DKK-1 Is Underexpressed in Mesenchymal Stem Cells from Patients with Ankylosing Spondylitis and Further Downregulated by IL-17
Abstract
:1. Introduction
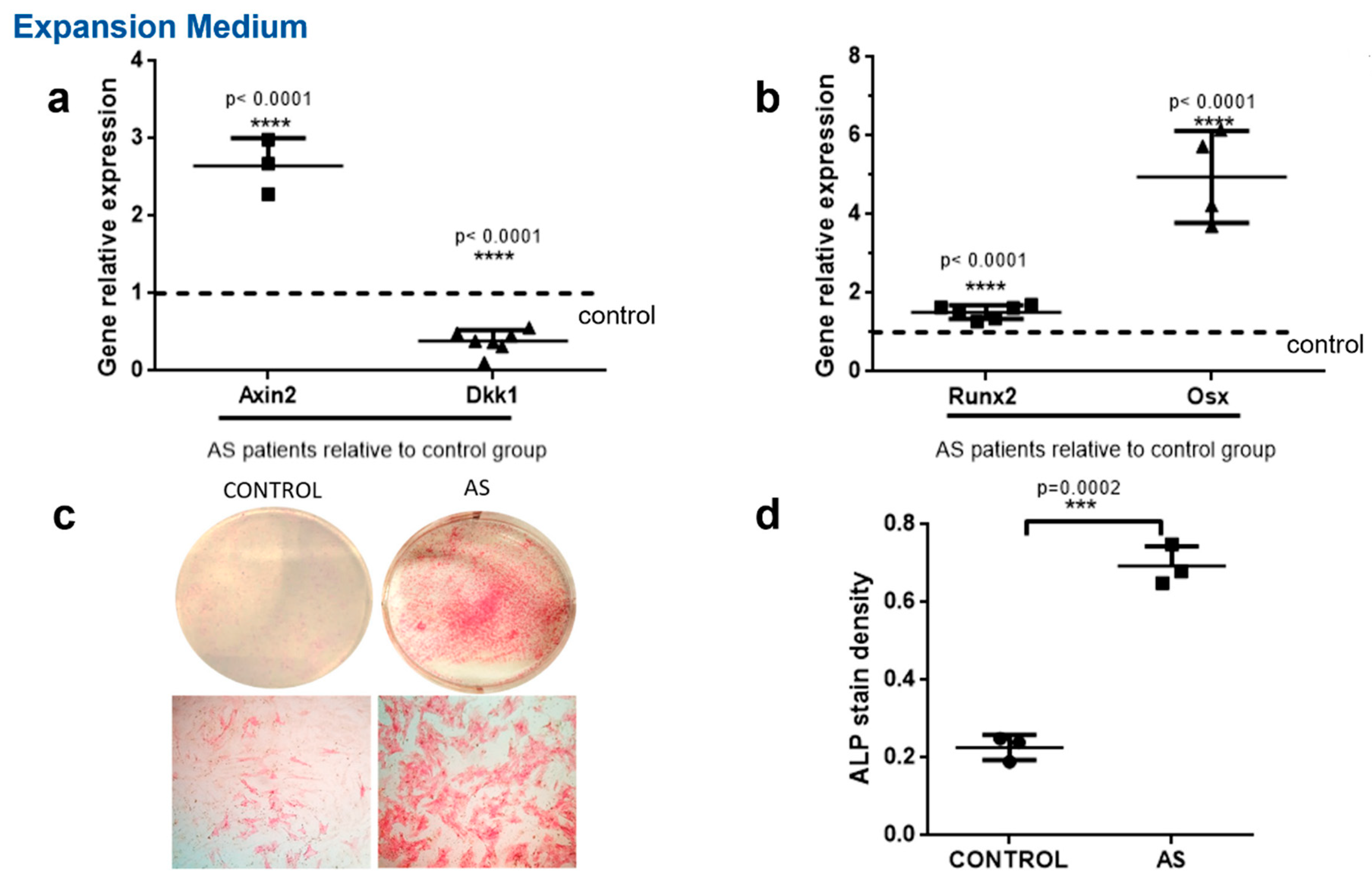
2. Results
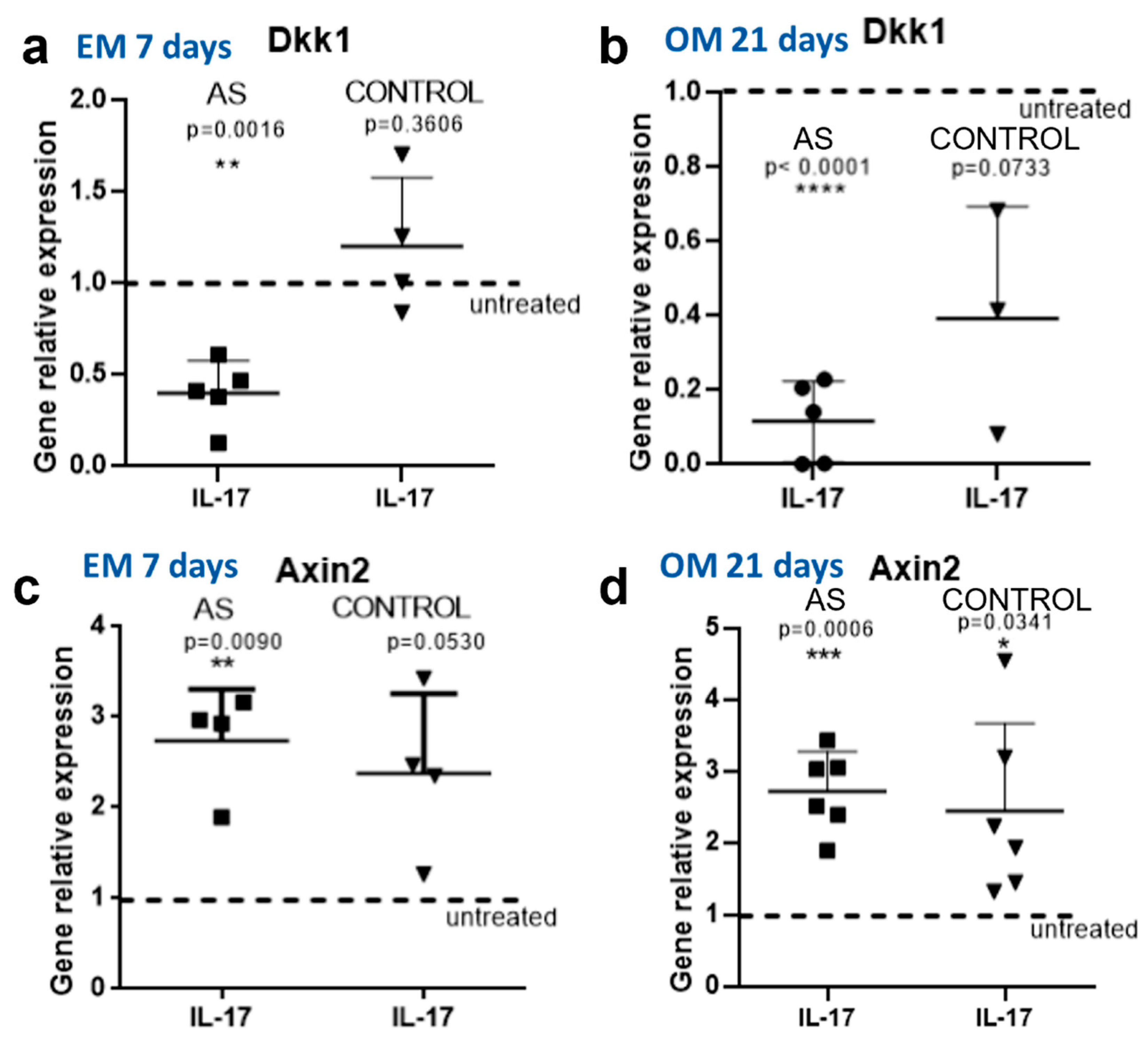
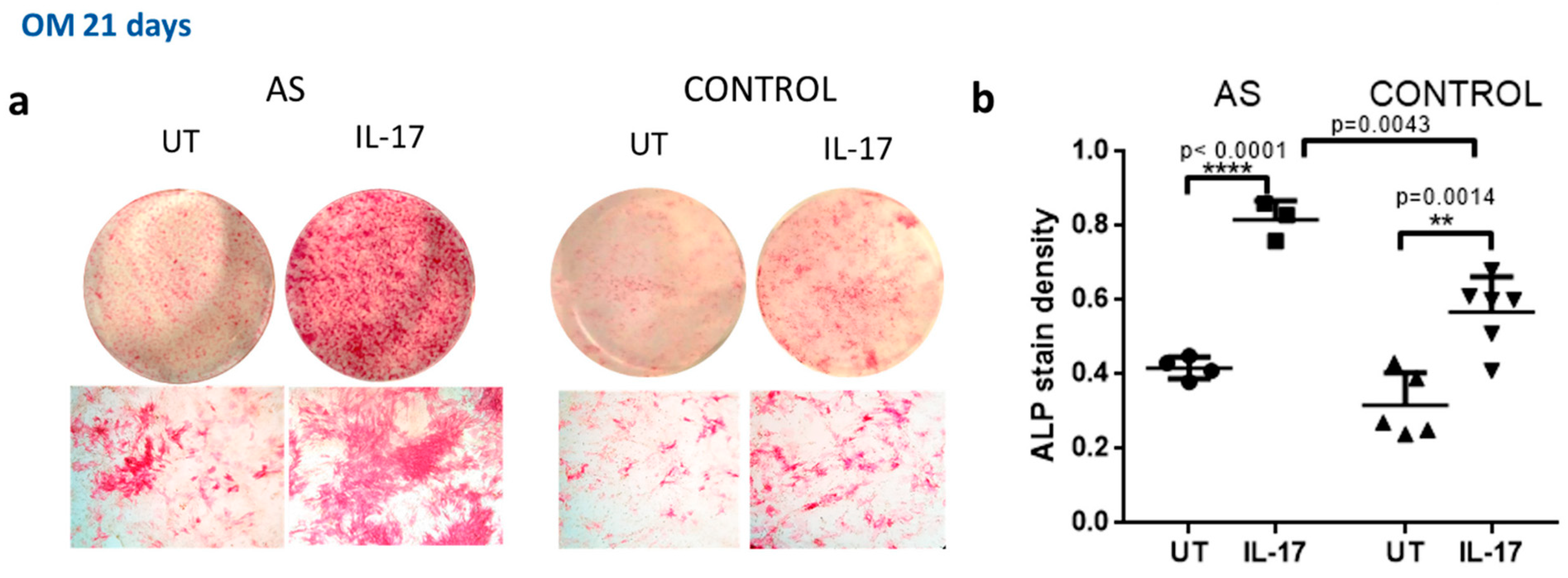
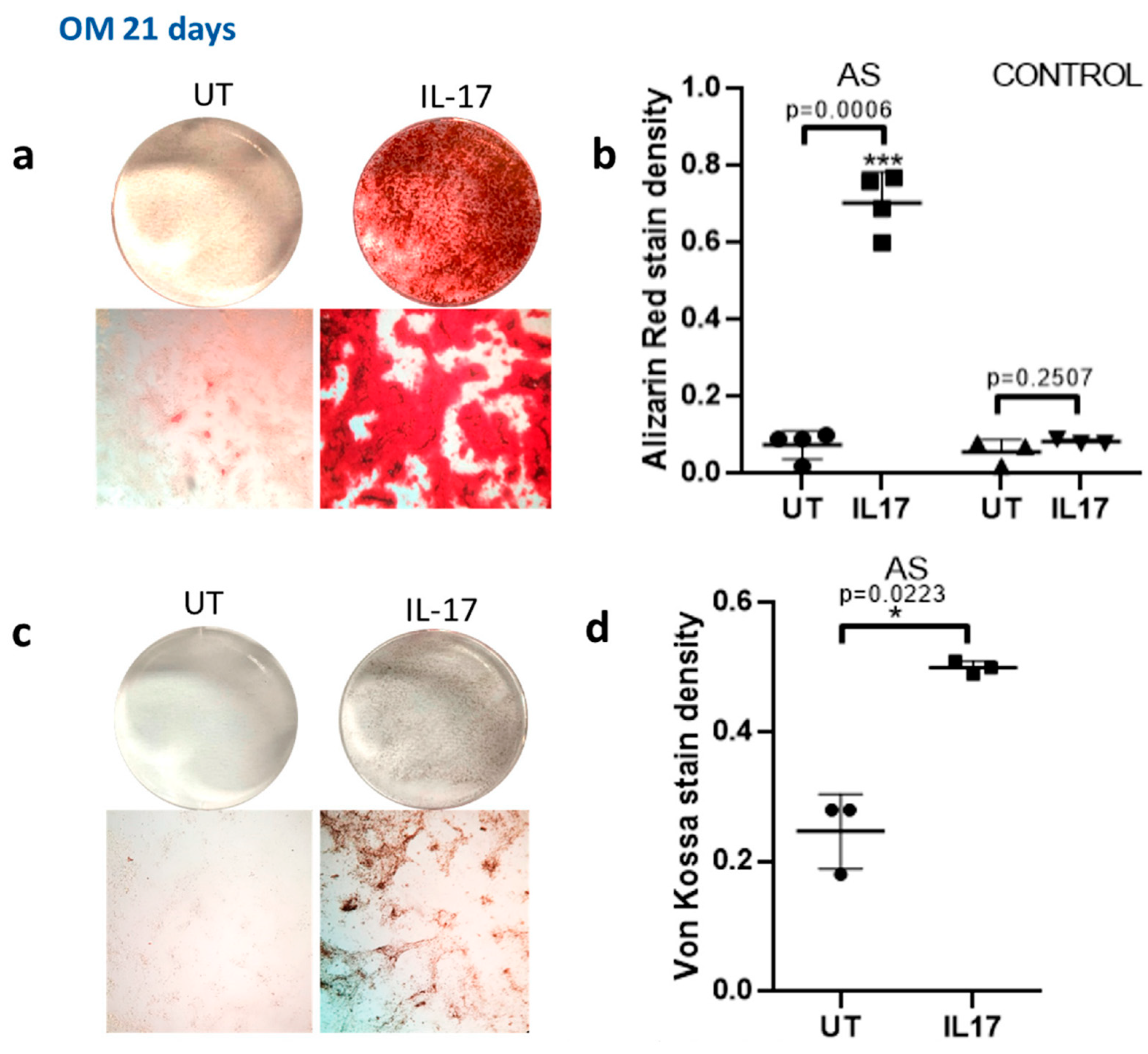
IL-17 Further Downregulates DKK-1 in MSCs from AS Patients
3. Discussion
4. Materials and Methods
4.1. Cell Isolation
4.2. In Vitro IL-17 Stimulation
4.3. Quantitative RT-PCR Analysis
4.4. Alkaline Phosphatase (ALP) Staining
4.5. Alizarin Red Staining
4.6. Von Kossa Staining
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Daoussis, D.; Andonopoulos, A.P.; Liossis, S.-N.C. Wnt Pathway and IL-17: Novel Regulators of Joint Remodeling in Rheumatic Diseases. Looking Beyond the RANK-RANKL-OPG Axis. Semin. Arthritis Rheum. 2008, 39, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Klavdianou, K.; Liossis, S.-N.; Sakkas, L.; Daoussis, D. The role of Dickkopf-1 in joint remodeling and fibrosis: A link connecting spondyloarthropathies and scleroderma? Semin. Arthritis Rheum. 2016, 46, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Heiland, G.; Horn, A.; Zerr, P.; Hofstetter, W.; Baum, W.; Stock, M.; Distler, J.H.; Nimmerjahn, F.; Schett, G.; Zwerina, J. Blockade of the hedgehog pathway inhibits osteophyte formation in arthritis. Ann. Rheum. Dis. 2012, 71, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Dittmann, K.; Wuelling, M.; Uhmann, A.; Dullin, C.; Hahn, H.; Schweyer, S.; Vortkamp, A.; Wienands, J. Inactivation of Patched 1 in Murine Chondrocytes Causes Spinal Fusion without Inflammation. Arthritis Rheumatol. 2013, 66, 831–840. [Google Scholar] [CrossRef]
