Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis
Abstract
:1. Introduction
2. Clinical Criteria and Symptomatology
2.1. Lymphedema
2.2. Lipedema
2.3. Summary
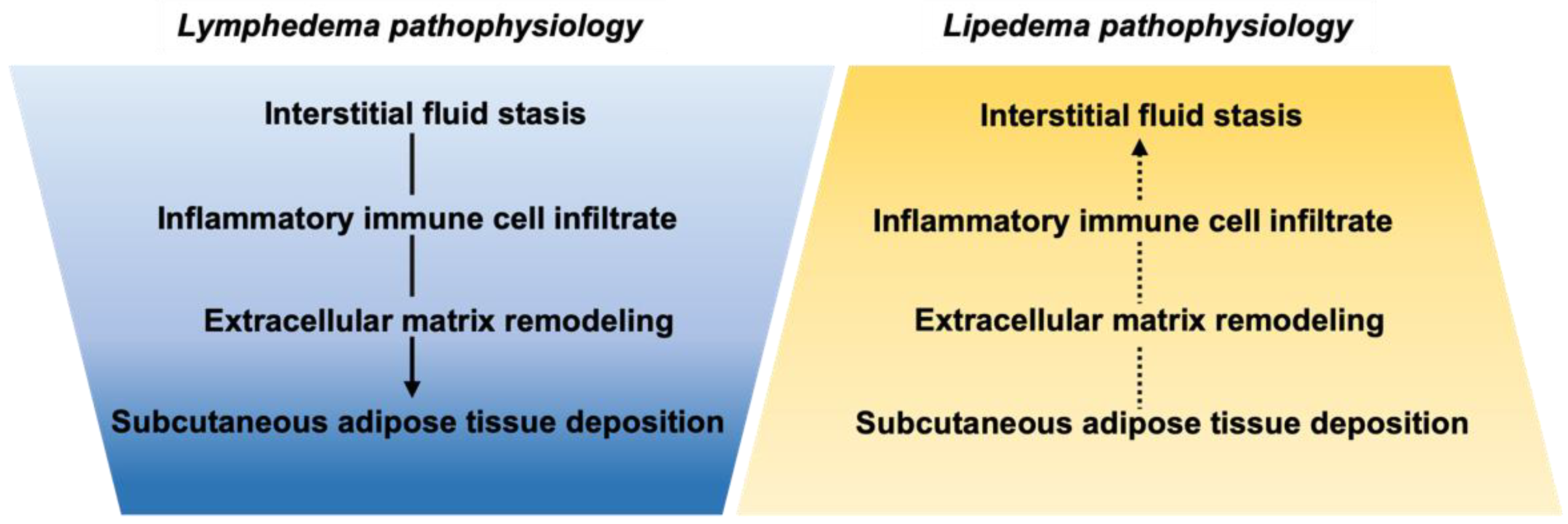
3. Molecular Regulators and Pathophysiology
3.1. Lymphedema
3.2. Lipedema
3.3. Summary
4. Genetic Causes or Indicators
4.1. Lymphedema
4.2. Lipedema
4.3. Summary
5. Summary and Call for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rockson, S.G. Advances in Lymphedema. Circ. Res. 2021, 128, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L.; Kahn, L.A.; Iker, E.; Ehrlich, C.; Wright, T.; McHutchison, L.; Schwartz, J.; Sleigh, M.; MC Donahue, P.; Lisson, K.H.; et al. Standard of care for lipedema in the United States. Phlebol. Phlebol. 2021, 36, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Kamali, P.; Lin, S.J. Lymphedema: Presentation, Diagnosis, and Treatment. Plast. Reconstr. Surg. 2016, 137, 1654–1655. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.K.; Grant, F.D.; Slavin, S.A. Lower-Extremity Lymphedema and Elevated Body-Mass Index. N. Engl. J. Med. 2012, 366, 2136–2137. [Google Scholar] [CrossRef]
- Rockson, S.G. Current concepts and future directions in the diagnosis and management of lymphatic vascular disease. Vasc. Med. 2010, 15, 223–231. [Google Scholar] [CrossRef]
- Cormier, J.N.; Askew, R.L.; Mungovan, K.S.; Xing, Y.; Ross, M.I.; Armer, J.M. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef]
- Fu, M.R.; Deng, J.; Armer, J.M. Putting Evidence into Practice: Cancer-Related Lymphedema. Clin. J. Oncol. Nurs. 2014, 18, 68–79. [Google Scholar] [CrossRef]
- Rockson, S.G.; Keeley, V.; Kilbreath, S.; Szuba, A.; Towers, A. Cancer-associated secondary lymphoedema. Nat. Rev. Dis. Prim. 2019, 5, 22. [Google Scholar] [CrossRef]
- Goss, J.A.; Greene, A.K. Diagnosis and Staging of Lymphedema. Semin. Plast. Surg. 2018, 32, 12–16. [Google Scholar] [CrossRef]
- Stemmer, R. Stemmer’s sign—Possibilities and limits of clinical diagnosis of lymphedema. Wien. Med. Wochenschr. 1999, 149, 85–86. [Google Scholar]
- Suehiro, K.; Mizumoto, Y.; Morikage, N.; Harada, T.; Samura, M.; Nagase, T.; Takeuchi, Y.; Mizoguchi, T.; Suzuki, R.; Kurazumi, H.; et al. Hardness Sensed by Skin Palpation in Legs with Lymphedema Is Predominantly Correlated with Dermal Thickening. Lymphat. Res. Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.A.; Phillips, T.J. Lymphedema: Diagnostic workup and management. J. Am. Acad. Dermatol. 2017, 77, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hoffner, M.; Brorson, H. Adipocytes are larger in lymphedematous extremities than in controls. J. Plast. Surg. Hand Surg. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, R.; Donahue, P.M.C.; Garza, M.; Lee, C.A.; Patel, N.J.; Gonzalez, V.; Jones, R.S.; Donahue, M.J. Elevated magnetic resonance imaging measures of adipose tissue deposition in women with breast cancer treatment-related lymphedema. Breast Cancer Res. Treat. 2021, 191, 115–124. [Google Scholar] [CrossRef]
- Mortimer, P.S.; Rockson, S.G. New developments in clinical aspects of lymphatic disease. J. Clin. Investig. 2014, 124, 915–921. [Google Scholar] [CrossRef]
- Rockson, S.G. Causes and consequences of lymphatic disease. Ann. N. Y. Acad. Sci. 2010, 1207, E2–E6. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Herbst, K.L. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 2012, 33, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Mihara, M.; Hara, H.; Zhou, H.P.; Tange, S.; Kikuchi, K. Lymphaticovenous Anastomosis Releases the Lower Extremity Lymphedema-associated Pain. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1205. [Google Scholar] [CrossRef]
- Fukushima, T.; Tsuji, T.; Sano, Y.; Miyata, C.; Kamisako, M.; Hohri, H.; Yoshimura, C.; Asakura, M.; Okitsu, T.; Muraoka, K.; et al. Immediate effects of active exercise with compression therapy on lower-limb lymphedema. Support. Care Cancer 2017, 25, 2603–2610. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Fu, M.R.; Armer, J.M.; Cormier, J.N.; Radina, E.M.; Thiadens, S.R.; Weiss, J.; Tuppo, C.M.; Dietrich, M.S.; Ridner, S.H. Factors Associated with Reported Infection and Lymphedema Symptoms among Individuals with Extremity Lymphedema. Rehabil. Nurs. 2015, 40, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.; Piedalue, K.-A.; Baydoun, M.; Carlson, L.E. The Quality of Life and Psychosocial Implications of Cancer-Related Lower-Extremity Lymphedema: A Systematic Review of the Literature. J. Clin. Med. 2020, 9, 3200. [Google Scholar] [CrossRef] [PubMed]
