A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Ionic Environment and Incubation Temperature
Abstract
:1. Introduction
2. Results and Discussion
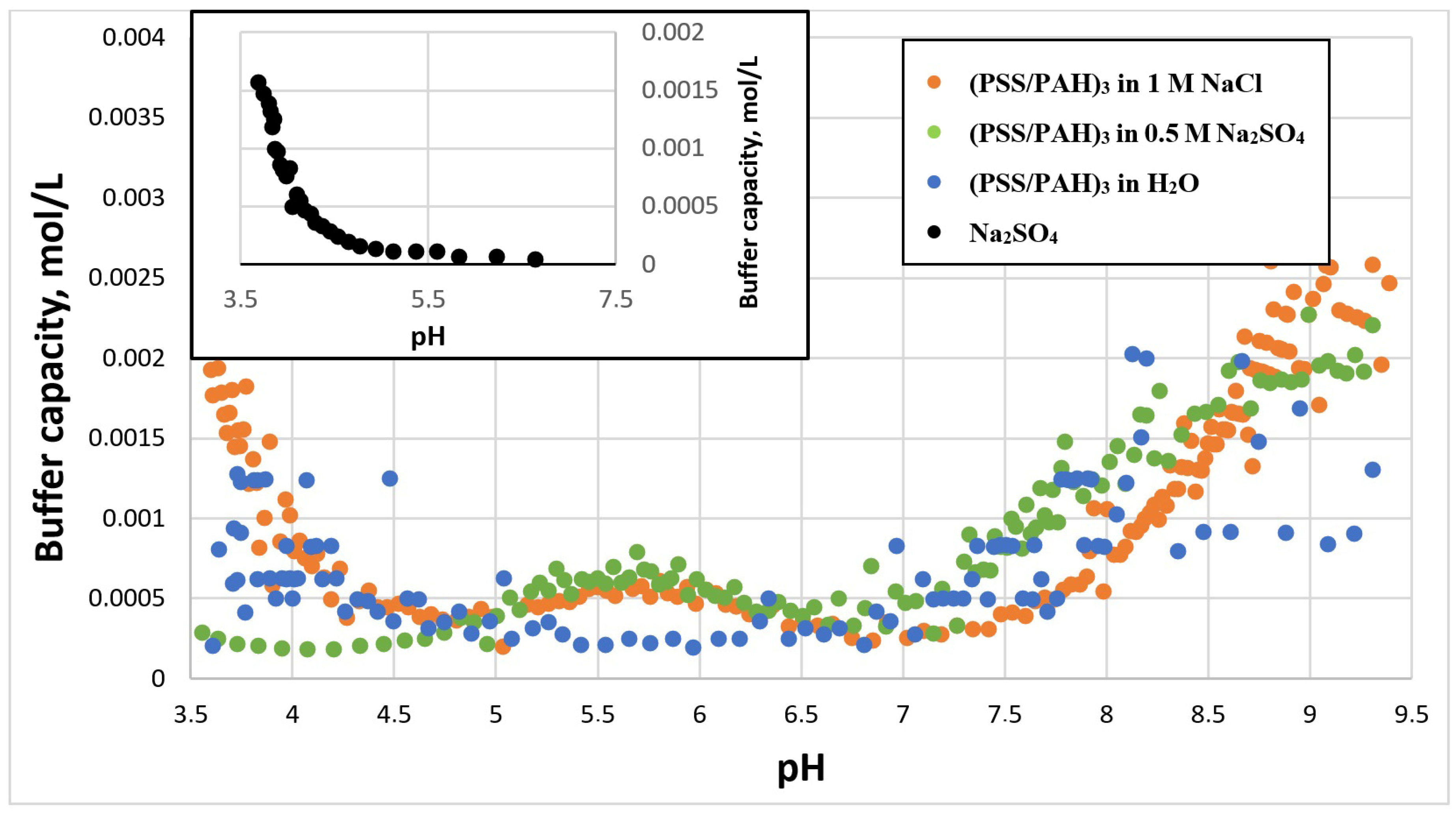
2.1. Study of the Buffer Capacity of Microcapsules Depending on the Type of Salt
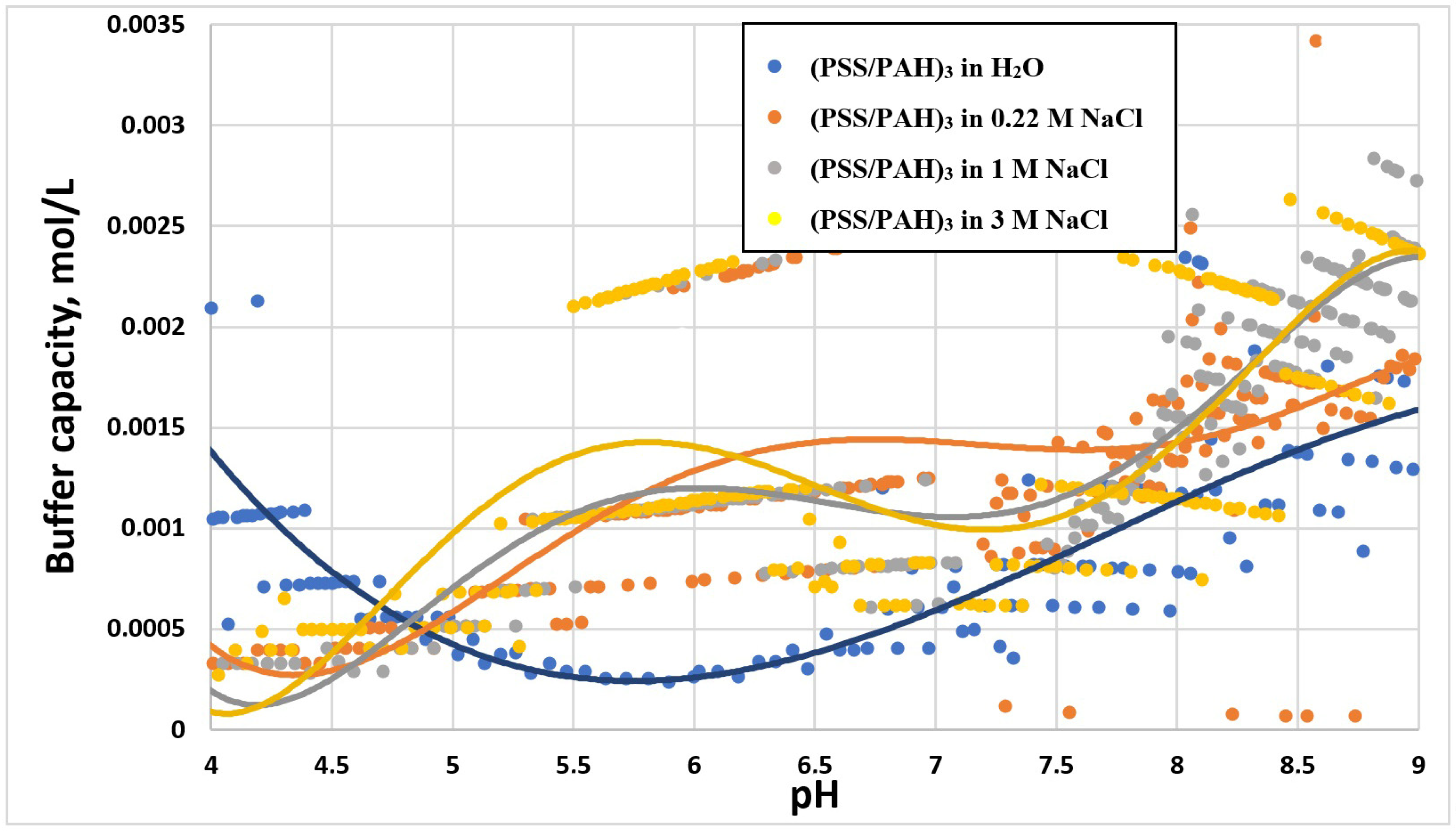
2.2. Study of the Buffer Capacity of PMC in Different Salt Concentrations
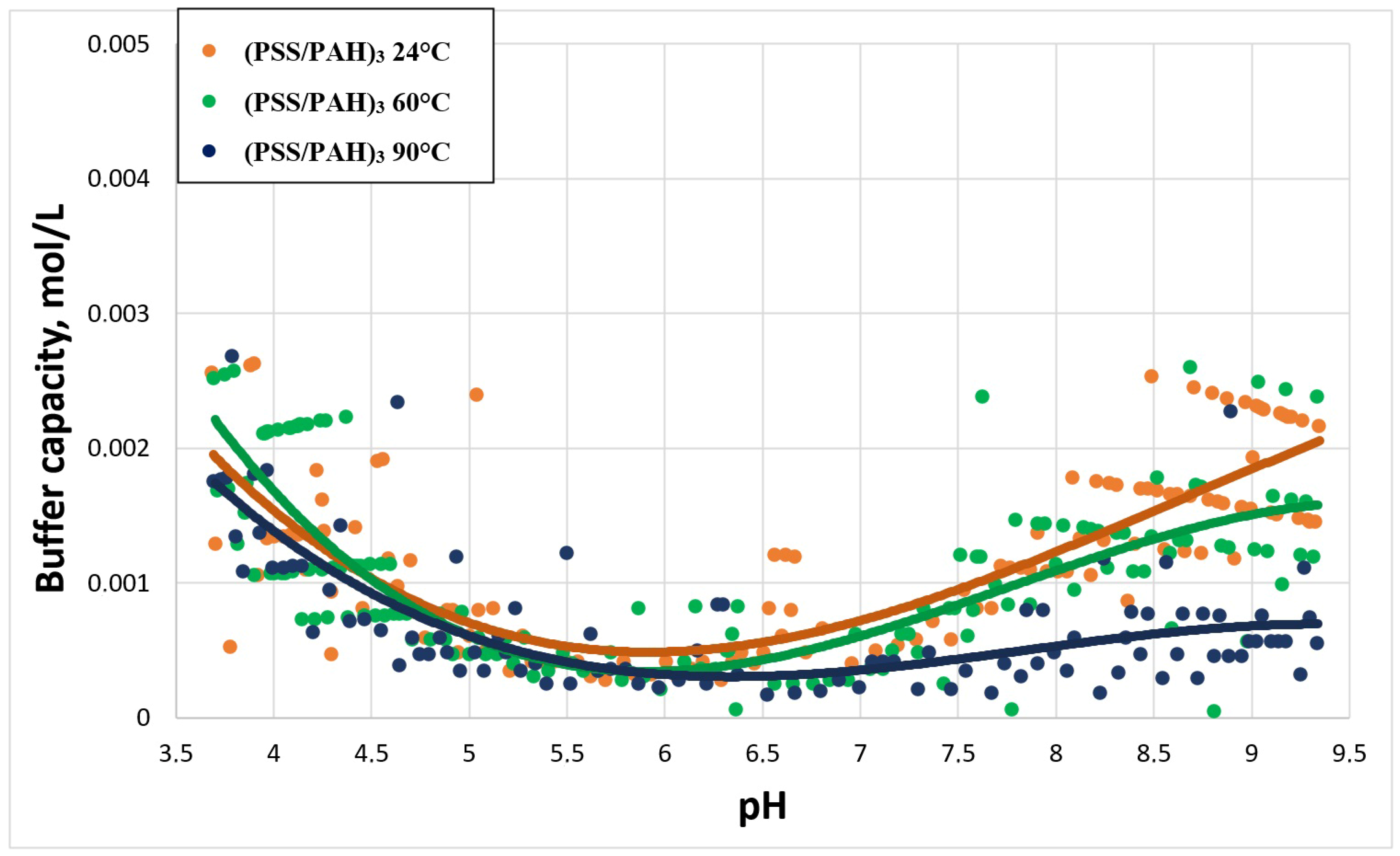
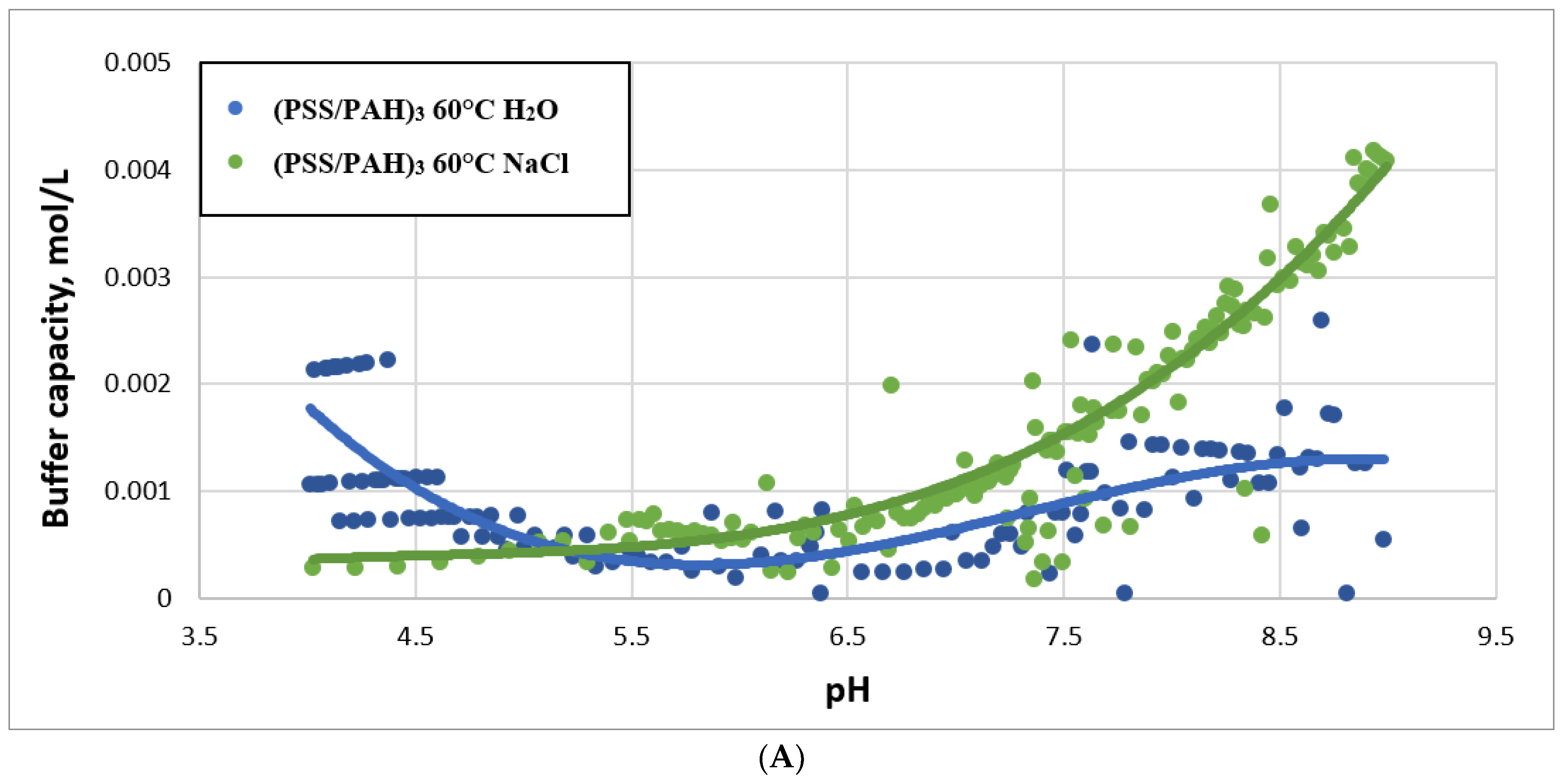
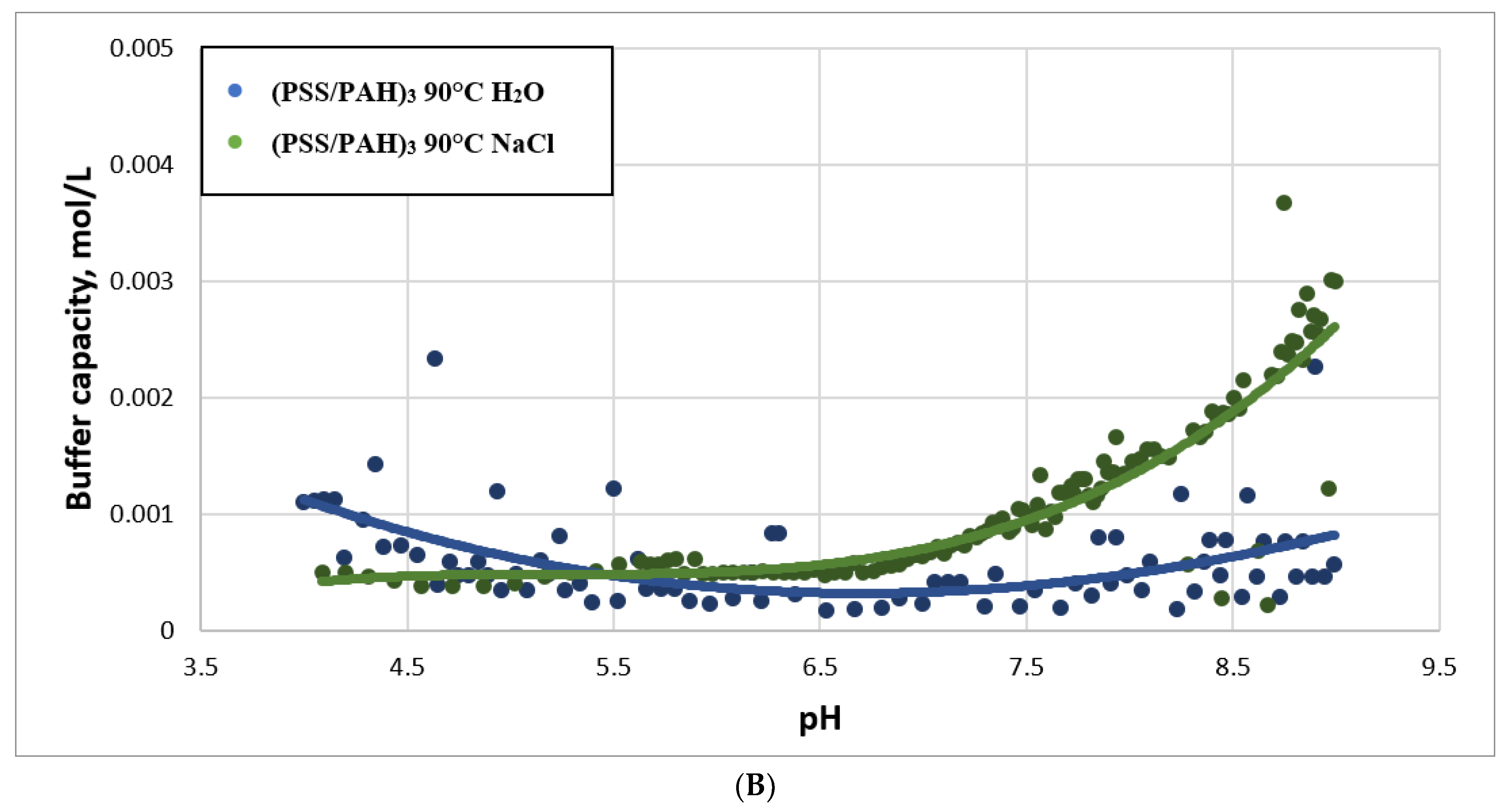
2.3. Study of Buffer Capacity of PMC Temperature Treatment
3. Materials and Methods
3.1. Materials
3.2. Preparation of CaCO3 Microspherulites
3.3. Preparation of Polyelectrolyte Microcapsules
3.4. Temperature Treatment
3.5. Measurement of Buffering Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Borodina, T.N.; Rumsh, L.D.; Kunizhev, S.M.; Sukhorukov, G.B.; Vorozhtsov, G.N.; Feldman, B.M.; Markvicheva, E.A. Polyelectrolyte microcapsules as the systems for delivery of biologically active substances. Biochem. Suppl. Ser. B Biomed. Chem. 2008, 2, 88–93. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Danilidis, I.L.; Pappas, G.S.; Kordas, G.C. Encapsulation and Release of Corrosion Inhibitors into Titania Nanocontainers. J. Nanosci. Nanotechnol. 2010, 10, 5912–5920. