Involvement of Ceramide Signalling in Radiation-Induced Tumour Vascular Effects and Vascular-Targeted Therapy
Abstract
:1. Introduction
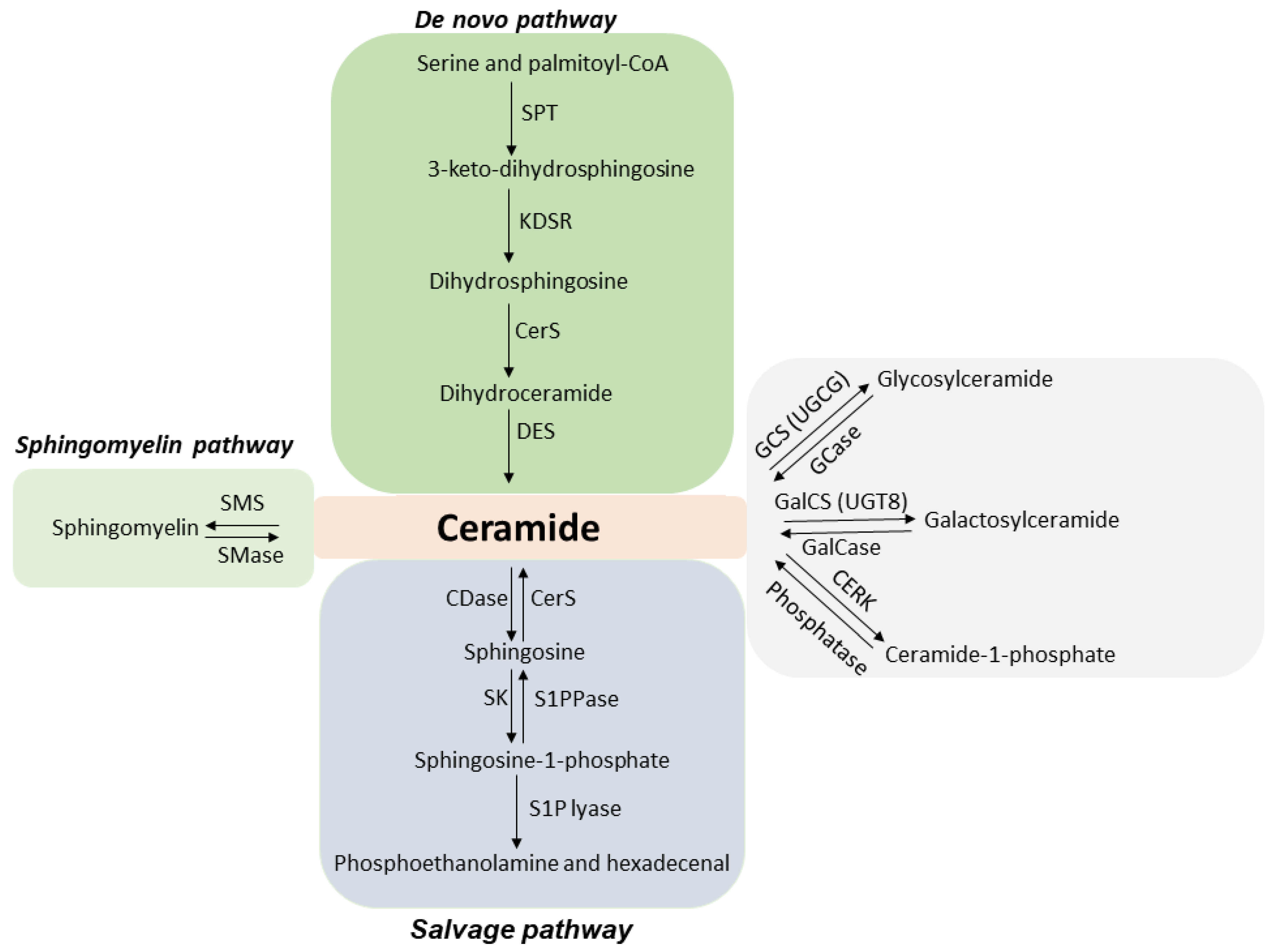
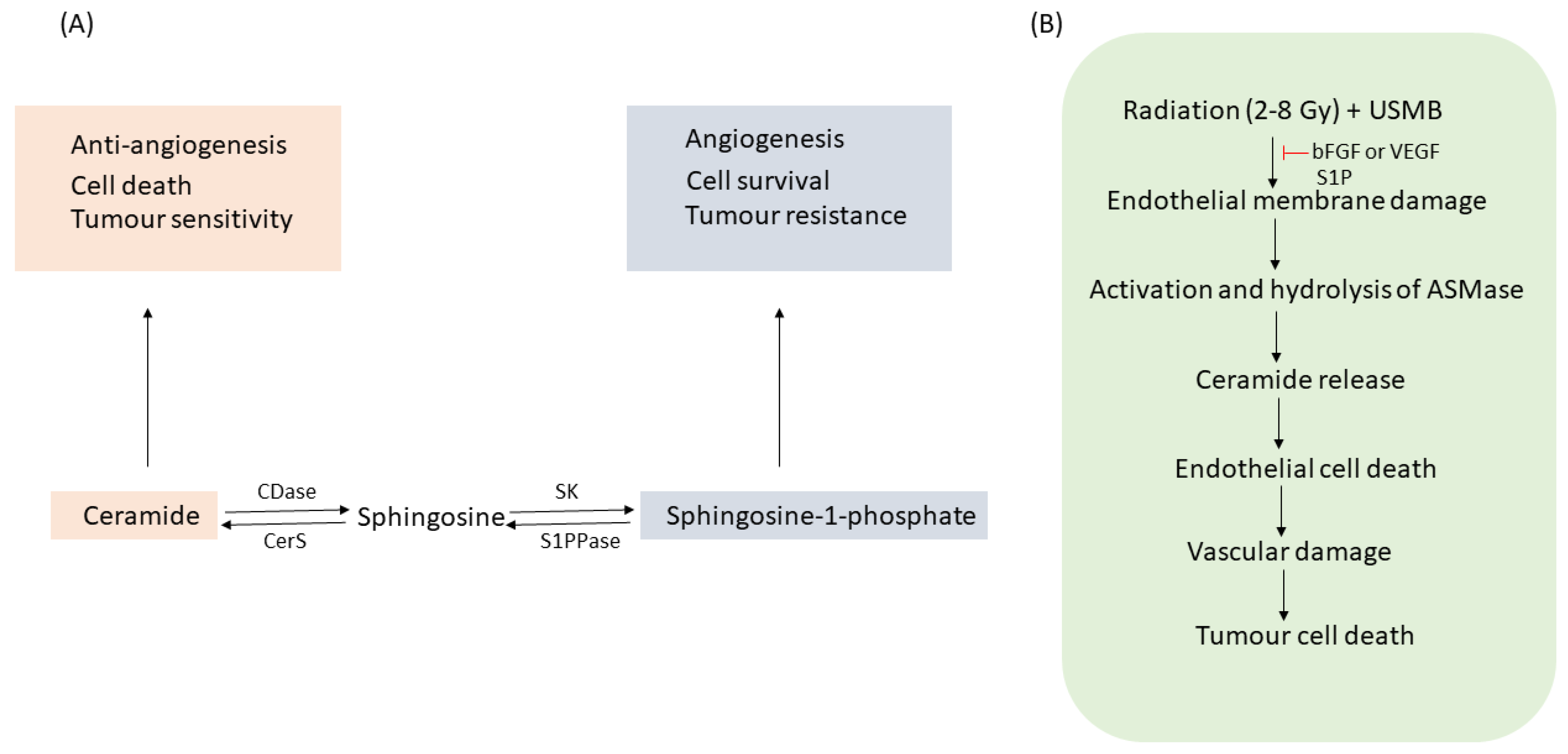
1.1. ASMase and Ceramide
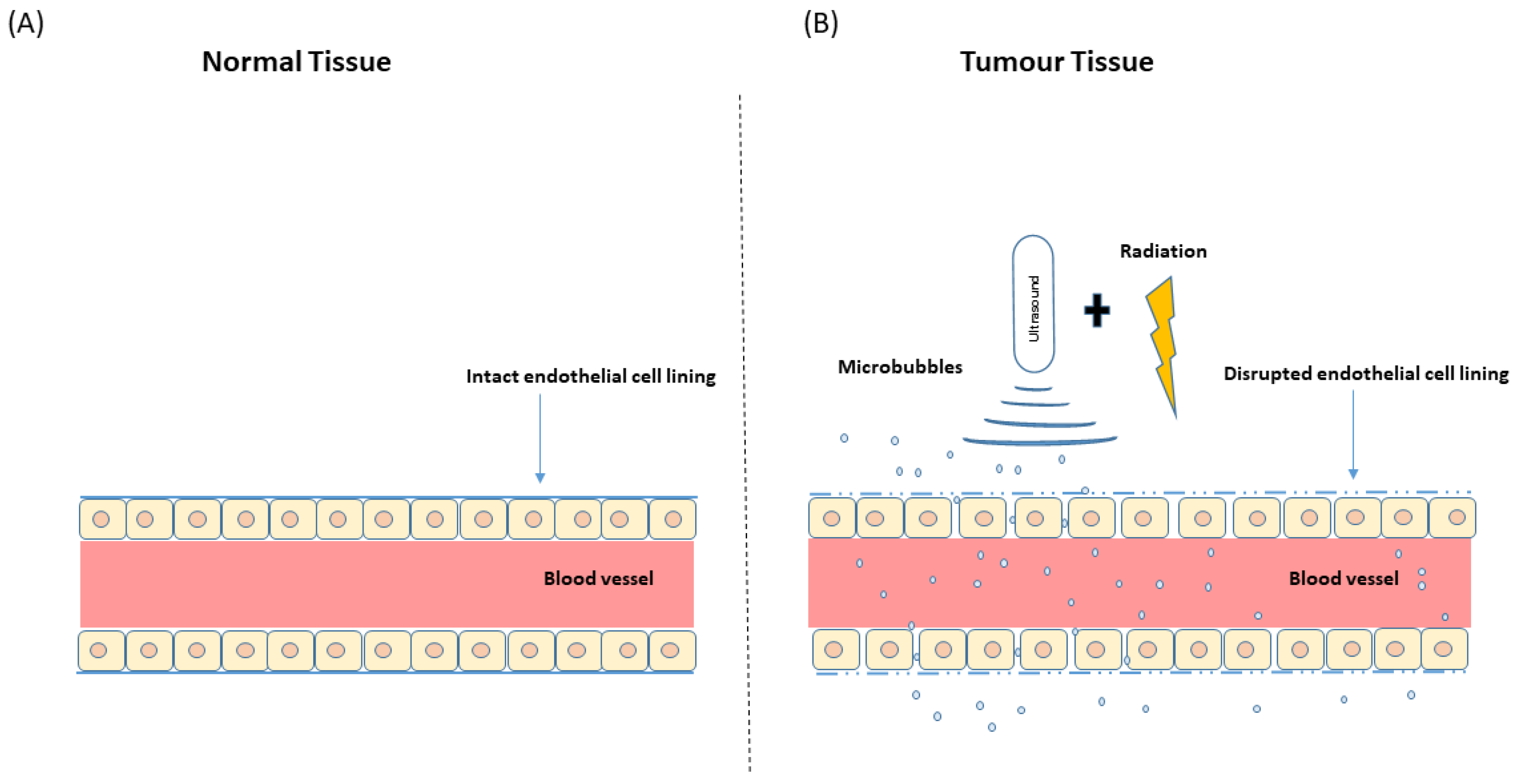
1.2. Microbubbles and Ultrasound: Ultrasound-Stimulated Microbubbles
1.3. Radiation-Induced Cell Death
2. Microbubble-Based Radiation Enhancement: Ceramide-Induced Cell Death
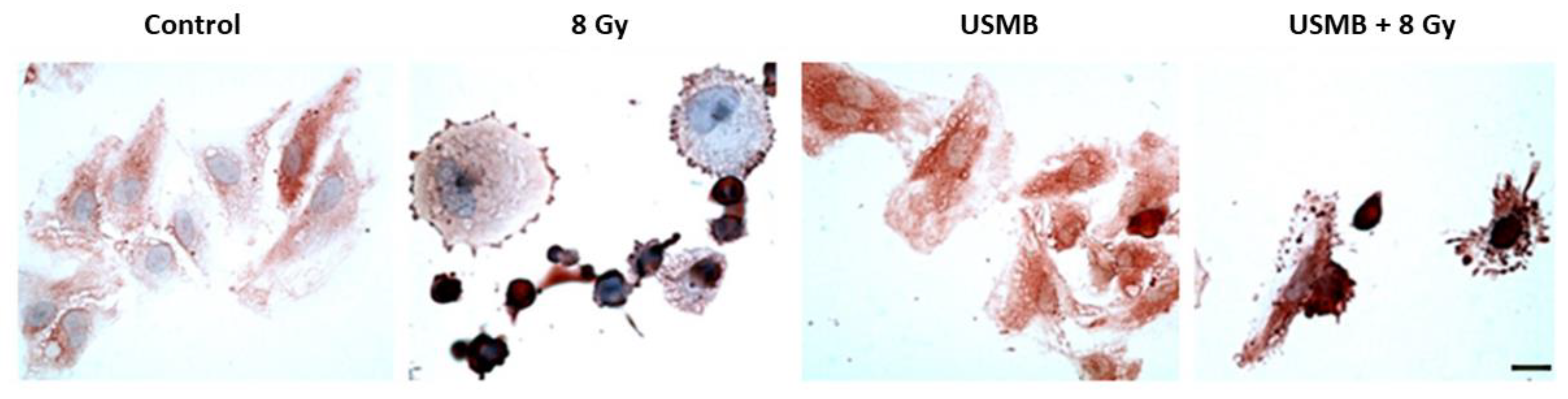
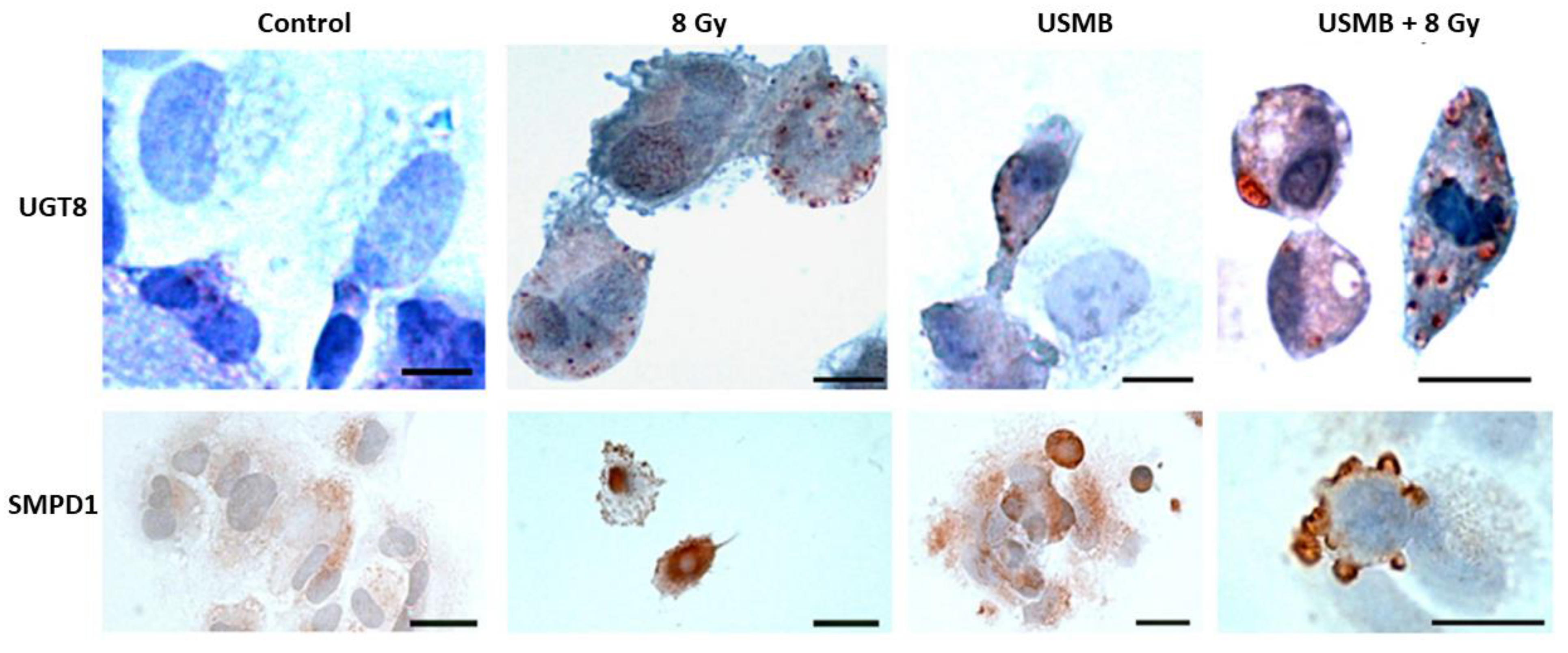
In Vitro Studies
3. Microbubble-Based Radiation Enhancement: Ceramide-Induced Cell Death
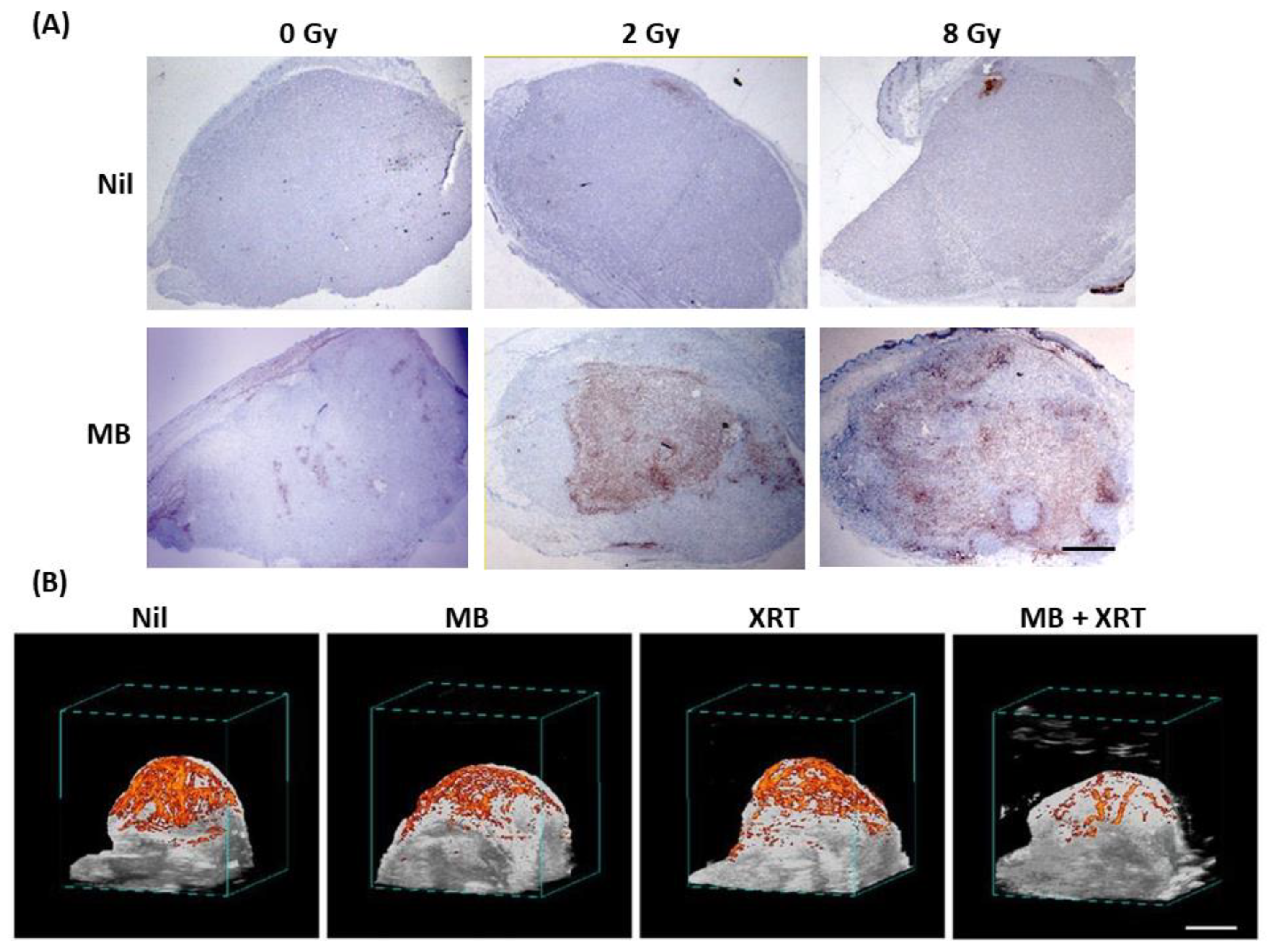
In Vivo Studies
4. USMB and Radiation Effect on Tumour Vasculature
5. Effects of Sequencing and Treatment Parameters
6. Clinical Relevance of USMB and Ceramide
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeidan, Y.H.; Hannun, Y.A. The Acid Sphingomyelinase/Ceramide Pathway: Biomedical Significance and Mechanisms of Regulation. Curr. Mol. Med. 2010, 999, 1–12. [Google Scholar] [CrossRef]
- Henry, B.; Ziobro, R.; Becker, K.A.; Kolesnick, R.; Gulbins, E. Acid Sphingomyelinase. In Handbook of Experimental Pharmacology; Springer Science and Business Media, LLC: Berlin/Heidelberg, Germany, 2013; pp. 77–88. [Google Scholar]
- Schneider, P.B.; Kennedy, E.P. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J. Lipid Res. 1967, 8, 202–209. [Google Scholar] [CrossRef]
- Schissel, S.L.; Jiang, X.-C.; Tweedie-Hardman, J.; Jeong, T.-S.; Camejo, E.H.; Najib, J.; Rapp, J.H.; Williams, K.J.; Tabas, I. Secretory Sphingomyelinase, a Product of the Acid Sphingomyelinase Gene, Can Hydrolyze Atherogenic Lipoproteins at Neutral pH: Implications for Atherosclerotic Lesion Development. J. Biol. Chem. 1998, 273, 2738–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schissel, S.L.; Keesler, G.A.; Schuchman, E.H.; Williams, K.J.; Tabas, I. The Cellular Trafficking and Zinc Dependence of Secretory and Lysosomal Sphingomyelinase, Two Products of the Acid Sphingomyelinase Gene. J. Biol. Chem. 1998, 273, 18250–18259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, R.W.; Canals, D.; Hannun, Y.A. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell. Signal. 2009, 21, 836–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassmé, H.; Jendrossek, V.; Riehle, A.; Von Kürthy, G.; Berger, J.; Schwarz, H.; Weller, M.; Kolesnick, R.; Gulbins, E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 2003, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, Y.H.; Jenkins, R.W.; Hannun, Y.A. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J. Cell Biol. 2008, 181, 335–350. [Google Scholar] [CrossRef] [Green Version]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef] [Green Version]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. In Sphingolipids as Signaling and Regulatory Molecules; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–23. [Google Scholar] [CrossRef] [Green Version]