- Halk, A.B.; Damstra, R.J. First Dutch guidelines on lipedema using the international classification of functioning, disability and health. Phlebol. J. Venous Dis. 2016, 32, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Hamburg, N.M.; Calligaro, K.D.; Casanegra, A.I.; Freeman, R.; Gordon, P.A.; Gornik, H.L.; Kim, E.S.H.; Leeper, N.J.; Merli, G.J.; et al. ACC/AHA/SVM/ACP Advanced Training Statement on Vascular Medicine (Revision of the 2004 ACC/ACP/SCAI/SVMB/SVS Clinical Competence Statement on Vascular Medicine and Catheter-Based Peripheral Vascular Interventions) A Report of the ACC Competency Management Committee. J. Am. Coll. Cardiol. 2021, 77, 998–1020. [Google Scholar] [PubMed]
- Reich-Schupke, S.; Schmeller, W.; Brauer, W.J.; Cornely, M.E.; Faerber, G.; Ludwig, M.; Lulay, G.; Miller, A.; Rapprich, S.; Richter, D.F.; et al. S1 guidelines: Lipedema. J. Dtsch. Dermatol. Ges. 2017, 15, 758–767. [Google Scholar] [CrossRef]
- Földi, M. Convincing evidence for the pumping activity of lymphatics. Acta Physiol. 2006, 186, 319. [Google Scholar] [CrossRef]
- Fonder, M.A.; Loveless, J.W.; Lazarus, G.S. Lipedema, a frequently unrecognized problem. J. Am. Acad. Dermatol. 2007, 57, S1–S3. [Google Scholar] [CrossRef]
- Crescenzi, R.; Donahue, P.; Petersen, K.; Garza, M.; Guerreso, K.; Luo, Y.; Beckman, J.; Donahue, M. Lymphatic insufficiency observed by noninvasive MR lymphangiography and multi-nuclear 23Na-MRI in patients with lymphedema and lipedema. In Proceedings of the 28th Annual Meeting of the International Society for Magnetic Resonance in Medicine (ISMRM), Oral Presentation at Scientific Sessions, Paris, France, 8–13 August 2020. [Google Scholar]
- Meier-Vollrath, I.; Schmeller, W. Lipoedema—Current status, new perspectives. J. Dtsch. Dermatol. Ges. 2004, 2, 181–186. [Google Scholar] [CrossRef]
- Herbst, K.L.; Mirkovskya, L.; Bharhagava, A.; Chava, Y.; Hanne, C.T. Lipedema fat and signs and symptoms of illness, increase with advancing stage. Arch. Med. 2015, 7, 10. [Google Scholar]
- Herbst, K.L. Subcutaneous Adipose Tissue Diseases: Dercum Disease, Lipedema, Familial Multiple Lipomatosis, and Madelung Disease. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Eds.; National Library of Medicine: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Romeijn, J.R.M.; de Rooij, M.J.M.; Janssen, L.; Martens, H. Exploration of Patient Characteristics and Quality of Life in Patients with Lipoedema Using a Survey. Dermatol. Ther. 2018, 8, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Beltran, K.; Herbst, K.L. Differentiating lipedema and Dercum’s disease. Int. J. Obes. 2016, 41, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C. Pressure-Volume relationships in the interstitial spaces. Investig. Ophthalmol. 1965, 4, 1075–1084. [Google Scholar]
- Buso, G.; Depairon, M.; Tomson, D.; Raffoul, W.; Vettor, R.; Mazzolai, L. Lipedema: A Call to Action! Obesity 2019, 27, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Karadag, A.S.; Wollina, U. Cause and management of lipedema-associated pain. Dermatol. Ther. 2020, 34, e14364. [Google Scholar] [CrossRef] [PubMed]
- Beighton, P.; Solomon, L.; Soskolne, C.L. Articular mobility in an African population. Ann. Rheum. Dis. 1973, 32, 413–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakim, A.J.; Grahame, R. A simple questionnaire to detect hypermobility: An adjunct to the assessment of patients with diffuse musculoskeletal pain. Int. J. Clin. Pract. 2003, 57, 163–166. [Google Scholar]
- Katzer, K.; Hill, J.L.; McIver, K.B.; Foster, M.T. Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation. Int. J. Mol. Sci. 2021, 22, 11720. [Google Scholar] [CrossRef]