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.L.; Musin, E.V.; Dubrovskii, A.V.; Tikhonenko, S.A. Determination of urea concentration using urease-containing polyelectrolyte microcapsules. Anal. Methods 2019, 11, 1585–1590. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Kim, A.L.; Tikhonenko, S.A. Method of determining the localization of charges on the surface. J. Electrostat. 2019, 102, 103376. [Google Scholar] [CrossRef]
- Reshetilov, A.N.; Plekhanova, Y.V.; Dubrovskii, A.V.; Tikhonenko, S.A. Detection of urea using urease and paramagnetic Fe3O4 particles incorporated into polyelectrolyte microcapsules. Process Biochem. 2016, 51, 277–281. [Google Scholar] [CrossRef]
- Nifontova, G.; Zvaigzne, M.; Baryshnikova, M.; Korostylev, E.; Ramos-Gomes, F.; Alves, F.; Nabiev, I.; Sukhanova, A. Next-Generation Theranostic Agents Based on Polyelectrolyte Microcapsules Encoded with Semiconductor Nanocrystals: Development and Functional Characterization. Nanoscale Res. Lett. 2018, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Reshetilov, A.; Plekhanova, Y.; Tarasov, S.; Tikhonenko, S.; Dubrovsky, A.; Kim, A.; Kashin, V.; Machulin, A.; Wang, G.J.; Kolesov, V.; et al. Bioelectrochemical properties of enzyme-containing multilayer polyelectrolyte microcapsules modified with multiwalled carbon nanotubes. Membranes 