- Merrill, A.H., Jr. Sphingolipid and Glycosphingolipid Metabolic Pathways in the Era of Sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Merrill, A.H. De Novo Sphingolipid Biosynthesis: A Necessary, but Dangerous, Pathway. J. Biol. Chem. 2002, 277, 25843–25846. [Google Scholar] [CrossRef] [Green Version]
- Summers, S.A.; Chaurasia, B.; Holland, W.L. Metabolic Messengers: Ceramides. Nat. Metab. 2019, 1, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Haimovitz-Friedman, A.; Kolesnick, R.N.; Fuks, Z. Ceramide signaling in apoptosis. Br. Med Bull. 1997, 53, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Kolesnick, R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J. Clin. Investig. 2002, 110, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Kolesnick, R. Acid Sphingomyelinase-derived Ceramide Signaling in Apoptosis. Subcell. Biochem. 2002, 36, 229–244. [Google Scholar] [CrossRef]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Ceramide in apoptosis: An overview and current perspectives. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2002, 1585, 114–125. [Google Scholar] [CrossRef]
- Kuzmenko, D.I.; Klimentyeva, T.K. Role of ceramide in apoptosis and development of insulin resistance. Biokhimiya 2016, 81, 913–927. [Google Scholar] [CrossRef]
- Dadsena, S.; Bockelmann, S.; Mina, J.G.M.; Hassan, D.G.; Korneev, S.; Razzera, G.; Jahn, H.; Niekamp, P.; Müller, D.; Schneider, M.; et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun. 2019, 10, 1832. [Google Scholar] [CrossRef]
- Zeidan, Y.H.; Hannun, Y.A. Activation of Acid Sphingomyelinase by Protein Kinase Cδ-mediated Phosphorylation. J. Biol. Chem. 2007, 282, 11549–11561. [Google Scholar] [CrossRef] [Green Version]
- Zeidan, Y.H.; Wu, B.X.; Jenkins, R.W.; Obeid, L.M.; Hannun, Y.A. A novel role for protein kinase Cδ-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. FASEB J. 2008, 22, 183–193. [Google Scholar] [CrossRef]
- Jin, S.; Yi, F.; Zhang, F.; Poklis, J.L.; Li, P.-L. Lysosomal Targeting and Trafficking of Acid Sphingomyelinase to Lipid Raft Platforms in Coronary Endothelial Cells. Arter. Thromb. Vasc. Biol. 2008, 28, 2056–2062. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gulbins, E.; Zhang, Y. Oxidative Stress Triggers Ca2+-Dependent Lysosome Trafficking and Activation of Acid Sphingomyelinase. Cell. Physiol. Biochem. 2012, 30, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Haimovitz-Friedman, A.; Kan, C.C.; Ehleiter, D.; Persaud, R.S.; McLoughlin, M.; Fuks, Z.; Kolesnick, R.N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J. Exp. Med. 1994, 180, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassmé, H.; Riethmüller, J.; Gulbins, E. Biological aspects of ceramide-enriched membrane domains. Prog. Lipid Res. 2007, 46, 161–170. [Google Scholar] [CrossRef]