- Child, A.H.; Gordon, K.D.; Sharpe, P.; Brice, G.; Ostergaard, P.; Jeffery, S.; Mortimer, P.S. Lipedema: An inherited condition. Am. J. Med Genet. Part A 2010, 152A, 970–976. [Google Scholar] [CrossRef]
- Rockson, S.G. Lymphedema. Am. J. Med. 2001, 110, 288–295. [Google Scholar] [CrossRef]
- Reed, R.K.; Laurent, T.C.; Taylor, A.E. Hyaluronan in prenodal lymph from skin: Changes with lymph flow. Am. J. Physiol. Circ. Physiol. 1990, 259, H1097–H1100. [Google Scholar] [CrossRef]
- Olszewski, W.L. The Pathophysiology of Lymphedema—2012. Handchir. Mikrochir. Plast. Chir. 2012, 44, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G. The Lymphatics and the Inflammatory Response: Lessons Learned from Human Lymphedema. Lymphat. Res. Biol. 2013, 11, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.V.; Karkkainen, M.J.; Alitalo, K.; Wiig, H. Transcapillary fluid balance consequences of missing initial lymphatics studied in a mouse model of primary lymphoedema. J. Physiol. 2006, 574, 583–596. [Google Scholar] [CrossRef]
- Bates, D.O.; Levick, J.R.; Mortimer, P.S. Change in macromolecular composition of interstitial fluid from swollen arms after breast cancer treatment, and its implications. Clin. Sci. 1993, 85, 737–746. [Google Scholar] [CrossRef]
- Zampell, J.C.; Aschen, S.; Weitman, E.S.; Yan, A.; Elhadad, S.; De Brot Andrade, M.; Mehrara, B.J. Regulation of adipogenesis by lymphatic fluid stasis: Part I. Adipogenesis, fibrosis, and inflammation. Plast. Reconstr. Surg. 2012, 129, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Mehrara, B.J.; Park, H.J.; Kataru, R.P.; Bromberg, J.; Coriddi, M.; Baik, J.E.; Shin, J.; Li, C.; Cavalli, M.R.; Encarnacion, E.M.; et al. Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema. Biology 2021, 10, 934. [Google Scholar] [CrossRef]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2012, 27, 1114–1126. [Google Scholar] [CrossRef] [Green Version]
- Savetsky, I.L.; Ghanta, S.; Gardenier, J.C.; Torrisi, J.S.; Nores, G.D.G.; Hespe, G.E.; Nitti, M.D.; Kataru, R.P.; Mehrara, B.J. Data from: Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 2015, 10, e0126908. [Google Scholar] [CrossRef]
- Gousopoulos, E.; Proulx, S.T.; Bachmann, S.B.; Scholl, J.; Dionyssiou, D.; Demiri, E.; Halin, C.; Dieterich, L.C.; Detmar, M. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI Insight 2016, 1, e89081. [Google Scholar] [CrossRef] [Green Version]
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.; Torrisi, J.S.; Nores, G.D.G.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C.; et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 14345. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.; Fujiu, K.; Matsumoto, S.; Nakayama, Y.; Shibata, M.; Oike, Y.; Koshima, I.; Watabe, T.; Nagai, R.; Manabe, I. Excess Lymphangiogenesis Cooperatively Induced by Macrophages and CD4+ T Cells Drives the Pathogenesis of Lymphedema. J. Investig. Dermatol. 2015, 136, 706–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gousopoulos, E.; Proulx, S.; Scholl, J.; Uecker, M.; Detmar, M. Prominent Lymphatic Vessel Hyperplasia with Progressive Dysfunction and Distinct Immune Cell Infiltration in Lymphedema. Am. J. Pathol. 2016, 186, 2193–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koc, M.; Wald, M.; Varaliová, Z.; Ondrůjová, B.; Čížková, T.; Brychta, M.; Kračmerová, J.; Beranová, L.; Pala, J.; Šrámková, V.; et al. Lymphedema alters lipolytic, lipogenic, immune and angiogenic properties of adipose tissue: A hypothesis-generating study in breast cancer survivors. Sci. Rep. 2021, 11, 8171. [Google Scholar] [CrossRef] [PubMed]
- Sedger, L.M.; Tull, D.; McConville, M.J.; DE Souza, D.; Rupasinghe, T.W.T.; Williams, S.; Dayalan, S.; Lanzer, D.; Mackie, H.; Lam, T.C.; et al. Lipidomic Profiling of Adipose Tissue Reveals an Inflammatory Signature in Cancer-Related and Primary Lymphedema. PLoS ONE 2016, 11, e0154650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Nicolls, M.R.; Tian, W.; Rockson, S.G. Lymphatic Dysfunction, Leukotrienes, and Lymphedema. Annu. Rev. Physiol. 2018, 80, 49–70. [Google Scholar] [CrossRef]