2019, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Sukhorukov, B.I.; Tikhonenko, S.A.; Saburova, E.A.; Dubrovskii, A.V.; Dybovskaya, Y.N.; Shabarchina, L.I. Protein-filled polyelectrolyte microcapsules in the design of enzymic microdiagnostics. Biophysics 2007, 52, 575–581. [Google Scholar] [CrossRef]
- Sukhorukov, B.I.; Tikhonenko, S.A.; Saburova, E.A.; Dubrovskiĭ, A.V.; Dybovskaia, I.N.; Shabarchina, L.I. Incapsulation of enzymes into polyelectrolyte nano- and microcapsules and the problem of the development of enzymatic microdiagnostics. Biofizika 2007, 52, 1041–1048. [Google Scholar]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The Future of Layer-by-Layer Assembly: A Tribute to ACS Nano Associate Editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef] [Green Version]
- Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Ramazanov, B.R.; Tikhonenko, S.A. The Discovery of the Buffer Capacity of Various Types of Polyelectrolyte Microcapsules. Polymers 2021, 13, 4026. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Maharjan, S.; Park, T.-E.; Jiang, T.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Tuning the Buffering Capacity of Polyethylenimine with Glycerol Molecules for Efficient Gene Delivery: Staying in or out of the Endosomes. Macromol. Biosci. 2015, 15, 622–635. [Google Scholar] [CrossRef]
