydrogel Structures Using Suspended Layer Additive Manufacturing (SLAM). Adv. Funct. Mater. 2019, 29, 1904845. [Google Scholar] [CrossRef]
- Cidonio, G.; Cooke, M.; Glinka, M.; Dawson, J.I.; Grover, L.; Oreffo, R.O.C. Printing Bone in a Gel: Using Nanocomposite Bioink to Print Functionalised Bone Scaffolds. Mater. Today Bio 2019, 4, 100028. [Google Scholar] [CrossRef] [PubMed]
- Aydin, L.; Kucuk, S.; Kenar, H. A Universal Self-eroding Sacrificial Bioink That Enables Bioprinting at Room Temperature. Polym. Adv. Technol. 2020, 31, 1634–1647. [Google Scholar] [CrossRef]
- Butler, H.M.; Naseri, E.; MacDonald, D.S.; Tasker, R.A.; Ahmadi, A. Investigation of Rheology, Printability, and Biocompatibility of N,O-Carboxymethyl Chitosan and Agarose Bioinks for 3D Bioprinting of Neuron Cells. Materialia 2021, 18, 101169. [Google Scholar] [CrossRef]
- Kothari, D.; Das, D.; Patel, S.; Goyal, A.; Tripura, W.; Informatics, M.; Diego, S. Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-319-03751-6. [Google Scholar]
- Choe, G.; Park, J.; Park, H.; Lee, J. Hydrogel Biomaterials for Stem Cell Microencapsulation. Polymers 2018, 10, 997. [Google Scholar] [CrossRef]
- Lee, H.-R.; Jung, S.M.; Yoon, S.; Yoon, W.H.; Park, T.H.; Kim, S.; Shin, H.W.; Hwang, D.S.; Jung, S. Immobilization of Planktonic Algal Spores by Inkjet Printing. Sci. Rep. 2019, 9, 12357. [Google Scholar] [CrossRef]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium Alginate/Gelatine Hydrogels for Direct Bioprinting—The Effect of Composition Selection and Applied Solvents on the Bioink Properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef]
- Huettner, N.; Dargaville, T.R.; Forget, A. Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD. Trends Biotechnol. 2018, 36, 372–383. [Google Scholar] [CrossRef]
- Datta, P.; Ayan, B.; Ozbolat, I.T. Bioprinting for Vascular and Vascularized Tissue Biofabrication. Acta Biomater. 2017, 51, 1–20. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological Properties, Chemical Modifications and Structural Analysis – A Review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Geonzon, L.C.; Descallar, F.B.A.; Du, L.; Bacabac, R.G.; Matsukawa, S. Gelation Mechanism and Network Structure in Gels of Carrageenans and Their Mixtures Viewed at Different Length Scales – A Review. Food Hydrocoll. 2020, 108, 106039. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan Based Hydrogels for Drug Delivery, Tissue Engineering and Wound Healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Jafari, A.; Farahani, M.; Sedighi, M.; Rabiee, N.; Savoji, H. Carrageenans for Tissue Engineering and Regenerative Medicine Applications: A Review. Carbohydr. Polym. 2022, 281, 119045. [Google Scholar] [CrossRef] [PubMed]
- KapMA. Available online: https://www.adbioink.com/product/kappa-carrageenan-bioink/ (accessed on 19 May 2022).
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive Polysaccharides and Their Thermoreversible Physical Hydrogel Networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Pokusaev, B.; Vyazmin, A.; Zakharov, N.; Karlov, S.; Nekrasov, D.; Reznik, V.; Khramtsov, D. Thermokinetics and Rheology of Agarose Gel Applied to Bioprinting Technology. Therm. Sci. 2020, 24, 347–353. [Google Scholar] [CrossRef]
- Topuz, F.; Nadernezhad, A.; Caliskan, O.S.; Menceloglu, Y.Z.; Koc, B. Nanosilicate Embedded Agarose Hydrogels with Improved Bioactivity. Carbohydr. Polym. 2018, 201, 105–112. [Google Scholar] [CrossRef]
- Heidari, H.; Taylor, H. Multilayered Microcasting of Agarose–Collagen Composites for Neurovascular Modeling. Bioprinting 2020, 17, e00069. [Google Scholar] [CrossRef]
- BeMiller, J.N. Polysaccharides. In Carbohydrate Chemistry for Food Scientists; Elsevier: Amesterdam, The Netherlands, 2019; pp. 75–101. ISBN 9780128120699. [Google Scholar]
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in Biomedical Applications of Pectin Gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Zolfagharian, A.; Varley, R. Dynamic Plant-Derived Polysaccharide-Based Hydrogels. Carbohydr. Polym. 2020, 231, 115743. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D Printability of Alginate-Carboxymethyl Cellulose Hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Hodder, E.; Duin, S.; Kilian, D.; Ahlfeld, T.; Seidel, J.; Nachtigall, C.; Bush, P.; Covill, D.; Gelinsky, M.; Lode, A. Investigating the Effect of Sterilisation Methods on the Physical Properties and Cytocompatibility of Methyl Cellulose Used in Combination with Alginate for 3D-Bioplotting of Chondrocytes. J. Mater. Sci. Mater. Med. 2019, 30, 10. [Google Scholar] [CrossRef]
- Im, S.; Choe, G.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. An Osteogenic Bioink Composed of Alginate, Cellulose Nanofibrils, and Polydopamine Nanoparticles for 3D Bioprinting and Bone Tissue Engineering. Int. J. Biol. Macromol. 2022, 205, 520–529. [Google Scholar] [CrossRef]
- Ronzoni, F.L.; Aliberti, F.; Scocozza, F.; Benedetti, L.; Auricchio, F.; Sampaolesi, M.; Cusella, G.; Redwan, I.N.; Ceccarelli, G.; Conti, M. Myoblast 3D Bioprinting to Burst in Vitro Skeletal Muscle Differentiation. J. Tissue Eng. Regen. Med. 2022, 16, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Gómez-Florit, M.; Hamilton, A.G.; Detamore, M.S.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Human Platelet Lysate-Based Nanocomposite Bioink for Bioprinting Hierarchical Fibrillar Structures. Biofabrication 2019, 12, 015012. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N. Characterization of Κ-carrageenan/Methylcellulose/Cellulose Nanocrystal Hydrogels for 3D Bioprinting. Polym. Int. 2022, 71, 181–191. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, S.; Kang, Y.; Shan, X.; Li, Q.; Cai, Z. Biocompatibility Evaluation of a 3D-Bioprinted Alginate-GelMA-Bacteria Nanocellulose (BNC) Scaffold Laden with Oriented-Growth RSC96 Cells. Mater. Sci. Eng. C 2021, 129, 112393. [Google Scholar] [CrossRef]
- Das, R.; Lee, C.P.; Prakash, A.; Hashimoto, M.; Fernandez, J.G. Geometrical Control of Degradation and Cell Delivery in 3D Printed Nanocellulose Hydrogels. Mater. Today Commun. 2022, 30, 103023. [Google Scholar] [CrossRef]
- García-Lizarribar, A.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Castaño, A.G.; Samitier, J.; Ramon-Azcon, J. Composite Biomaterials as Long-Lasting Scaffolds for 3D Bioprinting of Highly Aligned Muscle Tissue. Macromol. Biosci. 2018, 18, 1800167. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yue, Z.; Lucarelli, E.; Wallace, G.G. Hybrid Printing Using Cellulose Nanocrystals Reinforced GelMA/HAMA Hydrogels for Improved Structural Integration. Adv. Healthc. Mater. 2020, 9, 2001410. [Google Scholar] [CrossRef]
- Ji, S.; Abaci, A.; Morrison, T.; Gramlich, W.M.; Guvendiren, M. Novel Bioinks from UV-Responsive Norbornene-Functionalized Carboxymethyl Cellulose Macromers. Bioprinting 2020, 18, e00083. [Google Scholar] [CrossRef]
- Li, X.; Deng, Q.; Zhuang, T.; Lu, Y.; Liu, T.; Zhao, W.; Lin, B.; Luo, Y.; Zhang, X. 3D Bioprinted Breast Tumor Model for Structure–Activity Relationship Study. Bio-Design Manuf. 2020, 3, 361–372. [Google Scholar] [CrossRef]
- Montheil, T.; Maumus, M.; Valot, L.; Lebrun, A.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. Inorganic Sol–Gel Polymerization for Hydrogel Bioprinting. ACS Omega 2020, 5, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Liu, M.; Zhang, Y.; Cao, Y.; Pei, R. 3D Bioprinting of Bone Marrow Mesenchymal Stem Cell-Laden Silk Fibroin Double Network Scaffolds for Cartilage Tissue Repair. Bioconjug. Chem. 2020, 31, 1938–1947. [Google Scholar] [CrossRef]
- Trachsel, L.; Johnbosco, C.; Lang, T.; Benetti, E.M.; Zenobi-Wong, M. Double-Network Hydrogels Including Enzymatically Crosslinked Poly-(2-Alkyl-2-Oxazoline)s for 3D Bioprinting of Cartilage-Engineering Constructs. Biomacromolecules 2019, 20, 4502–4511. [Google Scholar] [CrossRef]
- Gantumur, E.; Nakahata, M.; Kojima, M.; Sakai, S. Extrusion-Based Bioprinting through Glucose-Mediated Enzymatic Hydrogelation. Int. J. Bioprinting 2020, 6, 43–52. [Google Scholar] [CrossRef]
