Binding of Citrate-Fe3+ to Plastic Culture Dishes, an Artefact Useful as a Simple Technique to Screen for New Iron Chelators
Abstract
1. Introduction
2. Results
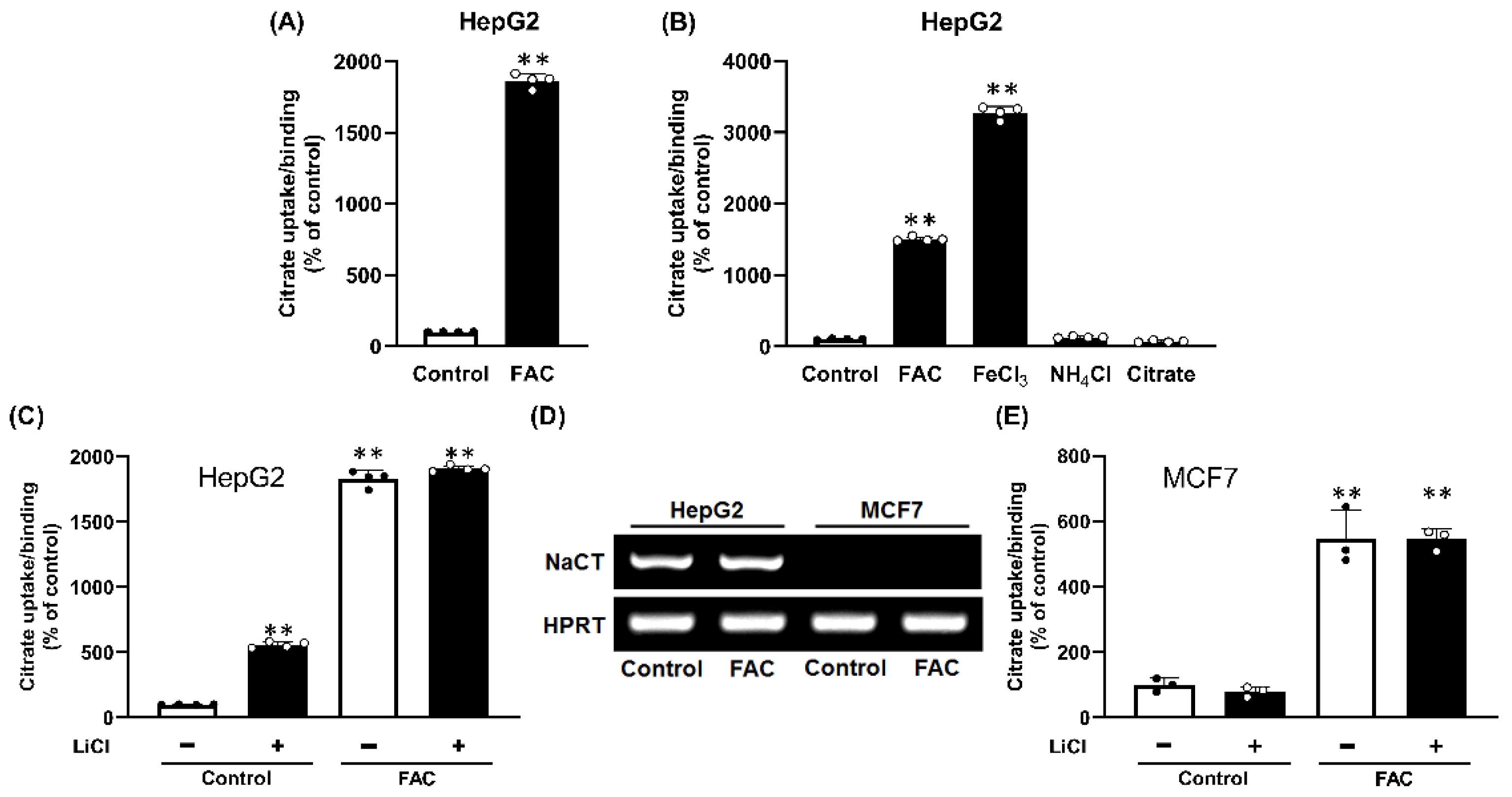
2.1. Citrate Uptake/Binding in Control and FAC (Ferric Ammonium Citrate)-Treated Liver Cells
2.2. Non-Involvement of NaCT in Citrate Uptake/Binding Induced by FAC Treatment
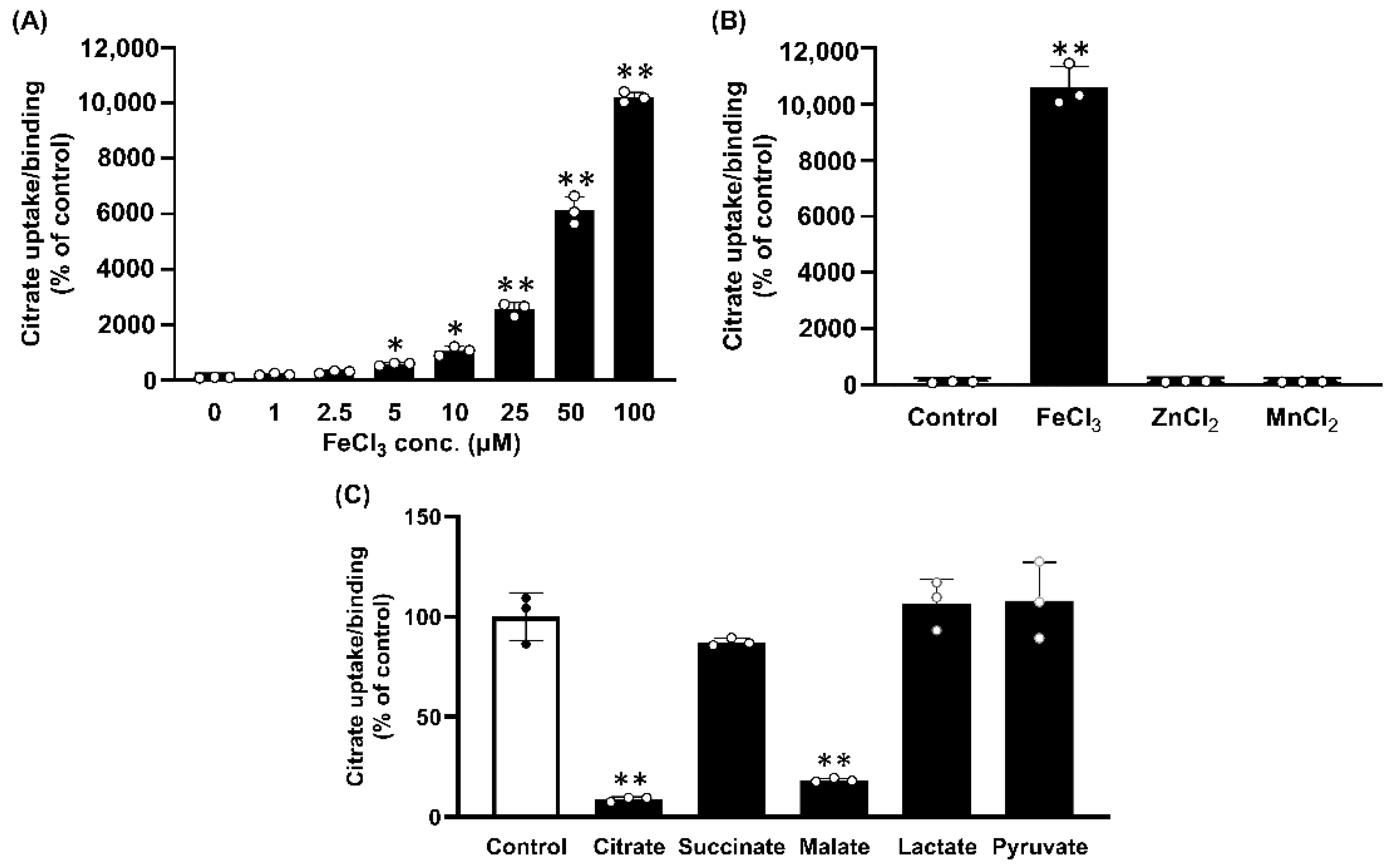
2.3. Dose-Response Relationship for the Stimulation of Citrate Uptake/Binding by Fe3+
2.4. Substrate Selectivity of Fe3+-Stimulated Citrate Uptake/Binding
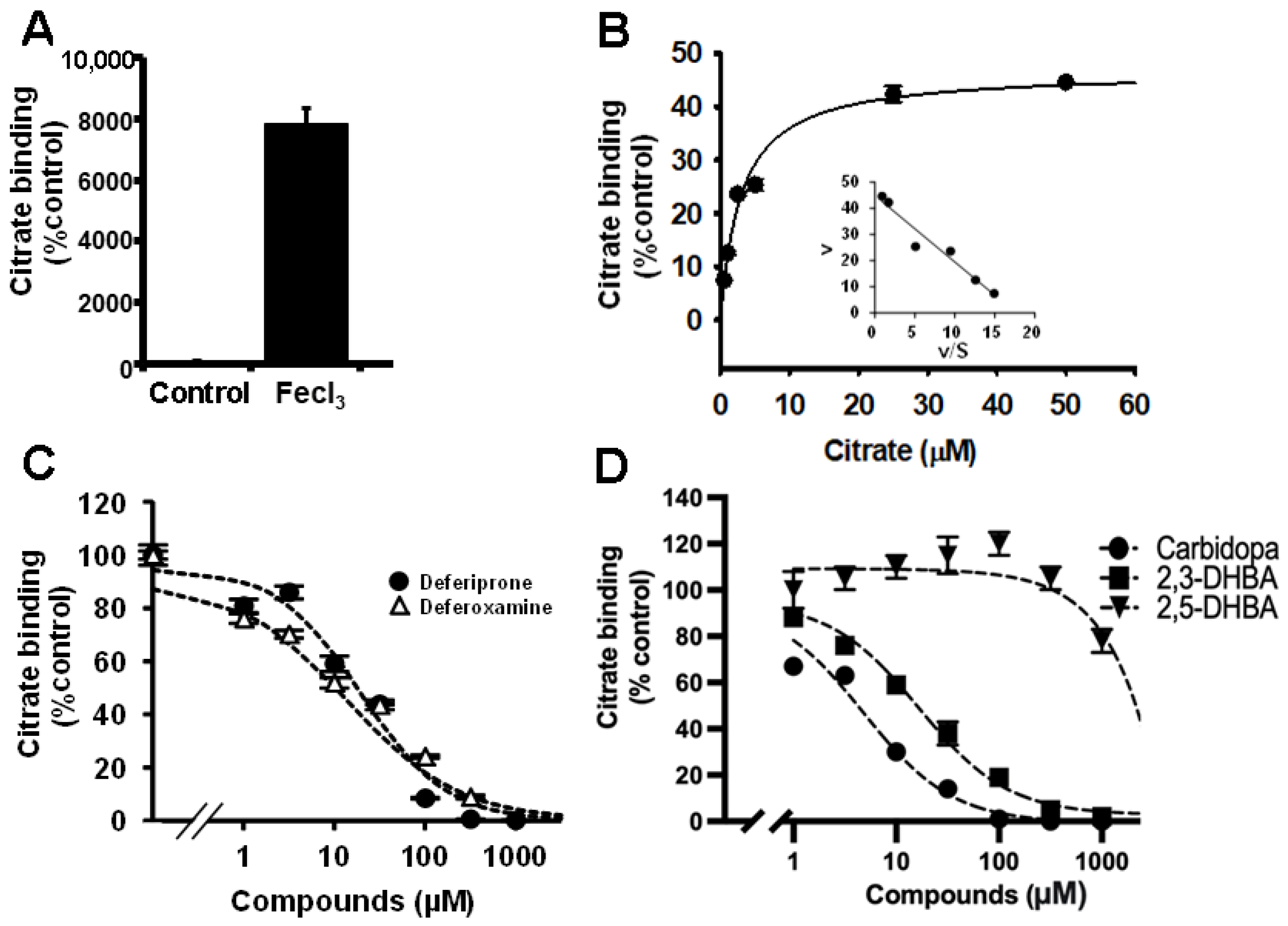
2.5. Mechanism of Fe3+-Stimulated Citrate Uptake/Binding
2.6. A Simple Screening Method for Potential Iron Chelators
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, K.; Zhuang, L.; Maddox, D.M.; Smith, S.B.; Ganapathy, V. Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J. Biol. Chem. 2002, 277, 39469–39476. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Zhuang, L.; Ganapathy, V. Human Na+-coupled citrate transporter: Primary structure, genomic organization, and transport function. Biochem. Biophys. Res. Commun. 2002, 299, 465–471. [Google Scholar] [CrossRef]
- Inoue, K.; Fei, Y.J.; Zhuang, L.; Gopal, E.; Miyauchi, S.; Ganapathy, V. Functional features and genomic organization of mouse NaCT, a sodium-coupled transporter for tricarboxylic acid cycle intermediates. Biochem. J. 2004, 378, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Gopal, E.; Miyauchi, S.; Martin, P.M.; Ananth, S.; Srinivas, S.R.; Smith, S.B.; Prasad, P.D.; Ganapathy, V. Expression and functional features of NaCT, a sodium-coupled citrate transporter, in human and rat livers and cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G402–G408. [Google Scholar] [CrossRef]