- Saikaew, R.; Meesorn, W.; Zoppe, J.O.; Weder, C.; Dubas, S.T. Influence of the Salt Concentration on the Properties of Salt-Free Polyelectrolyte Complex Membranes. Macromol. Mater. Eng. 2019, 304, 1900245. [Google Scholar] [CrossRef]
- Ali, S.; Bleuel, M.; Prabhu, V.M. Lower Critical Solution Temperature in Polyelectrolyte Complex Coacervates. ACS Macro Lett. 2019, 8, 289–293. [Google Scholar] [CrossRef]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual Salt and pH Induced Changes in Polyethylenimine Solutions. PLoS ONE 2016, 11, e0158147. [Google Scholar] [CrossRef] [Green Version]
- Gallops, C.E.; Yu, C.; Ziebarth, J.D.; Wang, Y. Effect of the Protonation Level and Ionic Strength on the Structure of Linear Polyethyleneimine. ACS Omega 2019, 4, 7255–7264. [Google Scholar] [CrossRef]
- Marciel, A.B.; Srivastava, S.; Tirrell, M.V. Structure and rheology of polyelectrolyte complex coacervates. Soft Matter 2018, 14, 2454–2464. [Google Scholar] [CrossRef]
- Cundan, R.B.; Lawton, J.B.; Murray, D.; Phillips, G. 0 Polyelectrolyte Complexes, 1 the Effect of pH and Ionic Strength on the Stoichiometry of Model Polycation-Polyanion Complexes. Makromol. Chem. 1979, 180, 2913. [Google Scholar]