- Zidarič, T.; Milojević, M.; Gradišnik, L.; Stana Kleinschek, K.; Maver, U.; Maver, T. Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis. Nanomaterials 2020, 10, 733. [Google Scholar] [CrossRef]
- Lan, X.; Ma, Z.; Szojka, A.R.A.; Kunze, M.; Mulet-Sierra, A.; Vyhlidal, M.J.; Boluk, Y.; Adesida, A.B. TEMPO-Oxidized Cellulose Nanofiber-Alginate Hydrogel as a Bioink for Human Meniscus Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. A Single-Component Hydrogel Bioink for Bioprinting of Bioengineered 3D Constructs for Dermal Tissue Engineering. Mater. Horizons 2018, 5, 1100–1111. [Google Scholar] [CrossRef]
- Hu, S.; Martinez-Garcia, F.D.; Moeun, B.N.; Burgess, J.K.; Harmsen, M.C.; Hoesli, C.; de Vos, P. An Immune Regulatory 3D-Printed Alginate-Pectin Construct for Immunoisolation of Insulin Producing β-Cells. Mater. Sci. Eng. C 2021, 123, 112009. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chemie Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.B.; Figueiredo, A.R.P.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Development and Applications of Cellulose Nanofibres Based Polymer Nanocomposites. In Advanced Composite Materials: Properties and Applications; De Gruyter Open: Aveiro, Portugal, 2017; Volume 4, pp. 1–65. [Google Scholar]
- Li, Y.-Y.; Wang, B.; Ma, M.-G.; Wang, B. Review of Recent Development on Preparation, Properties, and Applications of Cellulose-Based Functional Materials. Int. J. Polym. Sci. 2018, 2018, 1–18. [Google Scholar] [CrossRef]
- Hon, D.N.-S. Cellulose: Chemistry and Technology. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1039–1045. ISBN 0-08-0431526. [Google Scholar]
- Jedvert, K.; Heinze, T. Cellulose Modification and Shaping—A Review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key Advances of Carboxymethyl Cellulose in Tissue Engineering & 3D Bioprinting Applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent Advancements in 3D Bioprinting Technology of Carboxymethyl Cellulose-Based Hydrogels: Utilization in Tissue Engineering. Adv. Colloid Interface Sci. 2021, 292, 102415. [Google Scholar] [CrossRef]
- Dufresne, A. Preparation of Microfibrillated Cellulose. In Nanocellulose: From Nature to High Performance Tailored Materials, 2nd ed.; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2012; pp. 43–75. ISBN 9783110480412. [Google Scholar]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chemie Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering Nanocellulose Hydrogels for Biomedical Applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D Printing with Cellulose Materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- CELLINK Bioink. Available online: https://www.cellink.com/product/cellink-bioink/ (accessed on 19 May 2022).
- Wu, Y.; Lin, Z.Y.; Wenger, A.C.; Tam, K.C.; Tang, X. 3D Bioprinting of Liver-Mimetic Construct with Alginate/Cellulose Nanocrystal Hybrid Bioink. Bioprinting 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Flutto, L. PECTIN Properties and Determination. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4440–4449. [Google Scholar]
- Narasimman, P.; Sethuraman, P. An Overview on the Fundamentals of Pectin. Int. J. Adv. Res. 2016, 4, 1855–1860. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Mishra, R.K.; Banthia, A.K.; Majeed, A.B.A. Pectin Based Formulations for Biomedical Applications: A Review. Asian J. Pharm. Clin. Res. 2012, 5, 1–7. [Google Scholar]
- Li, D.; Li, J.; Dong, H.; Li, X.; Zhang, J.; Ramaswamy, S.; Xu, F. Pectin in Biomedical and Drug Delivery Applications: A Review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef]
- Jovic, T.H.; Kungwengwe, G.; Mills, A.C.; Whitaker, I.S. Plant-Derived Biomaterials: A Review of 3D Bioprinting and Biomedical Applications. Front. Mech. Eng. 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Indurkar, A.; Pandit, A.; Jain, R.; Dandekar, P. Plant-Based Biomaterials in Tissue Engineering. Bioprinting 2021, 21, e00127. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Bean, S.R.; Zhu, L.; Smith, B.M.; Wilson, J.D.; Ioerger, B.P.; Tilley, M. Starch and Protein Chemistry and Functional Properties. In Sorghum and Millets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–170. ISBN 9780128115275. [Google Scholar]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A Review of Natural Polysaccharides for Drug Delivery Applications: Special Focus on Cellulose, Starch and Glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and Its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Carrow, J.K.; Kerativitayanan, P.; Jaiswal, M.K.; Lokhande, G.; Gaharwar, A.K. Polymers for Bioprinting. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 229–248. ISBN 9780128009727. [Google Scholar]
- Maniglia, B.C.; Lima, D.C.; Matta Junior, M.D.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Hydrogels Based on Ozonated Cassava Starch: Effect of Ozone Processing and Gelatinization Conditions on Enhancing 3D-Printing Applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Noè, C.; Tonda-Turo, C.; Chiappone, A.; Sangermano, M.; Hakkarainen, M. Light Processable Starch Hydrogels. Polymers 2020, 12, 1359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, T.; Wu, L.; Han, Q.; Chen, S.; Kong, Y.; Li, G.; Ma, L.; Wu, H.; Zhao, Y.; et al. Fabrication and Characterization of 3D-Printed Gellan Gum/Starch Composite Scaffold for Schwann Cells Growth. Nanotechnol. Rev. 2021, 10, 50–61. [Google Scholar] [CrossRef]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.M.; Seesuriyachan, P. Microbial Exopolysaccharides for Immune Enhancement: Fermentation, Modifications and Bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Busuioc, M.; Mackiewicz, K.; Buttaro, B.A.; Piggot, P.J. Role of Intracellular Polysaccharide in Persistence of Streptococcus Mutans. J. Bacteriol. 2009, 191, 7315–7322. [Google Scholar] [CrossRef]
- Turner, P.R.; Murray, E.; McAdam, C.J.; McConnell, M.A.; Cabral, J.D. Peptide Chitosan/Dextran Core/Shell Vascularized 3D Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 32328–32339. [Google Scholar] [CrossRef]
- Lim, W.; Shin, S.Y.; Cha, J.M.; Bae, H. Optimization of Polysaccharide Hydrocolloid for the Development of Bioink with High Printability/Biocompatibility for Coextrusion 3D Bioprinting. Polymers 2021, 13, 1773. [Google Scholar] [CrossRef]
- Kapr, J.; Petersilie, L.; Distler, T.; Lauria, I.; Bendt, F.; Sauter, C.M.; Boccaccini, A.R.; Rose, C.R.; Fritsche, E. Human Induced Pluripotent Stem Cell-Derived Neural Progenitor Cells Produce Distinct Neural 3D In Vitro Models Depending on Alginate/Gellan Gum/Laminin Hydrogel Blend Properties. Adv. Healthc. Mater. 2021, 10, 2100131. [Google Scholar] [CrossRef]
- Piola, B.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Renò, F. 3D Bioprinting of Gelatin–Xanthan Gum Composite Hydrogels for Growth of Human Skin Cells. Int. J. Mol. Sci. 2022, 23, 539. [Google Scholar] [CrossRef]
- Muthusamy, S.; Kannan, S.; Lee, M.; Sanjairaj, V.; Lu, W.F.; Fuh, J.Y.H.; Sriram, G.; Cao, T. 3D Bioprinting and Microscale Organization of Vascularized Tissue Constructs Using Collagen-based Bioink. Biotechnol. Bioeng. 2021, 118, 3150–3163. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of Tissue Constructs by 3D Bioprinting of Cell-Laden Microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R.; Stewart, E.M.; Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3D Printing of Layered Brain-like Structures Using Peptide Modified Gellan Gum Substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Hu, D.; Wu, D.; Huang, L.; Jiao, Y.; Li, L.; Lu, L.; Zhou, C. 3D Bioprinting of Cell-Laden Scaffolds for Intervertebral Disc Regeneration. Mater. Lett. 2018, 223, 219–222. [Google Scholar] [CrossRef]
- Wu, D.; Yu, Y.; Tan, J.; Huang, L.; Luo, B.; Lu, L.; Zhou, C. 3D Bioprinting of Gellan Gum and Poly (Ethylene Glycol) Diacrylate Based Hydrogels to Produce Human-Scale Constructs with High-Fidelity. Mater. Des. 2018, 160, 486–495. [Google Scholar] [CrossRef]