- Bollinger, C.R.; Teichgräber, V.; Gulbins, E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2005, 1746, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blitterswijk, W.J.; van Luit, A.H.; van der Veldman, R.J.; Verheij, M.; Borst, J. Ceramide: Second messenger or modulator of membrane structure and dynamics? Biochem. J. 2003, 369, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Kolesnick, R. Raft ceramide in molecular medicine. Oncogene 2003, 22, 7070–7077. [Google Scholar] [CrossRef] [Green Version]
- Gulbins, E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacol. Res. 2003, 47, 393–399. [Google Scholar] [CrossRef]
- Grassme, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 Signaling via Ceramide-rich Membrane Rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef] [Green Version]
- Grassmé, H.; Jendrossek, V.; Bock, J.; Riehle, A.; Gulbins, E. Ceramide-Rich Membrane Rafts Mediate CD40 Clustering. J. Immunol. 2002, 168, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Grassmé, H.; Cremesti, A.; Kolesnick, R.; Gulbins, E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene 2003, 22, 5457–5470. [Google Scholar] [CrossRef] [Green Version]
- Viola, A.; Schroeder, S.; Sakakibara, Y.; Lanzavecchia, A. T Lymphocyte Costimulation Mediated by Reorganization of Membrane Microdomains. Science 1999, 283, 680–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremesti, A.; Paris, F.; Grassmé, H.; Holler, N.; Tschopp, J.; Fuks, Z.; Gulbins, E.; Kolesnick, R. Ceramide Enables Fas to Cap and Kill. J. Biol. Chem. 2001, 276, 23954–23961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolesnick, R. Ceramide: A novel second messenger. Trends Cell. Biol. 1992, 2, 232–236. [Google Scholar] [CrossRef]
- Mathias, S.; Kolesnick, R. Ceramide: A novel second messenger. Adv. Lipid Res. 1993, 25, 65–90. [Google Scholar] [PubMed]
- Okazaki, T.; Kondo, T.; Kitano, T.; Tashima, M. Diversity and Complexity of Ceramide Signalling in Apoptosis. Cell. Signal. 1998, 10, 685–692. [Google Scholar] [CrossRef]
- Woodcock, J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life. Int. Union Biochem. Mol. Biol. Life 2006, 58, 462–466. [Google Scholar] [CrossRef]
- Beach, J.A.; Aspuria, P.-J.P.; Cheon, D.-J.; Lawrenson, K.; Agadjanian, H.; Walsh, C.S.; Karlan, B.Y.; Orsulic, S. Sphingosine kinase 1 is required for TGF-β mediated fibroblast-to-myofibroblast differentiation in ovarian cancer. Oncotarget 2015, 7, 4167–4182. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, Y.; Zhang, C.; Fan, F.; Yang, W. Sphingosine Kinase 1 and Sphingosine-1-Phosphate Signaling in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 2109. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Qi, Y.; Zhao, Y.; Kaczorowski, D.; Couttas, T.; Coleman, P.R.; Don, A.; Bertolino, P.; Gamble, J.R.; Vadas, M.A.; et al. Deletion of sphingosine kinase 1 inhibits liver tumorigenesis in diethylnitrosamine-treated mice. Oncotarget 2018, 9, 15635–15649. [Google Scholar] [CrossRef] [Green Version]