- Zhang, Y.; Batys, P.; O’Neal, J.T.; Li, F.; Sammalkorpi, M.; Lutkenhaus, J.L. Molecular Origin of the Glass Transition in Polyelectrolyte Assemblies. ACS Cent. Sci. 2018, 4, 638–644. [Google Scholar] [CrossRef]
- Neitzel, A.E.; De Hoe, G.X.; Tirrell, M.V. Expanding the structural diversity of polyelectrolyte complexes and polyzwitterions. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100897. [Google Scholar] [CrossRef]
- Gao, C.; Donath, E.; Moya, S.; Dudnik, V.; Möhwald, H. Elasticity of hollow polyelectrolyte capsules prepared by the layer-by-layer technique. Eur. Phys. J. E 2001, 5, 21–27. [Google Scholar] [CrossRef]
- Dubrovskii, A.V.; Shabarchina, L.I.; Kim, Y.A.; Sukhorukov, B.I. Influence of the temperature on polyelectrolyte microcapsules: Light scattering and confocal microscopy data. Russ. J. Phys. Chem. A 2006, 80, 1703–1707. [Google Scholar] [CrossRef]
- Musin, E.V.; Kim, A.L.; Dubrovskii, A.V.; Tikhonenko, S.A. New sight at the organization of layers of multilayer polyelectrolyte microcapsules. Sci. Rep. 2021, 11, 14040. [Google Scholar] [CrossRef] [PubMed]
- Musin, E.V.; Kim, A.L.; Tikhonenko, S.A. Destruction of polyelectrolyte microcapsules formed on CaCO3 microparticles and the release of a protein included by the adsorption method. Polymers 2020, 12, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, K.; Shchukin, D.G.; Möhwald, H.; Sukhorukov, G.B. Thermal Behavior of Polyelectrolyte Multilayer Microcapsules. 1. The Effect of Odd and Even Layer Number. J. Phys. Chem. B 2005, 109, 18250–18259. [Google Scholar] [CrossRef]