- Lin, S.; Kim, J.; Lee, M.-J.; Roche, L.; Yang, N.L.; Tsao, P.S.; Rockson, S.G. Prospective Transcriptomic Pathway Analysis of Human Lymphatic Vascular Insufficiency: Identification and Validation of a Circulating Biomarker Panel. PLoS ONE 2012, 7, e52021. [Google Scholar] [CrossRef] [Green Version]
- Leung, G.; Baggott, C.; West, C.; Elboim, C.; Paul, S.M.; Cooper, B.A.; Abrams, G.; Dhruva, A.; Schmidt, B.L.; Kober, K.; et al. Cytokine Candidate Genes Predict the Development of Secondary Lymphedema Following Breast Cancer Surgery. Lymphat. Res. Biol. 2014, 12, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Gil, H.J.; Escobedo, N.; Benito-Martín, A.; Ximénez-Embún, P.; Muñoz, J.; Peinado, H.; Rockson, S.G.; Oliver, G. Platelet factor 4 is a biomarker for lymphatic-promoted disorders. JCI Insight 2020, 5, e135109. [Google Scholar] [CrossRef]
- Földi, E.; Sauerwald, A.; Hennig, B. Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema. Lymphology 2000, 33, 19–23. [Google Scholar]
- Tian, W.; Rockson, S.G.; Jiang, X.; Kim, J.; Begaye, A.; Shuffle, E.M.; Tu, A.B.; Cribb, M.; Nepiyushchikh, Z.; Feroze, A.H.; et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci. Transl. Med. 2017, 9, eaal3920. [Google Scholar] [CrossRef] [Green Version]
- Rockson, S.G.; Tian, W.; Jiang, X.; Kuznetsova, T.; Haddad, F.; Zampell, J.; Mehrara, B.; Sampson, J.P.; Roche, L.; Kim, J.; et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 2018, 3, e123775. [Google Scholar] [CrossRef]
- Schirger, A.; Harrison, E.G.; Janes, J.M. Idiopathic Lymphedema. JAMA 1962, 182, 14–22. [Google Scholar] [CrossRef]
- Zampell, J.C.; Elhadad, S.; Avraham, T.; Weitman, E.; Aschen, S.; Yan, A.; Mehrara, B.J. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am. J. Physiol. Physiol. 2012, 302, C709–C719. [Google Scholar] [CrossRef] [Green Version]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef] [Green Version]
- Suami, H.; Pan, W.-R.; Taylor, G.I. Changes in the Lymph Structure of the Upper Limb after Axillary Dissection: Radiographic and Anatomical Study in a Human Cadaver. Plast. Reconstr. Surg. 2007, 120, 982–991. [Google Scholar] [CrossRef]
- Barron, L.; Wynn, T.A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Liver Physiol. 2011, 300, G723–G728. [Google Scholar] [CrossRef] [Green Version]
- Ridner, S.H.; Dietrich, M.S.; Sonis, S.T.; Murphy, B. Biomarkers Associated with Lymphedema and Fibrosis in Patients with Cancer of the Head and Neck. Lymphat. Res. Biol. 2018, 16, 516–524. [Google Scholar] [CrossRef]
- Di, S.; Ziyou, Y.; Liu, N.-F. Pathological Changes of Lymphedematous Skin: Increased Mast Cells, Related Proteases, and Activated Transforming Growth Factor-β1. Lymphat. Res. Biol. 2016, 14, 162–171. [Google Scholar] [CrossRef]
- Fain, J.N.; Tichansky, D.S.; Madan, A.K. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism 2005, 54, 1546–1551. [Google Scholar] [CrossRef]
- Avraham, T.; Daluvoy, S.; Zampell, J.; Yan, A.; Haviv, Y.S.; Rockson, S.G.; Mehrara, B.J. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 2010, 177, 3202–3214. [Google Scholar] [CrossRef]
- Clavin, N.W.; Avraham, T.; Fernandez, J.; Daluvoy, S.V.; Soares, M.A.; Chaudhry, A.; Mehrara, B.J. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2113–H2127. [Google Scholar] [CrossRef] [Green Version]
- Brorson, H.; Ohlin, K.; Olsson, G.; Nilsson, M. Adipose Tissue Dominates Chronic Arm Lymphedema Following Breast Cancer: An Analysis Using Volume Rendered CT Images. Lymphat. Res. Biol. 2006, 4, 199–210. [Google Scholar] [CrossRef]
- Trinh, L.; Peterson, P.; Brorson, H.; Månsson, S. Assessment of Subfascial Muscle/Water and Fat Accumulation in Lymphedema Patients Using Magnetic Resonance Imaging. Lymphat. Res. Biol. 2019, 17, 340–346. [Google Scholar] [CrossRef]
- Borri, M.; Gordon, K.; Hughes, J.C.; Scurr, E.D.; Koh, D.-M.; Leach, M.O.; Mortimer, P.S.; Schmidt, M.A. Magnetic Resonance Imaging–Based Assessment of Breast Cancer–Related Lymphoedema Tissue Composition. Investig. Radiol. 2017, 52, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Sen, Y.; Qian, Y.; Koelmeyer, L.; Borotkanics, R.; Ricketts, R.; Mackie, H.; Lam, T.C.; Shon, K.H.; Suami, H.; Boyages, J. Breast Cancer-Related Lymphedema: Differentiating Fat from Fluid Using Magnetic Resonance Imaging Segmentation. Lymphat. Res. Biol. 2018, 16, 20–27. [Google Scholar] [CrossRef]