- Pyne, N.J.; El Buri, A.; Adams, D.R.; Pyne, S. Sphingosine 1-phosphate and cancer. Adv. Biol. Regul. 2018, 68, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Pitman, M.R.; Powell, J.A.; Coolen, C.; Moretti, P.A.B.; Zebol, J.R.; Pham, D.H.; Finnie, J.W.; Don, A.S.; Ebert, L.M.; Bonder, C.S.; et al. A selective ATP-competitive sphingosine kinase inhibitor demonstrates anti-cancer properties. Oncotarget 2015, 6, 7065–7083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mena, H.A.; Zubiry, P.R.; Dizier, B.; Mignon, V.; Parborell, F.; Schattner, M.; Boisson-Vidal, C.; Negrotto, S. Ceramide 1-phosphate protects endothelial colony–forming cells from apoptosis and increases vasculogenesis in vitro and in vivo. Arter. Thromb. Vasc. Biol. 2019, 39, e219–e232. [Google Scholar] [CrossRef] [PubMed]
- Truman, J.-P.; García-Barros, M.; Kaag, M.; Hambardzumyan, D.; Stancevic, B.; Chan, M.; Fuks, Z.; Kolesnick, R.; Haimovitz-Friedman, A. Endothelial Membrane Remodeling Is Obligate for Anti-Angiogenic Radiosensitization during Tumor Radiosurgery. PLoS ONE 2010, 5, e12310. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, J.; García-Barros, M.; Rao, S.; A Rotolo, J.; Thompson, C.; Mizrachi, A.; Feldman, R.; Manova, K.; Bielawska, A.; Bielawska, J.; et al. Targeting acid sphingomyelinase with anti-angiogenic chemotherapy. Cell. Signal. 2016, 29, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kaffas, A.; Al-Mahrouki, A.; Hashim, A.; Law, N.; Giles, A.; Czarnota, G.J. Role of acid sphingomyelinase and ceramide in mechano-acoustic enhancement of tumor radiation responses. J. Natl. Cancer Inst. 2018, 110, 1009–1018. [Google Scholar] [CrossRef]
- Dijkmans, P.; Juffermans, L.; Musters, R.; van Wamel, A.; Cate, F.T.; van Gilst, W.; Visser, C.; De Jong, N.; Kamp, O. Microbubbles and ultrasound: From diagnosis to therapy. Eur. J. Echocardiogr. 2004, 5, 245–256. [Google Scholar] [CrossRef]
- Sirsi, S.; Borden, M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci. Eng. Technol. 2009, 1, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Han, H.; Lee, M.; Lee, S.; Yoo, H.; Chang, J.H.; Kim, H. Microbubbles used for contrast enhanced ultrasound and theragnosis: A review of principles to applications. Biomed. Eng. Lett. 2017, 7, 59–69. [Google Scholar] [CrossRef]
- Al-Jawadi, S.; Thakur, S.S. Ultrasound-responsive lipid microbubbles for drug delivery: A review of preparation techniques to optimise formulation size, stability and drug loading. Int. J. Pharm. 2020, 585, 119559. [Google Scholar] [CrossRef]
- Lee, P.; Matkar, P.; Kuliszewski, M.; Leong-Poi, H. Ultrasound-targeted cardiovascular gene therapy. Card. Regen. Repair 2014, 2014, 380–407. [Google Scholar] [CrossRef]
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble Agents: New Directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.K.; Papadopoulou, V.; Dayton, P.A. Imaging with ultrasound contrast agents: Current status and future. Abdom. Radiol. 2018, 43, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, Q.; Tantawi, M.; Leeper, D.B.; Wheatley, M.A.; Eisenbrey, J.R. Emerging Applications of Ultrasound-Contrast Agents in Radiation Therapy. Ultrasound Med. Biol. 2021, 47, 1465–1474. [Google Scholar] [CrossRef]