- Bergeron, M.J.; Clemencon, B.; Hediger, M.A.; Markovich, D. SLC13 family of Na+-coupled di- and tri-carboxylate/sulfate transporters. Mol. Asp. Med. 2013, 34, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Pajor, A.M. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflug. Arch. 2014, 466, 119–130. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.R.; Neves, J.; Mleczko-Sanecka, K.; Tandon, A.; Sauer, S.W.; Hentze, M.W.; Muckenthaler, M.U. Cellular citrate levels establish a regulatory link between energy metabolism and the hepatic iron hormone hepcidin. J. Mol. Med. 2017, 95, 851–860. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Xu, Y.; Alfaro-Magallanes, V.M.; Babitt, J.L. Physiological and pathological mechanisms of hepcidin regulation: Clinical implications for iron disorders. Br. J. Haematol. 2021, 193, 882–893. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Inoue, K.; Zhuang, L.; Maddox, D.M.; Smith, S.B.; Ganapathy, V. Human sodium-coupled citrate transporter, the orthologue of Drosophila Indy, as a novel target for lithium action. Biochem. J. 2003, 374, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kopel, J.; Higuchi, K.; Ristic, B.; Sato, T.; Ramachandran, S.; Ganapathy, V. The hepatic plasma membrane citrate transporter NaCT (SLC13A5) as a molecular target for metformin. Sci. Rep. 2020, 10, 8536. [Google Scholar] [CrossRef] [PubMed]
- Gopal, E.; Babu, E.; Ramachandran, S.; Bhutia, Y.D.; Prasad, P.D.; Ganapathy, V. Species-specific influence of lithium on the activity of SLC13A5 (NaCT): Lithium-induced activation is specific for the transporter in humans. J. Pharmacol. Exp. Ther. 2015, 353, 17–26. [Google Scholar] [CrossRef]