- Gousopoulos, E.; Karaman, S.; Proulx, S.T.; Leu, K.; Buschle, D.; Detmar, M. High-Fat Diet in the Absence of Obesity Does Not Aggravate Surgically Induced Lymphoedema in Mice. Eur. Surg. Res. 2017, 58, 180–192. [Google Scholar] [CrossRef]
- Garcia Nores, G.D.; Cuzzone, D.A.; Albano, N.J.; Hespe, G.E.; Kataru, R.P.; Torrisi, J.S.; Gardenier, J.C.; Savetsky, I.L.; Aschen, S.Z.; Nitti, M.D.; et al. Obesity but not high-fat diet impairs lymphatic function. Int. J. Obes. 2016, 40, 1582–1590. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, J.M.; Markhus, C.E.; Gyenge, C.C.; Alitalo, K.; Wiig, H.; Swartz, M.A. Dermal Collagen and Lipid Deposition Correlate with Tissue Swelling and Hydraulic Conductivity in Murine Primary Lymphedema. Am. J. Pathol. 2010, 176, 1122–1129. [Google Scholar] [CrossRef]
- Escobedo, N.; Proulx, S.T.; Karaman, S.; Dillard, M.E.; Johnson, N.; Detmar, M.; Oliver, G. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 2016, 1, e85096. [Google Scholar] [CrossRef]
- Rutkowski, J.; Davis, K.E.; Scherer, P.E. Mechanisms of obesity and related pathologies: The macro- and microcirculation of adipose tissue. FEBS J. 2009, 276, 5738–5746. [Google Scholar] [CrossRef] [Green Version]
- Polomska, A.K.; Proulx, S.T. Imaging technology of the lymphatic system. Adv. Drug Deliv. Rev. 2021, 170, 294–311. [Google Scholar] [CrossRef]
- Pouwels, S.; Huisman, S.; Smelt, H.J.M.; Said, M.; Smulders, J.F. Lipoedema in patients after bariatric surgery: Report of two cases and review of literature. Clin. Obes. 2018, 8, 147–150. [Google Scholar] [CrossRef]
- Fife, C.E.; Maus, E.A.; Carter, M.J. Lipedema: A frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv. Ski. Wound Care 2010, 23, 81–92. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 8747461. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hägerling, R.; Wang, A.; Ströbel, P.; Hollmén, M.; Lindenblatt, N.; Gousopoulos, E. Adipose Tissue Hypertrophy, An Aberrant Biochemical Profile and Distinct Gene Expression in Lipedema. J. Surg. Res. 2020, 253, 294–303. [Google Scholar] [CrossRef]
- Suga, H.; Araki, J.; Aoi, N.; Kato, H.; Higashino, T.; Yoshimura, K. Adipose tissue remodeling in lipedema: Adipocyte death and concurrent regeneration. J. Cutan. Pathol. 2009, 36, 1293–1298. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Pursell, I.; Diaz, Z.; Herbst, K.; Bunnell, B. 3D Spheroids Derived from Human Lipedema ASCs Demonstrated Similar Adipogenic Differentiation Potential and ECM Remodeling to Non-Lipedema ASCs In Vitro. Int. J. Mol. Sci. 2020, 21, 8350. [Google Scholar] [CrossRef]
- Allen, M.; Schwartz, M.; Herbst, K.L. Interstitial Fluid in Lipedema and Control Skin. Womens Health Rep. 2020, 1, 480–487. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and Adipose Tissue Dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Park, J.; Gupta, O.T.; Holland, W.L.; Auerbach, P.; Zhang, N.; Marangoni, R.G.; Nicoloro, S.M.; Czech, M.P.; Varga, J.; et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 2014, 5, 3485. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, Y.; Chen, C.; Yang, L.; Lee, H.-H.; Wang, Z.; Zhang, N.; Kolonin, M.G.; An, Z.; Ge, X.; et al. Critical Role of Matrix Metalloproteinase 14 in Adipose Tissue Remodeling during Obesity. Mol. Cell. Biol. 2020, 40, e00564-19. [Google Scholar] [CrossRef]
- Crescenzi, R.; Marton, A.; Donahue, P.M.; Mahany, H.B.; Lants, S.K.; Wang, P.; Beckman, J.A.; Donahue, M.J.; Titze, J. Tissue Sodium Content is Elevated in the Skin and Subcutaneous Adipose Tissue in Women with Lipedema. Obesity 2017, 26, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, R.; Donahue, P.M.; Petersen, K.J.; Garza, M.; Patel, N.; Lee, C.; Beckman, J.A.; Donahue, M.J. Upper and Lower Extremity Measurement of Tissue Sodium and Fat Content in Patients with Lipedema. Obesity 2020, 28, 907–915. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Joffin, N.; Scherer, P.E. The MMP14–caveolin axis and its potential relevance for lipoedema. Nat. Rev. Endocrinol. 2020, 16, 669–674. [Google Scholar] [CrossRef]