- Hu, Y.; Wan, J.M.; Yu, A.C.H. Membrane Perforation and Recovery Dynamics in Microbubble-Mediated Sonoporation. Ultrasound Med. Biol. 2013, 39, 2393–2405. [Google Scholar] [CrossRef]
- Jia, C.; Shi, J.; Yao, Y.; Han, T.; Yu, A.C.; Qin, P. Plasma Membrane Blebbing Dynamics Involved in the Reversibly Perforated Cell by Ultrasound-Driven Microbubbles. Ultrasound Med. Biol. 2020, 47, 733–750. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tang, N.; Huang, L.; Qiao, W.; Zhu, Q.; Liu, Z. Effect of diagnostic ultrasound and microbubble-enhanced chemotherapy on metastasis of rabbit VX2 tumor. Med. Phys. 2021, 48, 3927–3935. [Google Scholar] [CrossRef]
- Shen, Z.; Shao, J.; Zhang, J.; Qu, W. Ultrasound cavitation enhanced chemotherapy: In vivo research and clinical application. Exp. Biol. Med. 2020, 245, 1200–1212. [Google Scholar] [CrossRef]
- Czarnota, G.J. Ultrasound-stimulated microbubble enhancement of radiation response. Biol. Chem. 2015, 396, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Delaney, L.J.; Ciraku, L.; Ms, B.E.O.; Bs, C.E.W.; Liu, J.; Li, J.; Nam, K.; Forsberg, F.; Leeper, D.B.; O’Kane, P.; et al. Breast Cancer Brain Metastasis Response to Radiation After Microbubble Oxygen Delivery in a Murine Model. J. Ultrasound Med. 2019, 38, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Czarnota, G.J.; Karshafian, R.; Burns, P.N.; Wong, S.; Al Mahrouki, A.; Lee, J.W.; Caissie, A.; Tran, W.; Kim, C.; Furukawa, M.; et al. Tumor radiation response enhancement by acoustical stimulation of the vasculature. Proc. Natl. Acad. Sci. USA 2012, 109, E2033–E2041. [Google Scholar] [CrossRef] [Green Version]
- Kotopoulis, S.; Dimcevski, G.; Mc Cormack, E.; Postema, M.; Gjertsen, B.T.; Gilja, O.H. Ultrasound- and microbubble-enhanced chemotherapy for treating pancreatic cancer: A phase I clinical trial. J. Acoust. Soc. Am. 2016, 139, 2092. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; Mc Cormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Yan, K.; Shen, L.; Yang, W.; Gong, J.; Ding, K. Clinical study of ultrasound and microbubbles for enhancing chemotherapeutic sensitivity of malignant tumors in digestive system. Chin. J. Cancer Res. 2018, 30, 553–563. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Forsberg, F.; Wessner, C.E.; Delaney, L.J.; Bradigan, K.; Gummadi, S.; Tantawi, M.; Lyshchik, A.; O’Kane, P.; Liu, J.-B.; et al. US-triggered Microbubble Destruction for Augmenting Hepatocellular Carcinoma Response to Transarterial Radioembolization: A Randomized Pilot Clinical Trial. Radiology 2021, 298, 450–457. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Prada, F.; Gennari, A.G.; Linville, I.M.; Mutersbaugh, M.E.; Chen, Z.; Sheybani, N.; DiMeco, F.; Padilla, F.; Hossack, J.A. Quantitative analysis of in-vivo microbubble distribution in the human brain. Sci. Rep. 2021, 11, 11797. [Google Scholar] [CrossRef]