- Zhuang, P.; Ng, W.L.; An, J.; Chua, C.K.; Tan, L.P. Layer-by-Layer Ultraviolet Assisted Extrusion-Based (UAE) Bioprinting of Hydrogel Constructs with High Aspect Ratio for Soft Tissue Engineering Applications. PLoS ONE 2019, 14, e0216776. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, X.; Liu, X.; Cui, R.; Wang, C.; Zhao, G.; Zhi, W.; Lu, M.; Duan, K.; Weng, J.; et al. 3D Bioprinting of Shear-Thinning Hybrid Bioinks with Excellent Bioactivity Derived from Gellan/Alginate and Thixotropic Magnesium Phosphate-Based Gels. J. Mater. Chem. B 2020, 8, 5500–5514. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.B.; Park, J.; Jorgensen, A.M.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Atala, A.; Yoo, J.J.; Lee, S.J. 3D Bioprinted Highly Elastic Hybrid Constructs for Advanced Fibrocartilaginous Tissue Regeneration. Chem. Mater. 2020, 32, 8733–8746. [Google Scholar] [CrossRef]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Leuconostoc Dextransucrase and Dextran: Production, Properties and Applications. J. Chem. Technol. Biotechnol. 2005, 80, 845–860. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Ng, J.Y.; Obuobi, S.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Biomimicry of Microbial Polysaccharide Hydrogels for Tissue Engineering and Regenerative Medicine – A Review. Carbohydr. Polym. 2020, 241, 116345. [Google Scholar] [CrossRef] [PubMed]
- García-Ochoa, F.; Santos, V.; Casas, J.; Gómez, E. Xanthan Gum: Production, Recovery, and Properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan Gum Derivatives: Review of Synthesis, Properties and Diverse Applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- GelXG. Available online: https://www.cellink.com/product/gelxg/ (accessed on 19 May 2022).
- GelXA. Available online: https://www.cellink.com/product/gelxa/ (accessed on 19 May 2022).
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan Gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, I.B.; Survase, S.A.; Saudagar, P.S.; Singhal, R.S. Gellan Gum: Fermentative Production, Downstream Processing and Applications. Food Technol. Biotechnol. 2007, 45, 341–354. [Google Scholar]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; Panhuis, M. Tissue Engineering with Gellan Gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef]
- GumMA. Available online: https://www.adbioink.com/product/gellan-gum-bioink/ (accessed on 19 May 2022).
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, Production, and Applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial Sources, Production and Applications. Carbohydr. Polym. 2008, 73, 515–531. [Google Scholar] [CrossRef]
- Qi, X.; Su, T.; Zhang, M.; Tong, X.; Pan, W.; Zeng, Q.; Shen, J. Sustainable, Flexible and Biocompatible Hydrogels Derived from Microbial Polysaccharides with Tailorable Structures for Tissue Engineering. Carbohydr. Polym. 2020, 237, 116160. [Google Scholar] [CrossRef]
- Della Giustina, G.; Gandin, A.; Brigo, L.; Panciera, T.; Giulitti, S.; Sgarbossa, P.; D’Alessandro, D.; Trombi, L.; Danti, S.; Brusatin, G. Polysaccharide Hydrogels for Multiscale 3D Printing of Pullulan Scaffolds. Mater. Des. 2019, 165, 107566. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Tokura, S.; Tamura, H. Chitin and Chitosan. In Comprehensive Glycoscience; Elsevier: Amsterdam, The Netherlands, 2007; Volume 2–4, pp. 449–475. ISBN 9780444519672. [Google Scholar]
- Parhi, R. Drug Delivery Applications of Chitin and Chitosan: A Review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, X.; Li, L.; Chen, Z.-N.; Gao, G.; Yao, R.; Sun, W. 3D Printing Human Induced Pluripotent Stem Cells with Novel Hydroxypropyl Chitin Bioink: Scalable Expansion and Uniform Aggregation. Biofabrication 2018, 10, 1–27. [Google Scholar] [CrossRef]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan Based Hydrogels: Characteristics and Pharmaceutical Applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, T.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Du, S.; Yang, X.; Pang, S.; Wang, X.; Yang, S. Preparation of a Recombinant Collagen-Peptide (RHC)-Conjugated Chitosan Thermosensitive Hydrogel for Wound Healing. Mater. Sci. Eng. C 2021, 119, 111555. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Chitoink. Available online: https://www.cellink.com/product/chitoink/ (accessed on 19 May 2022).
- Bergonzi, C.; Di Natale, A.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R.; Elviri, L. Study of 3D-Printed Chitosan Scaffold Features after Different Post-Printing Gelation Processes. Sci. Rep. 2019, 9, 362. [Google Scholar] [CrossRef]
- Ku, J.; Seonwoo, H.; Park, S.; Jang, K.-J.; Lee, J.; Lee, M.; Lim, J.W.; Kim, J.; Chung, J.H. Cell-Laden Thermosensitive Chitosan Hydrogel Bioinks for 3D Bioprinting Applications. Appl. Sci. 2020, 10, 2455. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. DLP Printing Photocurable Chitosan to Build Bio-Constructs for Tissue Engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Włodarczyk-Biegun, M.K.; del Campo, A.; Vázquez-Lasa, B.; San Román, J. 3D Printing of a Reactive Hydrogel Bio-Ink Using a Static Mixing Tool. Polymers 2020, 12, 1986. [Google Scholar] [CrossRef]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E.; et al. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable in Vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef]
- Hu, T.; Cui, X.; Zhu, M.; Wu, M.; Tian, Y.; Yao, B.; Song, W.; Niu, Z.; Huang, S.; Fu, X. 3D-Printable Supramolecular Hydrogels with Shear-Thinning Property: Fabricating Strength Tunable Bioink via Dual Crosslinking. Bioact. Mater. 2020, 5, 808–818. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Scocozza, F.; Mariotti, C.; Chiesa, E.; Bruni, G.; Genta, I.; Auricchio, F.; Conti, M.; Conti, B. Preliminary Investigation on a New Natural Based Poly(Gamma-Glutamic Acid)/Chitosan Bioink. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1–15. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Carmagnola, I.; Chiappone, A.; Feng, Z.; Ciardelli, G.; Hakkarainen, M.; Sangermano, M. Photocurable Chitosan as Bioink for Cellularized Therapies towards Personalized Scaffold Architecture. Bioprinting 2020, 18, e00082. [Google Scholar] [CrossRef]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological Production of Hyaluronic Acid: A Mini Review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef]
- Badri, A.; Williams, A.; Linhardt, R.J.; Koffas, M.A. The Road to Animal-Free Glycosaminoglycan Production: Current Efforts and Bottlenecks. Curr. Opin. Biotechnol. 2018, 53, 85–92. [Google Scholar] [CrossRef]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally Derived Proteins and Glycosaminoglycan Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 78, 1277–1299. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Lee, S.J.; Seok, J.M.; Lee, J.H.; Lee, J.; Kim, W.D.; Park, S.A. Three-Dimensional Printable Hydrogel Using a Hyaluronic Acid/Sodium Alginate Bio-Ink. Polymers 2021, 13, 794. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Wu, Y.; Yu, M.; Aazmi, A.; Gao, L.; Xue, Q.; Luo, Y.; Zhou, H.; Zhang, B.; et al. 3D Bioprinted Hyaluronic Acid-Based Cell-Laden Scaffold for Brain Microenvironment Simulation. Bio-Design Manuf. 2020, 3, 164–174. [Google Scholar] [CrossRef]
- Hauptstein, J.; Forster, L.; Nadernezhad, A.; Horder, H.; Stahlhut, P.; Groll, J.; Blunk, T.; Teßmar, J. Bioink Platform Utilizing Dual-Stage Crosslinking of Hyaluronic Acid Tailored for Chondrogenic Differentiation of Mesenchymal Stromal Cells. Macromol. Biosci. 2022, 22, 2100331. [Google Scholar] [CrossRef]
- Hauptstein, J.; Forster, L.; Nadernezhad, A.; Groll, J.; Teßmar, J.; Blunk, T. Tethered TGF-Β1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues. Int. J. Mol. Sci. 2022, 23, 924. [Google Scholar] [CrossRef]
- PhotoHATM-IRG. Available online: https://www.sigmaaldrich.com/PT/en/product/aldrich/917079 (accessed on 19 May 2022).