- Köhler, K.; Möhwald, H.; Sukhorukov, G.B. Thermal Behavior of Polyelectrolyte Multilayer Microcapsules: 2. Insight into Molecular Mechanisms for the PDADMAC/PSS System. J. Phys. Chem. B 2006, 110, 24002–24010. [Google Scholar] [CrossRef]
- Kazakova, L.; Dubrovskiĭ, A.V.; Moshkov, D.; Shabarchina, L.I.; Sukhorukov, B.I. An electron microscopy study of the structure of polyelectrolyte microcapsules containing protein and containing no protein. Biofizika 2007, 52, 850–854. [Google Scholar]
- Dubrovskii, A.V.; Kochetkova, O.Y.; Kim, A.L.; Musin, E.V.; Seraya, O.Y.; Tikhonenko, S.A. Destruction of shells and release of a protein from microcapsules consisting of non-biodegradable polyelectrolytes. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 160–164. [Google Scholar] [CrossRef]
- Duan, G.; Haase, M.F.; Stebe, K.J.; Lee, D. One-Step Generation of Salt-Responsive Polyelectrolyte Microcapsules via Surfactant-Organized Nanoscale Interfacial Complexation in Emulsions (SO NICE). Langmuir 2018, 34, 847–853. [Google Scholar] [CrossRef]