- Radford, I.R. The Level of Induced DNA Double-strand Breakage Correlates with Cell Killing after X-irradiation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1985, 48, 45–54. [Google Scholar] [CrossRef]
- Radford, I.R. Evidence for a General Relationship between the Induced Level of DNA Double-strand Breakage and Cell-killing after X-irradiation of Mammalian Cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1985, 49, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial Apoptosis as the Primary Lesion Initiating Intestinal Radiation Damage in Mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.A.; Fuks, Z.; Kolesnick, R.N. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000, 60, 321–327. [Google Scholar] [PubMed]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor Response to Radiotherapy Regulated by Endothelial Cell Apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef] [Green Version]
- Bonnaud, S.; Niaudet, C.; Pottier, G.; Gaugler, M.-H.; Millour, J.; Barbet, J.; Sabatier, L.; Paris, F. Sphingosine-1-Phosphate Protects Proliferating Endothelial Cells from Ceramide-Induced Apoptosis but not from DNA Damage–Induced Mitotic Death. Cancer Res. 2007, 67, 1803–1811. [Google Scholar] [CrossRef] [Green Version]
- Haimovitz-Friedman, A. Radiation-induced signal transduction and stress response. Radiat. Res. 1998, 150, S102. [Google Scholar] [CrossRef]
- Santana-Delgado, P.; A Peña, L.; Haimovitz-Friedman, A.; Martin, S.; Green, D.; McLoughlin, M.; Cordon-Cardo, C.; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Acid Sphingomyelinase–Deficient Human Lymphoblasts and Mice Are Defective in Radiation-Induced Apoptosis. Cell 1996, 86, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004, 5, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Moeller, B.J.; Dreher, M.R.; Rabbani, Z.; Schroeder, T.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 2005, 8, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Lai, P.; Tarapacki, C.; Tran, W.T.; El Kaffas, A.; Lee, J.; Hupple, C.; Iradji, S.; Giles, A.; Al-Mahrouki, A.; Czarnota, G.J. Breast tumor response to ultrasound mediated excitation of microbubbles and radiation therapy in vivo. Oncoscience 2016, 3, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, W.T.; Iradji, S.; Sofroni, E.; Giles, A.; Eddy, D.M.; Czarnota, G.J. Microbubble and ultrasound radioenhancement of bladder cancer. Br. J. Cancer 2012, 107, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nofiele, J.I.T.; Karshafian, R.; Furukawa, M.; Al Mahrouki, A.; Giles, A.; Wong, S.; Czarnota, G.J. Ultrasound-Activated Microbubble Cancer Therapy: Ceramide Production Leading to Enhanced Radiation Effect in vitro. Technol. Cancer Res. Treat. 2013, 12, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahrouki, A.A.; Wong, E.; Czarnota, G.J. Ultrasound-stimulated microbubble enhancement of radiation treatments: Endothelial cell function and mechanism. Oncoscience 2015, 2, 944–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mahrouki, A.A.; Karshafian, R.; Giles, A.; Czarnota, G.J. Bioeffects of Ultrasound-Stimulated Microbubbles on Endothelial Cells: Gene Expression Changes Associated with Radiation Enhancement In Vitro. Ultrasound Med. Biol. 2012, 38, 1958–1969. [Google Scholar] [CrossRef]