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink Properties before, during and after 3D Bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
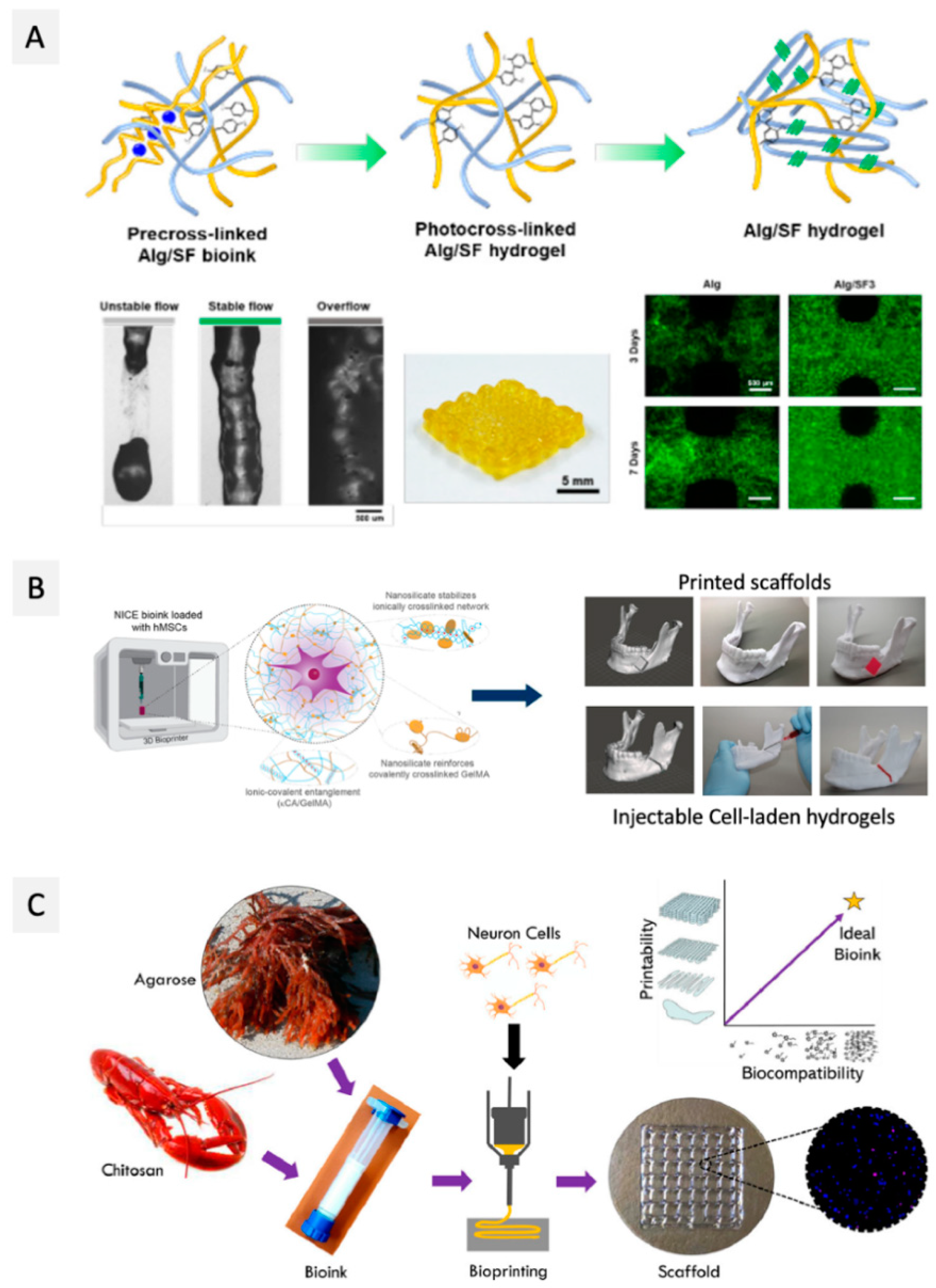




| Polysaccharide | Other Compounds | Cell Type | Bioink Formulation | Bioprinting Method | Conditions | Construct Properties | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Alginate | Gelatin Nano-apatite | rBMSC (1 × 105 cells/mL) | Alginate: 6 wt.% Gelatin: 10 wt.% Nano-apatite: 0.1 and 0.5 M Crosslinker: CaCl2 1 M | EB | Nozzle: 0.610 mm Printing speed: 5 mm/s Pressure: 0.5 MPa Temperature: 55 °C | Grid-like scaffolds with 10 × 10 × 5 mm3 Compressive strength: 20.7 ± 4.7 to 23.9 ± 1.5 MPa Young’s modulus: 119 ± 26 to 135 ± 36 MPa Cell viability: higher in nano-apatite coated scaffolds with osteogenic differentiation | Bone tissue engineering | [55] |
| Alginate | Agarose Collagen (Type I) | Primary chondrocytes (1 × 107 cells/mL) | Alginate: 0.1 g/mL Agarose: 15 mg/mL (Alg/Col blend in a ratio of 4:1 and Alg/Agr blend in a ratio of 3:1) Crosslinker: CaCl2 10% (w/v) | EB | Nozzle: 0.260 mm | Grid-like structures 2 × 2 cm2 with 6 layers Compressive modulus: ~50–65 kPa Tensile strength: ~40–45 kPa Cell viability: 95% at day 14 in the Alg/Col blend | Cartilage tissue engineering | [56] |
| Alginate | -- | SK-N-BE cells (1 × 107 cells/mL) | Alginate: 2% (w/v) Crosslinker: CaCl2 in a gelatin support medium | EB | Nozzle: 0.255 mm Printing speed: 8 mm/s Pressure: 12.5 psi | Grid-like geometries Cell viability: 83% at day 7 | – | [59] |
| Alginate | -- | RPSCs (2 × 105 cells/mL) | Alginate: 2% (w/v) engrafted with RGD and YIGSR peptides Crosslinker: CaCl2 50 mM | EB | Nozzle: 0.200 mm Printing speed: 18 mm/s Pressure: 0.3 Bar | Cubic shape scaffold (10 × 10 × 5 mm3) with 1 mm distance between strands Young’s modulus: 40.3 ± 2.2, 23.7 ± 3.5, 14.7 ± 3.6, and 14.5 ± 2.7 kPa at day 0, 7, 14, and 21, respectively Cell viability: ~100% at day 7 | Nerve tissue engineering | [60] |
| Alginate | Silk fibroin Pluronic F127 | C3A (1 × 106 cells/mL) | Alginate: 5% (w/v) SF: 5% (w/v) Pluronic F127: 13% (w/v) Crosslinker: CaCl2 5% (w/v) | Co-axial EB | Shell Nozzle: 1.070 mm Core Nozzle: 0.340 mm Printing speed: 15 mm/s Bioink extrusion rate: 7 mL/s Cross-linker solution extrusion rate: 5 mL/s | Grid-like scaffolds with the size of 20 × 20 × 3 mm3 Compressive modulus: 16 ± 2.5 kPa Cell viability: ~100% at day 14 | – | [61] |
| Alginate | Gelatin Carbon nanotubes | Fibroblasts (4 × 105 cells/mL) | CNTs: 0.5 and 1%(w/v) Crosslinker: CaCl2 | EB | Modified printer for bioprinting of hollow tubular scaffolds Non specified conditions | Circular tubes printed with a 3 mm diameter, an average wall thickness of 0.5 mm and a length of 7–10 cm Young’s modulus: ~1.4 and 0.7 MPa Tensile strength: ~0.9 and 0.5 MPa Cell viability: 85% survival rate until 5 days, with mild toxicity induced by CNTs | Vessels tissue engineering | [62] |
| Alginate | Albumen (Egg white) | HUVECs (6 × 106 cells/mL) | Alginate: 5% (w/v) Albumen (egg white) added in volume ratios of 1:1, 2:1, 3:1, 4:1, 5:1 and 6:1. Crosslinker: CaCl2 | EB | Nozzle: 0.160 mm Pressure: 2.5 Psi CaCl2 perfusion bath | Grid structures with 30 × 30 × 1 mm3 and 25 × 25 × 1 mm3 Cell viability: high up to 5 days with the formation of vascularized channels | – | [63] |
| Alginate | Albumen (Egg white) | HUVECs (1.25 × 106 cells/mL) | Alginate: 2–3% (w/v) Dissolved in Albumen (egg white) Crosslinker: CaCl2 500 mM | EB | Nozzle: 0.500 mm Printing speed: 9,10 and 11 mm/s Pressure: 0.3, 0.5 and 0.7 bar | Patches with 12 × 12 mm2 and 8 mm height Elastic modulus: 20–27 kPa Cell viability: 94% at day 7 | Endothelialized tissue engineering | [64] |
| Alginate | Collagen type I | hiMPCs (2.5 × 106 to 1 × 107 cells/mL) | Alginate: 2% (w/v) Collagen: 0.015% (w/v) With the addition of VEGF growth factor. Crosslinker: CaCl2 20 mM | EB | Nozzle: 0.455 mm Pressure: 100 kPa | Printed spherical discs Cell viability: after 21 days, it was observed the formation of small and large vessels that were transplanted into the chicken embryo chorioallantoic membrane (CAM) model and showed proper blood perfusion | Blood vessels tissue engineering | [65] |
| Alginate | SFMA | NIH-3T3 (5 × 106 cells/mL) | Alginate: 3 wt.% SFMA: 1,3 and 5 wt.% Crosslinker: CaCO3 and UV | EB | Pressure: 10 to 100 kPa Printing speed: 300 to 900 mm/s | Grid like scaffolds with the size of 20 × 20 × 3.5 mm3 Young’s modulus decreases with the increments of SFMA concentration. Cell viability: 95% at day 7 | – | [66] |
| Alginate | Hyaluronic acid Gelatin | Mel Im (1 × 106 cells/mL) ADSCs (1 × 107 cells/mL) | Alginate: 0.5% (w/v) HA: 0.1% (w/v) Gelatin: 3% (w/v) Crosslinker: CaCl2 100 mM | EB | Nozzle: 0.580 mm Printing speed: 400 mm/min Pressure: 10–15 kPa | Grids with 1 cm2 with 3 layers and 6 strands each Cell viability: ~100% at day 14 | In vitro and in vivo metastatic melanoma models | [58] |
| Alginate | Gelatin DCEL | Primary adult dermal fibroblasts (5 × 106 cells/mL) Primary epidermal keratinocytes (7 × 106 cells/mL) | Alginate: 2% (w/v) Gelatin: 3.3% (w/v) DCEL: 0.93% (w/v) Crosslinker: CaCl2 100 mM | EB | Nozzle: 0.410 mm Pressure: 120 kPa | Three-layered, disc-shaped constructs of 15 mm diameter, about 3 mm height for characterization Rectangular-shaped, single-layered construct (15 mm width, 15 mm length and 1 mm height) for cell culture Young’s modulus: 125 ± 22 kPa Elongation of break: 91.70 ± 9.36% Cell viability: at 21 days of culture, histological analysis showed the formation of both dermal and epidermal equivalent structures | Skin tissue engineering | [57] |