- Ito, S.; Ikuta, K.; Lynda, A.; Shibusa, K.; Niizeki, N.; Toki, V.; Hatayama, M.; Yamamoto, M.; Shindo, M.; Iizuka, N.; et al. In vivo behavior of NTBI revealed by automated quantification system. Int. J. Hematol. 2016, 104, 175–181. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stefanova, D.; Raychev, A.; Arezes, J.; Ruchala, P.; Gabayan, V.; Skurnik, M.; Dillon, B.J.; Horwitz, M.A.; Ganz, T.; Bulut, Y.; et al. Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron. Blood 2017, 130, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.L.; Gautier-Luneau, I. Iron and citric acid: A fuzzy chemistry of ubiquitous biological relevance. Biometals 2000, 13, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Gautier-Luneau, J.L.; Merle, C.; Phanon, D.; Lebrun, C.; Biaso, F.; Serratrice, G.; Pierre, J.L. New trends in the chemistry of iron(III) citrate complexes: Correlations between X-ray structures and solution species probed by electrospray mass spectrometry and kinetics of iron uptake from citrate by iron chelators. Chemistry 2005, 11, 2207–2219. [Google Scholar] [CrossRef]
- Silva, A.M.N.; Kong, X.; Parkin, M.C.; Cammack, R.; Hider, R.C. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 2009, 40, 8616–8625. [Google Scholar] [CrossRef]
- Devireddy, L.R.; Hart, D.O.; Goetz, D.H.; Green, M.R. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell 2010, 141, 1006–1017. [Google Scholar] [CrossRef]
- Crosa, J.H.; Walsh, C.T. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 223–249. [Google Scholar] [CrossRef]