- Daoussis, D.; Filippopoulou, A.; Liossis, S.-N.; Sirinian, C.; Klavdianou, K.; Bouris, P.; Karamanos, N.K.; Andonopoulos, A.P. Anti-TNFα treatment decreases the previously increased serum Indian Hedgehog levels in patients with ankylosing spondylitis and affects the expression of functional Hedgehog pathway target genes. Semin. Arthritis Rheum. 2015, 44, 646–651. [Google Scholar] [CrossRef]
- Lories, R.J.; Derese, I.; Luyten, F.P. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J. Clin. Investig. 2005, 115, 1571–1579. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, J.; Zhan, Z.; Li, S.; Zheng, Z.; Wang, T.; Zhang, K.; Pan, H.; Li, Z.; Zhang, N.; et al. Inflammation Intensity-Dependent Expression of Osteoinductive Wnt Proteins Is Critical for Ectopic New Bone Formation in Ankylosing Spondylitis. Arthritis Rheumatol. 2018, 70, 1056–1070. [Google Scholar] [CrossRef]
- Boyden, L.M.; Mao, J.; Belsky, J.; Mitzner, L.; Farhi, A.; Mitnick, M.A.; Wu, D.; Insogna, K.; Lifton, R.P. High Bone Density Due to a Mutation in LDL-Receptor–Related Protein 5. N. Engl. J. Med. 2002, 346, 1513–1521. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007, 13, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Uderhardt, S.; Diarra, D.; Katzenbeisser, J.; David, J.; Zwerina, J.; Richards, W.G.; Krönke, G.; Schett, G. Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann. Rheum. Dis. 2009, 69, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Pavy, S.; Boudaoud, S.; Seror, R.; Goupille, P.; Chanson, P.; Van Der Heijde, D.; Van Gaalen, F.; Berenbaum, F.; Mariette, X.; et al. Increase in Dickkopf-1 Serum Level in Recent Spondyloarthritis. Data from the DESIR Cohort. PLoS ONE 2015, 10, e0134974. [Google Scholar] [CrossRef] [Green Version]
- Daoussis, D.; Liossis, S.-N.C.; Solomou, E.E.; Tsanaktsi, A.; Bounia, K.; Karampetsou, M.; Yiannopoulos, G.; Andonopoulos, A.P. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Care Res. 2009, 62, 150–158. [Google Scholar] [CrossRef]
- Benjamin, M.; Toumi, H.; Suzuki, D.; Hayashi, K.; McGonagle, D. Evidence for a distinctive pattern of bone formation in enthesophytes. Ann. Rheum. Dis. 2008, 68, 1003–1010. [Google Scholar] [CrossRef]
- Sherlock, J.P.; Joyce-Shaikh, B.; Turner, S.P.; Chao, C.C.; Sathe, M.; Grein, J.; Cua, D.J. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat. Med. 2012, 18, 1069–1076. [Google Scholar] [CrossRef]
- Blair, H.A. Secukinumab: A Review in Ankylosing Spondylitis. Drugs 2019, 79, 433. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Poddubnyy, D.; Emery, P.; Delicha, E.M.; Talloczy, Z.; Porter, B. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology 2018, 58, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Yeremenko, N.; Zwerina, K.; Rigter, G.; Pots, D.; Fonseca, J.E.; Zwerina, J.; Schett, G.; Baeten, D. Brief Report: Tumor Necrosis Factor and Interleukin-6 Differentially Regulate Dkk-1 in the Inflamed Arthritic Joint. Arthritis Rheumatol. 2015, 67, 2071–2075. [Google Scholar] [CrossRef]