- Lebedeva, O.V.; Kim, B.-S.; Vasilev, K.; Vinogradova, O.I. Salt softening of polyelectrolyte multilayer microcapsules. J. Colloid Interface Sci. 2005, 284, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Lulevich, V.V.; Vinogradova, O.I. Effect of pH and Salt on the Stiffness of Polyelectrolyte Multilayer Microcapsules. Langmuir 2004, 20, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Köhler, K.; Déjugnat, C.; Dubois, M.; Zemb, T.; Sukhorukov, G.B.; Guttmann, P.; Möhwald, H. Soft X-ray microscopy to characterize polyelectrolyte assemblies. J. Phys. Chem. B 2007, 111, 8388–8393. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B.; Möhwald, H. Influence of the Ionic Strength on the Polyelectrolyte Multilayers’ Permeability. Langmuir 2003, 19, 2444–2448. [Google Scholar] [CrossRef]
- Musin, E.V.; Kim, A.L.; Tikhonenko, S.A. Substance Release from Polyelectrolyte Microcapsules. Encyclopedia 2022, 2, 428–440. [Google Scholar] [CrossRef]
- Balaskas, A.C.; Kartsonakis, I.A.; Tziveleka, L.-A.; Kordas, G.C. Improvement of anti-corrosive properties of epoxy-coated AA 2024-T3 with TiO2 nanocontainers loaded with 8-hydroxyquinoline. Prog. Org. Coat. 2012, 74, 418–426. [Google Scholar] [CrossRef]
- Katagiri, K.; Imai, Y.; Koumoto, K. Variable on-demand release function of magnetoresponsive hybrid capsules. J. Colloid Interface Sci. 2011, 361, 109–114. [Google Scholar] [CrossRef]