- Billings, J.L.; Gordon, S.L.; Rawling, T.; Doble, P.A.; Bush, A.I.; Adlard, P.A.; Finkelstein, D.I.; Hare, D.J. l-3,4-Dihydroxyphenylalanine (l-DOPA) modulates brain iron, dopaminergic neurodegeneration and motor dysfunction in iron overload and mutant alpha-synuclein mouse models of Parkinson’s disease. J. Neurochem. 2019, 150, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Alhassen, S.; Senel, M.; Alachker, A. Surface plasmon resonance identifies high-affinity binding of l-DOPA to siderocalin/lipocalin-2 through iron-siderophore action: Implications for Parkinson’s disease treatment. ACS Chem. Neurosci. 2022, 13, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Bozko, M.; Zarod, M.; Witecka, A.; Kocdemir, K.; Jagielski, A.K.; Drozak, J. Recharacterization of the mammalian cytosolic type 2 (R)-β-hydroxybutyrate dehydrogenase as 4-oxo-l-proline reductase (EC1.1.1.104). J. Biol. Chem. 2022, 298, 101708. [Google Scholar] [CrossRef] [PubMed]
- Ogura, J.; Miyauchi, S.; Shimono, K.; Yang, S.; Gonchigar, S.; Ganapathy, V.; Bhutia, Y.D. Carbidopa is an activator of aryl hydrocarbon receptor with potential for cancer therapy. Biochem. J. 2017, 474, 3391–3402. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Gnana-Prakasam, J.P.; Veeranan-Karmegam, R.; Coothankandaswamy, V.; Reddy, S.K.; Martin, P.M.; Thangaraju, M.; Smith, S.B.; Ganapathy, V. Loss of Hfe leads to progression of tumor phenotype in primary retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 63–71. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Ristic, B.; Mudaliar, N.; Hamood, A.N.; Colmer-Hamood, J.; Wachtel, M.S.; Nevels, A.G.; Kottapalli, K.R.; Ganapathy, V. Hereditary hemochromatosis promotes colitis and colon cancer and causes bacterial dysbiosis in mice. Biochem. J. 2020, 477, 3867–3883. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ogura, J.; Grippo, P.J.; Torres, C.; Sato, T.; Wachtel, M.; Ramachandran, S.; Babu, E.; Sivaprakasam, S.; Rajasekaran, D.; et al. Chronic exposure to excess iron promotes EMT and cancer via p53 loss in pancreatic cancer. Asian J. Pharm. Sci. 2020, 15, 237–251. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogura, J.; Sato, T.; Higuchi, K.; Sivaprakasam, S.; Kopel, J.; Bhutia, Y.D.; Ganapathy, V. Binding of Citrate-Fe3+ to Plastic Culture Dishes, an Artefact Useful as a Simple Technique to Screen for New Iron Chelators. Int. J. Mol. Sci. 2022, 23, 6657. https://doi.org/10.3390/ijms23126657
Ogura J, Sato T, Higuchi K, Sivaprakasam S, Kopel J, Bhutia YD, Ganapathy V. Binding of Citrate-Fe3+ to Plastic Culture Dishes, an Artefact Useful as a Simple Technique to Screen for New Iron Chelators. International Journal of Molecular Sciences. 2022; 23(12):6657. https://doi.org/10.3390/ijms23126657
Chicago/Turabian StyleOgura, Jiro, Toshihiro Sato, Kei Higuchi, Sathish Sivaprakasam, Jonathan Kopel, Yangzom D. Bhutia, and Vadivel Ganapathy. 2022. "Binding of Citrate-Fe3+ to Plastic Culture Dishes, an Artefact Useful as a Simple Technique to Screen for New Iron Chelators" International Journal of Molecular Sciences 23, no. 12: 6657. https://doi.org/10.3390/ijms23126657
APA StyleOgura, J., Sato, T., Higuchi, K., Sivaprakasam, S., Kopel, J., Bhutia, Y. D., & Ganapathy, V. (2022). Binding of Citrate-Fe3+ to Plastic Culture Dishes, an Artefact Useful as a Simple Technique to Screen for New Iron Chelators. International Journal of Molecular Sciences, 23(12), 6657. https://doi.org/10.3390/ijms23126657