- Jacques, P.; Lambrecht, S.; Verheugen, E.; Pauwels, E.; Kollias, G.; Armaka, M.; Verhoye, M.; Van der Linden, A.; Achten, R.; Lories, R.J.; et al. Proof of concept: Enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann. Rheum. Dis. 2013, 73, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Cambré, I.; Gaublomme, D.; Burssens, A.; Jacques, P.; Schryvers, N.; De Muynck, A.; Meuris, L.; Lambrecht, S.; Carter, S.; De Bleser, P.; et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotake, S.; Udagawa, N.; Takahashi, N.; Matsuzaki, K.; Itoh, K.; Ishiyama, S.; Saito, S.; Inoue, K.; Kamatani, N.; Gillespie, M.; et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 1999, 103, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Lubberts, E.; van den Bersselaar, L.; Oppers-Walgreen, B.; Schwarzenberger, P.; Coenen-de Roo, C.J.J.; Kolls, J.K.; van den Berg, W. BIL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J. Immunol. 2003, 170, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.-A.; Kannan, T.-P.; Norazmi, M.-N.; Nurul, A.-A. Interleukin-17A promotes osteogenic differentiation by increasing OPG/RANKL ratio in stem cells from human exfoliated deciduous teeth (SHED). J. Tissue Eng. Regen. Med. 2018, 12, 1856–1866. [Google Scholar] [CrossRef]
- Croes, M.; Öner, F.C.; van Neerven, D.; Sabir, E.; Kruyt, M.C.; Blokhuis, T.J.; Dhert, W.J.; Alblas, J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 2016, 84, 262–270. [Google Scholar] [CrossRef]