- Jagtman, A.B.; Kuiper, J.P.; Brakkee, A.J. Measurements of skin elasticity in patients with lipedema of the Moncorps “rusticanus” type. Phlebologie 1984, 37, 315–319. [Google Scholar]
- Szolnoky, G.; Nemes, A.; Gavallér, H.; Forster, T.; Kemény, L. Lipedema is associated with increased aortic stiffness. Lymphology 2012, 45, 71–79. [Google Scholar]
- Buso, G.; Favre, L.; Maufus, M.; Honorati, M.; Lessert, C.; Depairon, M.; Raffoul, W.; Tomson, D.; Mazzolai, L. Indocyanine green lymphography as novel tool to assess lymphatics in patients with lipedema. Microvasc. Res. 2021, 140, 104298. [Google Scholar] [CrossRef]
- Zetzmann, K.; Ludolph, I.; Horch, R.E.; Boos, A.M. Imaging for treatment planning in lipo-and lymphedema. Search Life Sci. Literature. 2018, 50, 386–392. [Google Scholar]
- Forner-Cordero, I.; Oliván-Sasot, P.; Ruiz-Llorca, C.; Muñoz-Langa, J. Lymphoscintigraphic findings in patients with lipedema. Rev. Esp. Med. Nucl. Imagen Mol. (Engl. Ed.) 2018, 37, 341–348. [Google Scholar] [CrossRef]
- Bilancini, S.; Lucchi, M.; Tucci, S.; Eleuteri, P. Functional Lymphatic Alterations in Patients Suffering from Lipedema. Angiology 1995, 46, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Boursier, V.; Pecking, A.; Vignes, S. Comparative analysis of lymphoscintigraphy between lipedema and lower limb lymphedema. J. Mal. Vasc. 2004, 29, 257–261. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hollmen, M.; Strobel, P.; Stepniewski, A.; Wang, A.; Frueh, F.S.; Kim, B.S.; Giovanoli, P.; Lindenblatt, N.; et al. Increased levels of VEGF-C and macrophage infiltration in lipedema patients without changes in lymphatic vascular morphology. Sci. Rep. 2020, 10, 10947. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, C.; Foeldi, E.; Langer, M. MR imaging of the lymphatic system in patients with lipedema and lipo-lymphedema. Microvasc. Res. 2009, 77, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Szolnoky, G.; Nagy, N.; Kovács, R.K.; Dósa-Rácz, E.; Szabó, A.; Bársony, K.; Balogh, M.; Kemény, L. Complex decongestive physiotherapy decreases capillary fragility in lipedema. Lymphology 2008, 41, 161–166. [Google Scholar] [PubMed]
- Schierke, F.; Wyrwoll, M.J.; Wisdorf, M.; Niedzielski, L.; Maase, M.; Ruck, T.; Meuth, S.G.; Kusche-Vihrog, K. Nanomechanics of the endothelial glycocalyx contribute to Na+-induced vascular inflammation. Sci. Rep. 2017, 7, srep46476. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Brouillard, P.; Witte, M.H.; Erickson, R.P.; Damstra, R.J.; Becker, C.; Quere, I.; Vikkula, M. Primary lymphoedema. Nat. Rev. Dis. Primers 2021, 7, 77. [Google Scholar] [CrossRef]
- Martin-Almedina, S.; Mortimer, P.S.; Ostergaard, P. Development and physiological functions of the lymphatic system: Insights from human genetic studies of primary lymphedema. Physiol. Rev. 2021, 101, 1809–1871. [Google Scholar] [CrossRef]
- Yang, Y.; Oliver, G. Development of the mammalian lymphatic vasculature. J. Clin. Investig. 2014, 124, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Wigle, J.; Harvey, N.; Detmar, M.; Lagutina, I.; Grosveld, G.; Gunn, M.; Jackson, D.G.; Oliver, G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002, 21, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Irrthum, A.; Devriendt, K.; Chitayat, D.; Matthijs, G.; Glade, C.; Steijlen, P.M.; Fryns, J.-P.; van Steensel, M.; Vikkula, M. Mutations in the Transcription Factor Gene SOX18 Underlie Recessive and Dominant Forms of Hypotrichosis-Lymphedema-Telangiectasia. Am. J. Hum. Genet. 2003, 72, 1470–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Dagenais, S.L.; Erickson, R.P.; Arlt, M.F.; Glynn, M.W.; Gorski, J.L.; Seaver, L.H.; Glover, T.W. Mutations in FOXC2 (MFH-1), a Forkhead Family Transcription Factor, Are Responsible for the Hereditary Lymphedema-Distichiasis Syndrome. Am. J. Hum. Genet. 2000, 67, 1382–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irrthum, A.; Karkkainen, M.J.; Devriendt, K.; Alitalo, K.; Vikkula, M. Congenital Hereditary Lymphedema Caused by a Mutation That Inactivates VEGFR3 Tyrosine Kinase. Am. J. Hum. Genet. 2000, 67, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrell, R.E.; Levinson, K.L.; Esman, J.H.; Kimak, M.A.; Lawrence, E.C.; Barmada, M.M.; Finegold, D. Hereditary lymphedema: Evidence for linkage and genetic heterogeneity. Hum. Mol. Genet. 1998, 7, 2073–2078. [Google Scholar] [CrossRef]