- Al-Mahrouki, A.; Giles, A.; Hashim, A.; Kim, H.C.; El-Falou, A.; Rowe-Magnus, D.; Farhat, G.; Czarnota, G.J. Microbubble-based enhancement of radiation effect: Role of cell membrane ceramide metabolism. PLoS ONE 2017, 12, e0181951. [Google Scholar] [CrossRef] [Green Version]
- Al-Mahrouki, A.A.; Iradji, S.; Tran, W.T.; Czarnota, G.J. Cellular characterization of ultrasound-stimulated microbubble radiation enhancement in a prostate cancer xenograft model. DMM Dis. Model Mech. 2014, 7, 363–372. [Google Scholar]
- Kim, H.C.; Al-Mahrouki, A.; Gorjizadeh, A.; Karshafian, R.; Czarnota, G.J. Effects of Biophysical Parameters in Enhancing Radiation Responses of Prostate Tumors with Ultrasound-Stimulated Microbubbles. Ultrasound Med. Biol. 2013, 39, 1376–1387. [Google Scholar] [CrossRef]
- Hida, K.; Maishi, N.; Torii, C.; Hida, Y. Tumor angiogenesis—characteristics of tumor endothelial cells. Int. J. Clin. Oncol. 2016, 21, 206–212. [Google Scholar] [CrossRef]
- Hida, K.; Kawamoto, T.; Ohga, N.; Akiyama, K.; Hida, Y.; Shindoh, M. Altered angiogenesis in the tumor microenvironment. Pathol. Int. 2011, 61, 630–637. [Google Scholar] [CrossRef]
- Dudley, A.C. Tumor Endothelial Cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006536. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Briggs, K.; Al Mahrouki, A.; Nofiele, J.; El-Falou, A.; Stanisz, M.; Kim, H.C.; Kolios, M.C.; Czarnota, G.J. Non-invasive Monitoring of Ultrasound-Stimulated Microbubble Radiation Enhancement Using Photoacoustic Imaging. Technol. Cancer Res. Treat. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kaffas, A.; Gangeh, M.J.; Farhat, G.; Tran, W.T.; Hashim, A.; Giles, A.; Czarnota, G.J. Tumour vascular shutdown and cell death following ultrasound-microbubble enhanced radiation therapy. Theranostics 2018, 8, 314–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, J.; Tran, W.; Lai, P.; Al-Mahrouki, A.; Giles, A.; Czarnota, G.J. Effect of Treatment Sequencing on the Tumor Response to Combined Treatment With Ultrasound-Stimulated Microbubbles and Radiotherapy. J. Ultrasound. Med. 2020, 39, 2415–2425. [Google Scholar] [CrossRef]
- Sathishkumar, S.; Boyanovski, B.; A Karakashian, A.; Rozenova, K.A.; Giltiay, N.V.; Kudrimoti, M.; Mohiuddin, M.; Ahmed, M.M.; Nikolova-Karakashian, M. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: Implications for endothelial apoptosis. Cancer Biol. Ther. 2005, 4, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Tsuchida, J.; Moro, K.; Hasegawa, M.; Tatsuda, K.; Woelfel, I.A.; Takabe, K.; Wakai, T. High levels of sphingolipids in human breast cancer. J. Surg. Res. 2016, 204, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, J.; Nagahashi, M.; Nakajima, M.; Moro, K.; Tatsuda, K.; Ramanathan, R.; Takabe, K.; Wakai, T. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. J. Surg. Res. 2016, 205, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Moro, K.; Kawaguchi, T.; Tsuchida, J.; Gabriel, E.; Qi, Q.; Yan, L.; Wakai, T.; Takabe, K.; Nagahashi, M. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 2018, 9, 19874–19890. [Google Scholar] [CrossRef] [Green Version]