| Carrageenan | nSi | MC3T3-E1 (N/A) | Carrageenan: 2.5 wt.% nSi: 6 wt.% Crosslinker: CaSO4 1% (w/v) Gelling temperature: 35 °C | EB | Nozzle: 0.340 mm Printing speed: 4 mm/s Extrusion flow rate: 0.3 mL/h | (i) Single fiber in a lattice network and a layered lattice network; (ii) 30-layer cylinder; (iii) nose and ear models. Compressive modulus: 208 ± 6.5 kPa G’ recovery: 95% Cell viability: 99% at day 7 | – | [68] |
| Carrageenan | nSi GelMA | MC3T3-E1 (1 × 106 cells/mL) | Carrageenan: 1% (w/v) nSi: 2% (w/v) GelMA: 10% (w/v) Crosslinker: KCl and UV | EB | Nozzle: 0.400 mm Printing speed: 20 mm/s Extrusion flow rate: 0.15 mL/h | (i) Single fiber in a lattice network and a layered lattice network; (ii) 30-layer cylinder; (iii) nose and ear models. Compressive modulus: 71.1 ± 4.9 kPa G’ recovery: 75% Cell viability: >90% at day 120 | – | [69] |
| Carrageenan | Gelatin | C2C12 (2.8 × 105 cells/mL in Gel) | Carrageenan: 2% (w/v) Gel: 8% (w/v) Gelling temperature: 25 °C | EB | Nozzle: 0.250 mm Temperature: 25 °C | Grid-like scaffolds with 25 × 25 mm2 Cell viability: 90% at day 1 | – | [71] |
| Carrageenan | GelMA | C2C12 (3 × 105 cells/mL in Gel MA) | Carrageenan: 2% (w/v) GelMA: 10% (w/v) Crosslinker: KCl and UV | EB | Nozzle: 0.250 mm Temperature: 25 °C | Grid-like scaffolds with 25 × 25 mm2, line space: 1.3 mm, and 4 layers Cell viability: 96% at day 7 | – | [70] |
| Carrageenan | Alginate | MSCs (5 × 105 cells/mL) | Carrageenan: 1.5% (w/v) Alginate: 2% (w/v) Crosslinker: CaSO4 1% (w/v) | EB | Nozzle: 0.510 mm Printing speed: 2 mm/s Pressure: 50 kPa | Grid-like scaffolds with 25 × 25 mm2, line space: 1.3 mm, and 4 layers Storage modulus: 900 Pa Cell viability: higher in Alg-Crg bioinks at day 3 | – | [73] |
| Carrageenan-MA | GelMA | ADSCs (1 × 105 cells/scaffold) | Crg-MA: 1% (w/v) GelMA: 10% (w/v) Crosslinker: UV | EB | Nozzle: 0.210 mm Printing speed: 650 mm/min | Grid-like structure with 10 × 10 mm2 and 10 layers in height Young’s modulus: 2.2 to 2.5 kPa Cell viability: >80% at day 14 | Adipose tissue regeneration | [72] |
| Carrageenan | nSi GelMA GAG’s proteoglycans | hMSCs | Carrageenan: 1% (w/v) nSi: 2% (w/v) GelMA: 7.5% (w/v) Crosslinker: KCl and UV | EB | Nozzle: 0.400 mm Printing speed: 20 mm/s Extrusion flow rate: 0.15 mL/h | Mandibular models Compressive modulus: 141 ± 8 kPa Cell viability: high with differentiation until day 90 (histological analysis) | Bone tissue engineering | [74] |
| Agarose | Alginate | Auricular cartilage digested with Collagenase Type 4 cell suspension | Agarose: 2, 3 and 4% (w/v), combined with alginate in a ratio of 3:2 Gelling temperature: 25 °C | EB | Nozzle: 0.160 mm Pressure: 65–75 Psi | Constructs printed as single lines (print width = 0.5 mm, length = 30 mm) Compressive yield: ~15–20 kPa Cell viability: >~70% cell survival at day 28 | Tissue engineering | [75] |
| Agarose | NOCC | neuro2A (1 × 105 cells/mL) | Agr stock solution: 1% (w/v) NOOC stock solution: 10% (w/v) Agr-NOCC 80:20, 60:40, 40:60 and 20:80 | EB | Nozzle: 0.410 mm Printing speed: 3 mm/s | Grid-like scaffolds with 20 × 20 × 0.5 mm3 Storage modulus: 20 Pa (Agr-NOCC 40:60); 25 Pa (Agr-NOCC 40:60) Printability numbers: 0.95 (Agr-NOCC 40:60); 0.99 (Agr-NOCC 40:60) Cell viability: 100% at day 14 (Agr-NOCC 40:60) | – | [79] |
| Polysaccharide | Other Compounds | Cell Type | Bioink Formulation | Bioprinting Method | Conditions | Construct Properties | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| CMC | Sodium alginate | Human pancreatic cancer cells (2 × 106 cells/mL) | Alginate: 4% (w/v) Alg:CMC: 4:1, 2:1, 4:3 and 1:1 (dry mass) Crosslinker: CaCl2 4% (w/v) | EB | Nozzle: 0.410 mm Printing speed: 5 mm/s Pressure: 8 psi | Cubic model (10 × 10 × 2 mm3 with 1 mm of filament distance) was printed Young’s modulus: >75 kPa for >4% CMC Cell viability: >70% for alginate/CMC, for 15 and 23 days after bioprinting | – | [100] |
| Sodium carboxymethyl cellulose methacrylate | GelMA, AlgMA PEGDA | C2C12 (1 × 107 cells/mL) | GelMA: 1 or 5% (w/v) CMCMA, AlgMA, or PEGDA: 1% (w/v) Crosslinker: UV | EB | Nozzle: 0.200 mm Printing speed: 7 mm/s Pressure: 2.5 bar Temperature: 10 °C | Cylindrical model (10 mm in diameter) Compressive modulus: 1.96 ± 0.16 kPa (GelMA-CMCMA) Cell viability: 60% | Muscle tissue engineering | [109] |
| Methyl cellulose | Alginate | Bovine primary chondrocytes (5 × 106 cells/g) | Alginate: 3 wt.% MC: 9 wt.% Crosslinker: CaCl2 100 mM | EB | Nozzle: 0.610 mm Printing speed: 10 mm/s Pressure: 70–80 kPa | Cubic model (9.5 × 9.5 × 1.4 mm3) Compressive strength: 45.2 ± 8.0 MPa for UV-treated, 32.1 ± 6.8 MPa for the autoclaved, and 27.7 ± 4.6 MPa for scCO2Cell viability: >50% for all samples, except for scCO2-treated | – | [101] |
| NorCMC and cCMC | N/A | hMSCs, NIH 3T3 and HUVECs (1 × 107 cells/mL) | cCMC: 15% (w/v) NorCMC: 10% (w/v) Thiol: norbornene: to 1:4, 1:2 and 1:1. Crosslinker: UV | EB | Printing speed: 5–10 mm/s Increased pressure from 1368 kPa (30 min) to 276 kPa (60 min) and 345 kPa (90 min) | Grid-like construct (15 × 15 mm2) Compression modulus: 46 to 316 kPa when increasing from 1:4 to 1:2, for cCMC, and from 40 to 133 kPa for NorCMC Cell viability: >80% for all cell lines | – | [111] |