- Alper, S. Genetic Diseases of PIEZO1 and PIEZO2 Dysfunction. Curr. Top. Membr. 2017, 79, 97–134. [Google Scholar] [CrossRef]
- Michelini, S.; Amato, B.; Ricci, M.; Serrani, R.; Veselenyiova, D.; Kenanoglu, S.; Kurti, D.; Dautaj, A.; Baglivo, M.; Compagna, R.; et al. SVEP1 is important for morphogenesis of lymphatic system: Possible implications in lymphedema. Lymphology 2021, 54, 12–22. [Google Scholar] [CrossRef]
- Maltese, P.E.; Michelini, S.; Ricci, M.; Maitz, S.; Fiorentino, A.; Serrani, R.; Lazzerotti, A.; Bruson, A.; Paolacci, S.; Benedetti, S.; et al. Increasing evidence of hereditary lymphedema caused by CELSR1 loss-of-function variants. Am. J. Med. Genet. Part A 2019, 179, 1718–1724. [Google Scholar] [CrossRef]
- Leppänen, V.-M.; Brouillard, P.; Korhonen, E.A.; Sipilä, T.; Jha, S.K.; Revencu, N.; Labarque, V.; Fastré, E.; Schlögel, M.; Ravoet, M.; et al. Characterization of ANGPT2 mutations associated with primary lymphedema. Sci. Transl. Med. 2020, 12, eaax8013. [Google Scholar] [CrossRef]
- Kajiya, K.; Hirakawa, S.; Ma, B.; Drinnenberg, I.; Detmar, M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005, 24, 2885–2895. [Google Scholar] [CrossRef] [Green Version]
- Frye, M.; Taddei, A.; Dierkes, C.; Martinez-Corral, I.; Fielden, M.; Ortsäter, H.; Kazenwadel, J.; Calado, D.; Ostergaard, P.; Salminen, M.; et al. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat. Commun. 2018, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; Adams, R.H.; Bailey, J.; Lu, Q.; Ziemiecki, A.; Alitalo, K.; Klein, R.; Wilkinson, G.A. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005, 19, 397–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelini, S.; Ricci, M.; Serrani, R.; Barati, S.; Kenanoglu, S.; Veselenyiova, D.; Kurti, D.; Baglivo, M.; Basha, S.H.; Priya, S.; et al. NOTCH1: Review of its role in lymphatic development and study of seven families with rare pathogenic variants. Mol. Genet. Genom. Med. 2020, 9, e1529. [Google Scholar] [CrossRef] [PubMed]
- Newman, B.; Lose, F.; Kedda, M.-A.; Francois, M.; Ferguson, K.; Janda, M.; Yates, P.; Spurdle, A.B.; Hayes, S.C. Possible Genetic Predisposition to Lymphedema after Breast Cancer. Lymphat. Res. Biol. 2012, 10, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miaskowski, C.; Dodd, M.; Paul, S.M.; West, C.; Hamolsky, D.; Abrams, G.; Cooper, B.A.; Elboim, C.; Neuhaus, J.; Schmidt, B.L.; et al. Lymphatic and Angiogenic Candidate Genes Predict the Development of Secondary Lymphedema following Breast Cancer Surgery. PLoS ONE 2013, 8, e60164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldrich, M.B.; Guilliod, R.; Fife, C.E.; Maus, E.A.; Smith, L.; Rasmussen, J.C.; Sevick-Muraca, E.M. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomed. Opt. Express 2012, 3, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Morfoisse, F.; Tatin, F.; Chaput, B.; Therville, N.; Vaysse, C.; Métivier, R.; Malloizel-Delaunay, J.; Pujol, F.; Godet, A.-C.; De Toni, F.; et al. Lymphatic Vasculature Requires Estrogen Receptor-α Signaling to Protect from Lymphedema. Arter. Thromb. Vasc. Biol. 2018, 38, 1346–1357. [Google Scholar] [CrossRef] [Green Version]
- Schook, C.C.; Mulliken, J.B.; Fishman, S.J.; Grant, F.D.; Zurakowski, D.; Greene, A.K. Primary Lymphedema: Clinical Features and Management in 138 Pediatric Patients. Plast. Reconstr. Surg. 2011, 127, 2419–2431. [Google Scholar] [CrossRef]
- Silha, J.V.; Kršek, M.; Sucharda, P.; Murphy, L.J. Angiogenic factors are elevated in overweight and obese individuals. Int. J. Obes. 2005, 29, 1308–1314. [Google Scholar] [CrossRef] [Green Version]
- Trincot, C.E.; Caron, K.M. Lymphatic Function and Dysfunction in the Context of Sex Differences. ACS Pharmacol. Transl. Sci. 2019, 2, 311–324. [Google Scholar] [CrossRef]