- Rae, J.; Ashokkumar, M.; Eulaerts, O.; von Sonntag, C.; Reisse, J.; Grieser, F. Estimation of ultrasound induced cavitation bubble temperatures in aqueous solutions. Ultrason. Sonochem. 2005, 12, 325–329. [Google Scholar] [CrossRef]
- Richard, I.; Thibault, M.; De Crescenzo, G.; Buschmann, M.D.; Lavertu, M. Ionization Behavior of Chitosan and Chitosan–DNA Polyplexes Indicate That Chitosan Has a Similar Capability to Induce a Proton-Sponge Effect as PEI. Biomacromolecules 2013, 14, 1732–1740. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubrovskii, A.V.; Kim, A.L.; Musin, E.V.; Tikhonenko, S.A. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Ionic Environment and Incubation Temperature. Int. J. Mol. Sci. 2022, 23, 6608. https://doi.org/10.3390/ijms23126608
Dubrovskii AV, Kim AL, Musin EV, Tikhonenko SA. A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Ionic Environment and Incubation Temperature. International Journal of Molecular Sciences. 2022; 23(12):6608. https://doi.org/10.3390/ijms23126608
Chicago/Turabian StyleDubrovskii, Alexey V., Aleksandr L. Kim, Egor V. Musin, and Sergey A. Tikhonenko. 2022. "A Study of the Buffer Capacity of Polyelectrolyte Microcapsules Depending on Their Ionic Environment and Incubation Temperature" International Journal of Molecular Sciences 23, no. 12: 6608. https://doi.org/10.3390/ijms23126608