- Schiffmann, S.; Sandner, J.; Birod, K.; Wobst, I.; Angioni, C.; Ruckhäberle, E.; Kaufmann, M.; Ackermann, H.; Lötsch, J.; Schmidt, H.; et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis 2009, 30, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Senkal, C.E.; Ponnusamy, S.; Rossi, M.J.; Bialewski, J.; Sinha, D.; Jiang, J.C.; Jazwinski, S.M.; Hannun, Y.A.; Ogretmen, B. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol. Cancer Ther. 2007, 6, 712–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, N.; Rio, E.; Ripoche, N.; Ferchaud-Roucher, V.; Gaugler, M.-H.; Campion, L.; Krempf, M.; Carrie, C.; Mahé, M.; Mirabel, X.; et al. Plasma ceramide, a real-time predictive marker of pulmonary and hepatic metastases response to stereotactic body radiation therapy combined with irinotecan. Radiother. Oncol. 2016, 119, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karam, M.; Ives, A.; Auclair, C. Is Sphingosine-1-Phosphate a Regulator of Tumor Vascular Functionality? Cancers 2022, 14, 1302. [Google Scholar] [CrossRef] [PubMed]
- Riboni, L.; Campanella, R.; Bassi, R.; Villani, R.; Gaini, S.M.; Boneschi, F.M.; Viani, P.; Tettamanti, G. Ceramide levels are inversely associated with malignant progression of human glial tumors. Glia 2002, 39, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Abuhusain, H.J.; Matin, A.; Qiao, Q.; Shen, H.; Kain, N.; Day, B.W.; Stringer, B.; Daniels, B.; Laaksonen, M.A.; Teo, C.; et al. A Metabolic Shift Favoring Sphingosine 1-Phosphate at the Expense of Ceramide Controls Glioblastoma Angiogenesis. J. Biol. Chem. 2013, 288, 37355–37364. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.; Czarnota, G.J. Involvement of Ceramide Signalling in Radiation-Induced Tumour Vascular Effects and Vascular-Targeted Therapy. Int. J. Mol. Sci. 2022, 23, 6671. https://doi.org/10.3390/ijms23126671
Sharma D, Czarnota GJ. Involvement of Ceramide Signalling in Radiation-Induced Tumour Vascular Effects and Vascular-Targeted Therapy. International Journal of Molecular Sciences. 2022; 23(12):6671. https://doi.org/10.3390/ijms23126671
Chicago/Turabian StyleSharma, Deepa, and Gregory J. Czarnota. 2022. "Involvement of Ceramide Signalling in Radiation-Induced Tumour Vascular Effects and Vascular-Targeted Therapy" International Journal of Molecular Sciences 23, no. 12: 6671. https://doi.org/10.3390/ijms23126671
APA StyleSharma, D., & Czarnota, G. J. (2022). Involvement of Ceramide Signalling in Radiation-Induced Tumour Vascular Effects and Vascular-Targeted Therapy. International Journal of Molecular Sciences, 23(12), 6671. https://doi.org/10.3390/ijms23126671