- Michelini, S.; Chiurazzi, P.; Marino, V.; Dell’Orco, D.; Manara, E.; Baglivo, M.; Fiorentino, A.; Maltese, P.E.; Pinelli, M.; Herbst, K.L.; et al. Aldo-Keto Reductase 1C1 (AKR1C1) as the First Mutated Gene in a Family with Nonsyndromic Primary Lipedema. Int. J. Mol. Sci. 2020, 21, 6264. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Mansour, S.; Brice, G.; Ostergaard, P.; Mortimer, P.S.; Jeffery, S.; Nussey, S. Pit-1 Mutation and Lipoedema in a Family. Exp. Clin. Endocrinol. Diabetes 2009, 118, 377–380. [Google Scholar] [CrossRef]
- Makinen, T.; Jussila, L.; Veikkola, T.; Karpanen, T.; Kettunen, M.; Pulkkanen, K.J.; Kauppinen, R.; Jackson, D.G.; Kubo, H.; Nishikawa, S.-I.; et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Waxler, J.L.; Guardino, C.; Feinn, R.S.; Lee, H.; Pober, B.R.; Stanley, T.L. Altered body composition, lipedema, and decreased bone density in individuals with Williams syndrome: A preliminary report. Eur. J. Med. Genet. 2017, 60, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Kozel, B.A.; Barak, B.; Kim, C.A.; Mervis, C.B.; Osborne, L.R.; Porter, M.; Pober, B.R. Williams syndrome. Nat. Rev. Dis. Primers 2021, 7, 42. [Google Scholar] [CrossRef]
- Stanley, T.L.; Leong, A.; Pober, B.R. Growth, body composition, and endocrine issues in Williams syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 28, 64–74. [Google Scholar] [CrossRef]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [Green Version]
- Michelini, S.; Herbst, K.L.; Precone, V.; Manara, E.; Marceddu, G.; Dautaj, A.; Maltese, P.E.; Paolacci, S.; Ceccarini, M.R.; Beccari, T.; et al. A Multi-Gene Panel to Identify Lipedema-Predisposing Genetic Variants by a Next-Generation Sequencing Strategy. J. Pers. Med. 2022, 12, 268. [Google Scholar] [CrossRef]
- Grigoriadis, D.; Sackey, E.; Riches, K.; van Zanten, M.; Brice, G.; England, R.; Mills, M.; Dobbins, S.E.; Consortium, L.; Consortium, G.E.R.; et al. Investigation of clinical characteristics and genome associations in the ‘UK Lipoedema’ cohort. medRxiv 2021. [Google Scholar] [CrossRef]
- Paolacci, S.; Precone, V.; Acquaviva, F.; Chiurazzi, P.; Fulcheri, E.; Pinelli, M.; Buffelli, F.; Michelini, S.; Herbst, K.L.; Unfer, V.; et al. Genetics of lipedema: New perspectives on genetic research and molecular diagnoses. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5581–5594. [Google Scholar]
| Lymphedema 1 | Lipedema 2 | |
|---|---|---|
| STAGE 0 | No observable limb swelling or pitting; limb is at risk for lymphedema following lymph node removal | N/A |
| STAGE 1 | Reversible lymphedema with symptoms of pitting edema; elevation reduces edema | Limbs exhibit smooth skin with enlarged subcutaneous adipose tissue |
| STAGE 2 | Stage 1 criteria plus fibrosis; pitting may be present; elevation does not fully reduce edema | Uneven skin with indentations in the adipose tissue and larger mounds of tissue able to be seen or felt |
| STAGE 3 | Tissue is hard and pitting can be absent; skin changes such as thickening and hyperpigmentation | Large extrusion of adipose tissue causing deformations especially around the thighs and knees |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duhon, B.H.; Phan, T.T.; Taylor, S.L.; Crescenzi, R.L.; Rutkowski, J.M. Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis. Int. J. Mol. Sci. 2022, 23, 6621. https://doi.org/10.3390/ijms23126621
Duhon BH, Phan TT, Taylor SL, Crescenzi RL, Rutkowski JM. Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis. International Journal of Molecular Sciences. 2022; 23(12):6621. https://doi.org/10.3390/ijms23126621
Chicago/Turabian StyleDuhon, Bailey H., Thien T. Phan, Shannon L. Taylor, Rachelle L. Crescenzi, and Joseph M. Rutkowski. 2022. "Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis" International Journal of Molecular Sciences 23, no. 12: 6621. https://doi.org/10.3390/ijms23126621
APA StyleDuhon, B. H., Phan, T. T., Taylor, S. L., Crescenzi, R. L., & Rutkowski, J. M. (2022). Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis. International Journal of Molecular Sciences, 23(12), 6621. https://doi.org/10.3390/ijms23126621