| Hydroxyethyl cellulose | Sodium alginate, Gelatin | MCF-7 (107 cells/mL) | Sodium alginate: 1% (w/v) Gelatin: 5% (w/v) Hydoxyethyl cellulose: 1% (w/v) Crosslinker: CaCl2 1.5% (w/v) | EB | Printing speed: 5 mm/s Temperature: 25ºC | Cylindrical model (9 × 8 mm2); spheroid model and human ear structure Compressive modulus: 13 kPa for hydroxyethyl cellulose-reinforced constructs Cell viability: 98% | Breast tumor model | [112] |
| Hydroxypropyl methyl cellulose-Si | NaF and glycine | hMSCs (1.106 cells/mL) | NaF and/or glycine was added to obtain a final HPMC-Si concentration of 135 g/L Gelation temperature: room temperature | EB | Nozzle: 0.210 mm Printing speed: 10 mm/s Pressure: 3 bar Temperature: 37 °C | Grid-like structures Young’s modulus: 99 ± 15 kPa Cell viability: LIVE/DEAD assay indicated that 3D bioprinting did not affect cells since most green living cells were observed at day 1 and till day 7 | – | [113] |
| Hydroxypropyl methyl cellulose methacrylate | Silk fibroin | BMSCs (1 × 106 cells/mL) | Hydroxypropyl methyl cellulose methacrylate: 5 wt.% Silk fibroin: Hydroxypropyl methyl cellulose methacrylate: 4:0, 3:1, 2:2, 1:4 and 0:4 Crosslinker: UV | EB | Nozzle: 0.160 mm Printing speed: 20 mm/s Pressure: 30–80 kPa | Ring-like structure (8 mm diameter); cylindrical (8 × 4 mm2) open structure and human ear structure Compressive stress: above 100 kPa for proportion 3:1 Cell viability: nonnegligible cell dead 46% at day 1 and decreased to 3% at day 10 | Cartilage tissue repair | [114] |
| NFC | Poly(2-ethyl-2-oxazoline), Sortase A and alginate | hACs (107 cells/mL) | Poly(2-ethyl-2-oxazoline): 5% (w/v) Alginate: 5% (w/v) NFC: 0.5, 1.0, 1.5 and 2.0% (w/v) Crosslinker: Sortase A 100 µM and CaCl2 10 mM | EB | Nozzle: 0.410 mm Pressure: 18–21 kPa | Grid-like structures Compressive modulus: ~30 kPa Cell viability: 90 ± 2% | Cartilage tissue engineering | [115] |
| NFC | Horseradish peroxidase, glucose, and alginate | 10T1/2 (5 × 105 cells/mL) | NFC: 0.5–1.5% (w/v) Alginate: 0.5% (w/v) Crosslinker: horseradish peroxidase 100 (units/mL) | EB | Nozzle: 0.210 mm Printing speed: 22 mm/s | Lattice structure (20 × 21 mm2) and human nose (12 × 15 mm2) Cell viability: 54.1 ± 0.6% at day 1 and 56.0 ± 2.4% at day 7 | – | [116] |
| NFC | Alginate, CMC | hSF (106 cells/mL) | Alginate: 3 wt.% CMC: wt. 3% NFC: 1.5 wt.% Crosslinker: CaCl2 2 wt.% | EB | Nozzle: 0.250 mm | Cylinder-shaped structure (10 × 0.8 mm2) Cell viability: LIVE/DEAD assay indicated a homogeneous cell distribution | In vitro model of the human dermis | [117] |
| NFC | Alginate | hMFC (107 cells/mL) | NFC:Alg: 01:00, 20:80, 50:50, 60:40, 70:30, 80:20 and 90:10, with a solid content of 3.5% (w/v) Crosslinker: CaCl2 100 mM | EB | Nozzle: 0.413 mm Printing speed: 10 mm/s Pressure: 55–200 kPa | Block (20 × 20 × 3 mm3) Peak modulus <10 kPa for the 10–40% cumulative strain Cell viability: >60% | Human meniscus tissue engineering | [118] |
| NFC | Alginate and polydopamine nanoparticles | MC3T3-E1 (6 × 103 cells/cm2) | Alginate: 2.1, 1.5 and 0.9% (w/v) NFC: 2.1, 1.5 and 0.9% (w/v) Polydopamine nanoparticles: 0.5% (w/v) Crosslinker: CaCl2 5% (w/v) | EB | Nozzle: 0.500 mm Printing speed: 5 mm/s | Grid structure (20 × 20 mm2) Compressive modulus: 2.03 ± 0.31 kPa (higher for 1.5% (w/v) of alginate and NFC with 0.5% (w/v) polydopamine nanoparticles) Cell viability: >75% | Bone tissue engineering | [102] |
| NFC | Alginate and fibrinogen | C2C12 (25 × 106 cells/mL) | Commercial inks: gelatin methacrylate and alginate crosslinked by UV light (CELLINK® GelMA A); (2) gelatin methacrylate, xanthan gum, and alginate- fibrinogen (CELLINK® GelXA FIBRIN); (3) nanofibrillated cellulose (NFC)/alginate-fibrinogen crosslinked with CaCl2 and thrombin (CELLINK ® FIBRIN) | EB | Nozzle: 0.250 mm Printing speed: 16 mm/s Pressure: 10–15 kPa | Lines (length: 20 mm and thickness: 0.35 mm) Cell viability: >90% | Skeletal muscle regeneration | [103] |
| CNC | Platelet lysate | hASCs (1 × 106 cells/mL) | Aldehyde CNC: 18 wt.% Platelet lysate: 2.88 wt.% Crosslinker: CaCl2 10 mM | EB | Nozzle: 0.210 mm Printing speed: 5 mm/s Temperature: 20 °C | Square lattice (1 × 1 × 0.25 cm2) Cell viability: >90% | – | [104] |
| CNC | Gelatin methacryloyl and hyaluronic acid methacrylate | ATDC5 (1 × 106 cells/mL) | CNC: 1, 5, 10 and 15% (w/v) GelMA: 10% (w/v) HAMA: 2% (w/v) Crosslinker: UV | EB | Nozzle: 0.200 mm Printing speed: 8–12 mm/s Pressure: 2–4 bar | Cuboid structures (10 × 10 × 1.5 mm3) Compressive modulus: 22.7 ± 2.8 kPa to 55.8 ± 2.1 kPa with increasing CNC loading to 10% (w/v) Cell viability: >90% until 7 days after bioprinting | – | [110] |
| CNC | Chitosan, hydroxyethyl cellulose | MC3T3-E1 (5 × 106 cells/mL) | Chitosan: 3% (w/v) Hydroxyethyl cellulose (0–0.5 mg/mL) CNC: 0–2% (w/v) Crosslinker: β-glycerophosphate 100 mM and | EB | Nozzle: 0.900 mm Printing speed: 2 mm/s Pressure: 20 kPa | Cylindrical scaffolds (7.5 × 4 mm2) Young’s modulus: 85.12 ± 4.31 Pa for chitosan, to 122.12 ± 13.84 Pa for 0.5% CNC and to 132.40 ± 2.55 Pa for 1.5% CNC Cell viability: qualitative analysis through LIVE/DEAD indicated that bioprinting cell-laden bioinks did not comprise cell viability | – | [105] |
| CNC | k-carrageenan and methylcellulose | L929 (3 × 105 cells/mL) | k-carrageenan: 0.3 wt.% Methylcellulose: 7 wt.% CNC: 2 or 4 wt.% | EB | Nozzle: 0.200 mm Printing speed: 1 mm/s Pressure: 110 kPa Temperature: 25 °C | Grid-like constructs (10 × 10 cm2) Compressive stress: 20.03 ± 0.02 and 23.28 ± 0.01 kPa when increasing CNC content from 2 to 4 wt.% Cell viability: >90% | – | [106] |