- Ono, T.; Okamoto, K.; Nakashima, T.; Nitta, T.; Hori, S.; Iwakura, Y.; Takayanagi, H. IL-17-producing γδ T cells enhance bone regeneration. Nat. Commun. 2016, 7, 10928. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.T.; Maeda, Y.; Gravallese, E.M. IL-17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis. Arthritis Res. Ther. 2016, 18, 104. [Google Scholar] [CrossRef] [Green Version]
- Uluçkan, Ö.; Jimenez, M.; Karbach, S.; Jeschke, A.; Graña, O.; Keller, J.; Wagner, E.F. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci. Transl. Med. 2016, 8, 330RA37. [Google Scholar] [CrossRef]
- DeSelm, C.; Takahata, Y.; Warren, J.; Chappel, J.C.; Khan, T.; Li, X.; Liu, C.; Choi, Y.; Kim, Y.F.; Zou, W.; et al. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. J. Cell. Biochem. 2012, 113, 2895–2902. [Google Scholar] [CrossRef] [Green Version]
- Osta, B.; Lavocat, F.; Eljaafari, A.; Miossec, P. Effects of Interleukin-17A on Osteogenic Differentiation of Isolated Human Mesenchymal Stem Cells. Front. Immunol. 2014, 5, 425. [Google Scholar] [CrossRef] [Green Version]
- van Tok, M.N.; van Duivenvoorde, L.M.; Kramer, I.; Ingold, P.; Pfister, S.; Roth, L.; Baeten, D.L. Interleukin-17A Inhibition Diminishes Inflammation and New Bone Formation in Experimental Spondyloarthritis. Arthritis Rheumatol. 2019, 71, 612–625. [Google Scholar] [PubMed]
- Jo, S.; Wang, S.E.; Lee, Y.L.; Kang, S.; Lee, B.; Han, J.; Kim, T.H. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res. Ther. 2018, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Maroof, A.; Gikas, P.; Mittal, G.; Keen, R.; Baeten, D.; Shaw, S.; Roberts, S.J. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open 2020, 6, e001306. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.; Maksymowych, W.P.; Wordsworth, B.P.; Inman, R.; Danoy, P.; Rahman, P.; Stone, M.A.; Corr, M.; Gensler, L.S.; Gladman, D.; et al. Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann. Rheum. Dis. 2014, 74, 1387–1393. [Google Scholar] [CrossRef] [Green Version]

| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| Runx2 | 5′-CGGAGTGGACGAGGCAAGA-3′ | 5′-GAGGCGGTCAGAGAACAAACT-3′ |
| Osterix | 5′-AGGTTCCCCCAGCTCTCT-3′ | 5′-TTCTTTGTGCCTGCTTTGCC-3′ |
| Osteonectin (sparc) | 5′-GGCAGAGGTGACTGAGGTATC-3′ | 5′-GTTTGCAGTGGTGGTTCTGG-3′ |
| Axin2 | 5′-TGTGAGGTCCACGGAAACTG-3′ | 5′-TTCCATCTACACTGCTGTCCG-3′ |
| Dkk1 | 5′-GAATAAGTACCAGACCATTGAC-3′ | 5′-CCATTTTTGCAGTAATTCCC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoussis, D.; Kanellou, A.; Panagiotopoulos, E.; Papachristou, D. DKK-1 Is Underexpressed in Mesenchymal Stem Cells from Patients with Ankylosing Spondylitis and Further Downregulated by IL-17. Int. J. Mol. Sci. 2022, 23, 6660. https://doi.org/10.3390/ijms23126660
Daoussis D, Kanellou A, Panagiotopoulos E, Papachristou D. DKK-1 Is Underexpressed in Mesenchymal Stem Cells from Patients with Ankylosing Spondylitis and Further Downregulated by IL-17. International Journal of Molecular Sciences. 2022; 23(12):6660. https://doi.org/10.3390/ijms23126660
Chicago/Turabian StyleDaoussis, Dimitrios, Anastasia Kanellou, Elias Panagiotopoulos, and Dionysios Papachristou. 2022. "DKK-1 Is Underexpressed in Mesenchymal Stem Cells from Patients with Ankylosing Spondylitis and Further Downregulated by IL-17" International Journal of Molecular Sciences 23, no. 12: 6660. https://doi.org/10.3390/ijms23126660