| BC | Alginate and GelMA | RSC96 (15 × 106 cells/mL) | Alginate: 5% (w/v) GelMA: 5% (w/v) BC: 0.3% (w/v) Crosslinker: CaCl2 50 mM and blue light | EB | Nozzle: 0.160 mm Printing speed: 30 mm/s Temperature: 20–25 °C | Cuboid structure (8 × 8 × 2 mm3), cylinder (5 × 4 mm2) Compressive modulus: 2.25 kPa to 10.92 kPa with the incorporation of BC Cell viability: the addition of BC did not affect cell proliferation as cell proliferation absorbance increase to > 3 at day 7 | – | [107] |
| TEMPO oxidized bacterial NFC | N/A | R1/E (3 × 107 cells/mL) | TEMPO oxidized bacterial NFC: 1% (w/v) Crosslinker: N/A | EB | Nozzle: 0.900 mm Room temperature | Grid-like constructs Cell viability: LIVE/DEAD assay showed that only at day 7 cells started to stretch and elongate | – | [108] |
| Pectin methacrylate | N/A | Human neonatal dermal fibroblasts (1.5 or 2.5 wt.%) | Pectin methacrylate: 1.5–2.5 wt.% Crosslinker: UV | EB | Nozzle: 0.642 mm | Cuboid structures (8 × 8 × 4.5 mm3 and 17 × 17 × 2.4 mm3) Cell viability: LIVE/DEAD showed that after 24 h post-printing the printed constructs displayed viable cells | Dermal tissue engineering | [119] |
| Pectin | Pluronic F127 and alginate | MIN6 (1 × 107 cells/mL) | Pectin: 2 wt.% Alginate: 6 wt.% Pluronic F127: 8 wt.% Crosslinker: CaCl2 5 mM | EB | Nozzle: 0.455 mm Printing speed: 4 mm/s Pressure: 50 psi Temperature: 30 ± 3 °C | Grid-like structures (8 × 2 mm2) Cell viability: ≥80 ± 3.7% during the 7 days of culture | – | [120] |
| Polysaccharide | Other Compounds | Cell Type | Bioink Formulation | Bioprinting Method | Conditions | Construct Properties | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Dextran | GelMA, succinylated chitosan and dextran aldehyde | hBMSC (1.0 × 106 cells/mL) and HUVEC (1.0 × 106 cells/mL) | Succinylated chitosan: 8% (w/v) Dextran aldehyde: 0.6% (w/v) GelMA: 13% (w/v) Crosslinker: UV | EB | Nozzle: 0.400 mm Printing speed: 5 mm/s Pressure: 150–225 kPa Temperature: 25 °C | Core/shell structure (12 × 12 × 4 mm3) Young’s modulus: 100 kPa for GelMA and 50 kPa for chitosan-dextran hydrogel Cell viability: cell growth increased until day 21 | Wound healing | [151] |
| Xanthan gum | GelMA, alginate and CMC | hMSCS (2.5 × 106 cells/mL) | GelMA: 10% (w/v) Alginate: 2% (w/v) CMC: 1 or 2% (w/v) Xanthan gum: 1 or 2% (w/v) Crosslinker: UV and CaCl2 | EB | Nozzle: 0.515 mm | Grid-like structures Young’s modulus: >40 kPa for UV + ionic (with Ca2+)-crosslinked hydrogels, >20 kPa for UV-crosslinked and <20 kPa ion-crosslinked Cell viability: >80% | – | [152] |
| Xanthan gum | Collagen type 1 | ECs/hESC-ECs (10 × 106 cells/mL) and hESC-FBs (2 × 106 cells/mL) | Xanthan gum: 10% (w/v) Collagen: 4.73 mg/mL | EB | Nozzle: 0.410 mm Printing speed: 15 mm/s Pressure: 29–35 kPa | Grid-like constructures (10 ×10 × 3 mm3) Cell viability: 92.39 ± 2.02% at 24 h post printing and 89.40 ± 2.58% at 48 h post printing | – | [155] |
| Xanthan gum | Gelatin | Primary human-derived-skin fibroblasts (0.5 × 106 cells/mL) and HaCaTs (5 × 106 cells/mL) | Xanthan gum: 0.3, 0.7 1 and 1.2% (w/v) Gelatin: 2.5 and 3% (w/v) Crosslinker: glutaraldehyde 0.3, 0.5 and 1% (v/v) | EB | Nozzle: 0.250 mm Pressure: 10–20 kPa | Grid-like constructs (1 cm2) Cell viability: bioprinting process did not affect the cell viability, as no sign of cell death was visible on day 1 after bioprinting | – | [154] |
| Gellan gum | Poly(lactic acid), GelMA | Mesenchymal stromal cells (10 × 106 cells/mL) | Gellan gum: 1% (w/v) GelMA: 10% (w/v) Crosslinker: UV | EB | Nozzle: 0.908 mm Printing speed: 7.9 mm/s Temperature: room temperature | Grid-like structures (2.25 mm line spacing) Cell viability: >80% after 3 days | – | [156] |
| Gellan gum modified with RGD | N/A | Primary cortical neurons (1 × 106 cells/mL) | RGD-gellan gum: 1% (w/v) Crosslinker: CaCl2 1 M | EB | Nozzle: 0.200 mm | Cylindrical structure Cell viability: >70% until day 7 after bioprinting. | – | [157] |
| Gellan gum | PEGDA | BMSCs (2 × 106 cells/mL) | Gellan gum: 0.75 wt.% PEGDA: 15 wt.% Crosslinker: UV | EB | Nozzle: 0.515 mm Temperature: 37 °C | Rectilinear and honeycomb structures Young’s modulus: higher values for honeycomb structures than for rectilinear Cell viability: >90% | Intervertebral disc regeneration | [158] |
| Gellan gum | PEGDA | BMSCs and MC3T3-E1 (2 × 106 cells/mL) | Gellan gum: 1.0, 1.5, 2.0 wt.% PEGDA: 0, 5.0, 10.0 and 15.0 wt.% Crosslinker: UV | EB | Nozzle: 0.515 mm Printing speed: 10 mm/s Temperature: 37 °C | Sharp cone (10 mm in diameter and height), square prism (bottom diameter 10 mm, top diameter 10 mm, height 10 mm) and human scale ear and nose Young’s modulus: UV crosslinking of G1.5P10 (chosen formulation) caused an improvement in the Young’s modulus from 40 kPa to 60 kPa Cell viability: >87% | – | [159] |
| Gellan gum | GelMA | C2C12 (4 × 106 cells/mL) | GelMA: 2, 4, 10, 15, 20, 30% (w/v) Gellan gum: 0, 0.2, 0.4, 1, 1.5% (w/v) Crosslinker: UV | EB | Nozzle: 0.410 mm Printing speed: 1.7 mm/s Pressure: 1.2 bar Temperature: 25 °C | Grid pattern (9 × 9 × 10 mm3) and tubular structure Compressive modulus: 9–16 kPa Cell viability: maintained > 95% at all time points (0, 7 and 14 days). | Soft tissue engineering | [160] |
| Gellan gum | Sodium alginate and thixotropic magnesium phosphate-based gel | MG-63 (1 × 106 cells/mL) | Sodium alginate: 2.5 or 4.0% (w/v) Gellan gum: 3.0 or 2.0% (w/v) Sodium alginate-gellan gum to thixotropic magnesium phosphate-based gel (1.5:1) Crosslinker: UV | EB | Nozzle: 0.410 mm Printing speed: 0.005 mL/s | Grid-like constructs (20 × 20 mm3), human mandible, university symbol abbreviation and human nose Compressive stiffness: 299 ± 71 kPa for 2.0% gellan gum and 4.0% (w/v) sodium alginate Cell viability: relative proliferation rate >100% 5 and 7 days after bioprinting | Osteochondral repair | [161] |
| Gellan gum | Fibrinogen | pMCs (1.5 × 107 cells/mL) | Gellan gum: 12 mg/mL Fibrinogen: 25, 50, 75, 100, 125, and 150 mg/mL Crosslinker: thrombin 20 (U/mL) and UV | EB | Nozzle: 0.240 mm Printing speed: 250 mm/min Pressure: 45–65 kPa | Cuboid structures (10 × 10 × 5 mm3) Compressive elastic modulus: ink with 100 mg/mL of fibrinogen had the highest with values increasing from 13.6 ± 1.5 to 23.1 ± 2.7 KPa at 3% strain, 14.8 ± 2.1 to 24.3 ± 1.8 KPa at 6% strain and 17.9 ± 3.2 to 27.5 ± 2.3 KPa at 12% Cell viability: >90% during the culture | Fibrocartilaginous tissue regeneration | [162] |
| Gellan gum | Alginate and laminin | hiNPCs | Two different alginate-gellan gum blends were prepared: 1.5% (w/v) alginate, 0.5% (w/v) gellan gum and 0.01% (w/v) laminin, and 0.3% (w/v) alginate, 0.8% (w/v) and 0.01% (w/v) laminin gellan gum Crosslinker: CaCl2 0.09 M | EB | Nozzle: 0.200 mm Printing speed: 4.1 mm/s Pressure: 450–550 kPa | Grid-like structures Elastic modulus: 0.3% Alg-0.8% gellan gum-0.01% laminin had the lowest elastic modulus (20 kPa) when compared to 1.5% Alg-0.5% gellan gum-0.01% laminin (35 kPa) Cell viability: 60% | In vitro neural models | [153] |
| Polysaccharide | Other Compounds | Cell Type | Bioink Formulation | Bioprinting Method | Conditions | Construct Properties | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chitosan | D-(+)-raffinose pentahydrate | Primary human skin fibroblasts | Chitosan: 6% (w/v) D-(+)-raffinose pentahydrate: 290 mM Crosslinkers: KOH 1.5 M Na2CO3 1.5 M, ammonia vapours | EB | Nozzle: 0.260 mm Printing speed: 3 mm/s | Grid structure with 1.6 × 1.6 cm2 Young’s modulus: KOH: 105 kPa ± 18 kPa; Na2CO3: 94 kPa ± 19 kPa; ammonia vapours 128 kPa ± 21 kPa Cell viability: enhanced cell growth up to 21 days | – | [190] |
| Chitosan | - | PDLSCs (5 × 105 cells/mL) | Chitosan: 1.67% (w/v) Crosslinker: K2HPO4 and NaHCO3 | EB | N/A | Lattice-type structure (thickness of 2 mm × 8-layer height) Cell viability: high viability for 7 days. | – | [191] |
| Chitosan methacrylate | LAP | HUVECs (1 × 106 cells/mL) | Chitosan: 1% (w/v) LAP: 0.2 wt.% Crosslinker: UV | EB/DLP | DLP photocuring conditions: 405 nm, 15 mW/cm2, 15 s | Lattice structure with 10 × 10 × 1 mm3 Compressive modulus: Increased from 315 kPa to 910 kPa, with CSMA. | Tissue engineering | [192] |
| Carboxymethyl chitosan | Oxidized and non-oxidized hyaluronic acid | L929 (1 × 106 cells/mL) | Carboxymethyl chitosan: 2 wt.% Hyaluronic acid: 0.4 wt.% Oxidized hyaluronic acid: 4 wt.% Crosslinker: FeCl3 20 mM | EB | Nozzle: 0.200 mm Printing speed: 5–25 mm/s | 2- and 4-layered grid square scaffolds (12 × 12 mm2 printed area) Cell viability: 96% at day 7 and 95% at day 14 | – | [193] |
| Chitosan | Gelatin PEG-Star-Ma | U87 (7 × 105 cells/mL) | Chitosan/Gelatin/PEG-Star-ma ratio = 1:3:0.05% (w/v) Gelling temperature: 37 °C | EB | Nozzle: 0.410 mm Pressure: 25–35 kPa Temperature: 37 °C | Grid-like structure Cell viability: maintained for 6 days | In vitro models | [194] |
| Chitosan | PEG, α-cyclodextrin and gelatin | MSCs (1 × 107 cells/mL) | CS-PEG at 30 mg.mL. Crosslinker: β-glycerophosphate | EB | Nozzle: 0.300–0.400 mm | 3D columnar structures (10 mm diameter × 3 mm thickness) Young’s modulus: 4 kPa to 130 kPa MSCs differentiated better towards adipose cells in bioink with a Young’s modulus of 60 kPa, while bioinks with 10–20 kPa favoured the MSCs differentiation towards neuron-like cells. | – | [195] |
| Chitosan | Gamma-PGA | Human adult fibroblasts (2 × 105 cells/mL) | Chitosan: 4.5% and 6% (w/v) Gamma-PGA: 2–20% (w/v) Gelling temperature: 37 °C | EB | Nozzle: 0.700 mm (CS) 0.500 mm (Gamma-PGA) Pressure: 25–40 kPa (CS) 5–10 kPa (Gamma-PGA) Temperature: 37 °C Printing speed: 10 mm/s | Rectangular grid structure with 20 × 10 × 1.2 mm3 Cell viability: ~70% after 24 h | – | [196] |
| Chitosan methacrylate | β-glycerol phosphate salt | NIH 3T3 (1 × 106 cells/mL) | Chitosan: 1.5% (w/v) Crosslinker: UV | EB | Nozzle: 0.720, 0.510 and 0.410 mm Temperature: 37 °C | Grid-like structure The developed hydrogel was non-cytotoxic After bioprinting, NIH 3T3 cells were well dispersed and proliferation was observed. | – | [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. Int. J. Mol. Sci. 2022, 23, 6564. https://doi.org/10.3390/ijms23126564
Teixeira MC, Lameirinhas NS, Carvalho JPF, Silvestre AJD, Vilela C, Freire CSR. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. International Journal of Molecular Sciences. 2022; 23(12):6564. https://doi.org/10.3390/ijms23126564
Chicago/Turabian StyleTeixeira, Maria C., Nicole S. Lameirinhas, João P. F. Carvalho, Armando J. D. Silvestre, Carla Vilela, and Carmen S. R. Freire. 2022. "A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications" International Journal of Molecular Sciences 23, no. 12: 6564. https://doi.org/10.3390/ijms23126564
APA StyleTeixeira, M. C., Lameirinhas, N. S., Carvalho, J. P. F., Silvestre, A. J. D., Vilela, C., & Freire, C. S. R. (2022). A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. International Journal of Molecular Sciences, 23(12), 6564. https://doi.org/10.3390/ijms23126564










