Action Mechanisms of Effectors in Plant-Pathogen Interaction
Abstract
:1. Introduction
2. Plant-Pathogen Interaction Models
3. Action Mechanisms of Effectors in Plant-Pathogen Interaction of Pathogen Effectors
3.1. Breaking down Physical Barriers
3.1.1. Manipulation of Host Plant Stomatal Defenses
3.1.2. Degradation of Plant Cell Walls
3.1.3. Attacking Plasmodesmata–Callose Regulation
3.1.4. Destruction of the Host Plant Cytoskeleton
3.2. Creating Conditions Favorable to Infestation
3.2.1. Construction of Hydrophobic Space
3.2.2. Induction of Extracellular Alkalinization
3.3. Protecting or Masking Themselves
3.3.1. Inhibition of PTI
3.3.2. Antagonism with Anti-Microbial Compounds in Plants
3.4. Interfering with Host Plant Cell Physiological Activities
3.4.1. Regulation of Plant Gene Transcription
3.4.2. Degradation of Host Plant RNA
3.4.3. Interference with Plant Cell Degradation Pathways
3.4.4. Interference with Host Plant Protein Function
3.4.5. Interference with Host Vesicle Transport
3.5. Manipulating Plant Downstream Immune Responses
3.5.1. Interference with Plant Hormone Signaling
3.5.2. Utilization of RNA Silencing Strategy
3.5.3. Regulation of Reactive Oxygen Species Generation
3.5.4. Manipulation of Plant Cell Death
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Wit, P.J.G.M.; Mehrabi, R.; Van den Burg, H.A.; Stergiopoulos, I. Fungal Effector Proteins: Past, Present and Future. Mol. Plant Pathol. 2009, 10, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty Years of Resistance: Zig-Zag through the Plant Immune System. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling Mechanisms in Pattern-Triggered Immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Feng, B.; He, P.; Shan, L. From Chaos to Harmony: Responses and Signaling upon Microbial Pattern Recognition. Annu. Rev. Phytopathol. 2017, 55, 109–137. [Google Scholar] [CrossRef]
- Ghosh, S.; Malukani, K.K.; Chandan, R.K.; Sonti, R.V.; Jha, G. How Plants Respond to Pathogen Attack: Interaction and Communication. In Sensory Biology of Plants; Sopory, S., Ed.; Springer: Singapore, 2019; pp. 537–568. ISBN 9789811389214. [Google Scholar]
- Koeck, M.; Hardham, A.R.; Dodds, P.N. The Role of Effectors of Biotrophic and Hemibiotrophic Fungi in Infection. Cell Microbiol. 2011, 13, 1849–1857. [Google Scholar] [CrossRef] [Green Version]
- Duplessis, S.; Cuomo, C.A.; Lin, Y.-C.; Aerts, A.; Tisserant, E.; Veneault-Fourrey, C.; Joly, D.L.; Hacquard, S.; Amselem, J.; Cantarel, B.L.; et al. Obligate Biotrophy Features Unraveled by the Genomic Analysis of Rust Fungi. Proc. Natl. Acad. Sci. USA 2011, 108, 9166–9171. [Google Scholar] [CrossRef] [Green Version]
- Saitoh, H.; Fujisawa, S.; Mitsuoka, C.; Ito, A.; Hirabuchi, A.; Ikeda, K.; Irieda, H.; Yoshino, K.; Yoshida, K.; Matsumura, H.; et al. Large-Scale Gene Disruption in Magnaporthe oryzae Identifies MC69, a Secreted Protein Required for Infection by Monocot and Dicot Fungal Pathogens. PLoS Pathog. 2012, 8, e1002711. [Google Scholar] [CrossRef] [Green Version]
- Gan, P.; Ikeda, K.; Irieda, H.; Narusaka, M.; O’Connell, R.J.; Narusaka, Y.; Takano, Y.; Kubo, Y.; Shirasu, K. Comparative Genomic and Transcriptomic Analyses Reveal the Hemibiotrophic Stage Shift of Colletotrichum Fungi. New Phytol. 2013, 197, 1236–1249. [Google Scholar] [CrossRef]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two Distinct Secretion Systems Facilitate Tissue Invasion by the Rice Blast Fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1996. [Google Scholar] [CrossRef] [Green Version]
- Stergiopoulos, I.; de Wit, P.J.G.M. Fungal Effector Proteins. Annu. Rev. Phytopathol. 2009, 47, 233–263. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, K.; Roberto, S.R.; Tiepo, A.N.; Constantino, L.V.; de Resende, J.T.V.; Abo-Elyousr, K.A.M. Salt Solution Treatments Trigger Antioxidant Defense Response against Gray Mold Disease in Table Grapes. JoF 2020, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Lachhab, N.; Sanzani, S.M.; Fallanaj, F.; Youssef, K.; Nigro, F.; Boselli, M.; Ippolito, A. Protein Hydrolysates As Resistance Inducers For Controlling Green Mould Of Citrus Fruit. Acta Hortic. 2015, 1065, 1593–1598. [Google Scholar] [CrossRef]
- Salem, E.A.; Youssef, K.; Sanzani, S.M. Evaluation of Alternative Means to Control Postharvest Rhizopus Rot of Peaches. Sci. Hortic. 2016, 198, 86–90. [Google Scholar] [CrossRef]
- Liu, T.; Song, T.; Zhang, X.; Yuan, H.; Su, L.; Li, W.; Xu, J.; Liu, S.; Chen, L.; Chen, T.; et al. Unconventionally Secreted Effectors of Two Filamentous Pathogens Target Plant Salicylate Biosynthesis. Nat. Commun. 2014, 5, 4686. [Google Scholar] [CrossRef] [Green Version]
- Collemare, J.; Lebrun, M. Fungal Secondary Metabolites: Ancient Toxins and Novel Effectors in Plant–Microbe Interactions. In Effectors in Plant–Microbe Interactions; Martin, F., Kamoun, S., Eds.; Wiley: Hoboken, NJ, USA, 2011; pp. 377–400. ISBN 978-0-470-95822-3. [Google Scholar]
- Ciuffetti, L.M.; Manning, V.A.; Pandelova, I.; Betts, M.F.; Martinez, J.P. Host-selective Toxins, Ptr ToxA and Ptr ToxB, as Necrotrophic Effectors in the Pyrenophora Tritici-repentis—Wheat Interaction. New Phytol. 2010, 187, 911–919. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.-M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.-D.; Jin, H. Fungal Small RNAs Suppress Plant Immunity by Hijacking Host RNA Interference Pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Chanclud, E.; Morel, J.-B. Plant Hormones: A Fungal Point of View. Mol. Plant Pathol. 2016, 17, 1289–1297. [Google Scholar] [CrossRef]
- Collemare, J.; O’Connell, R.; Lebrun, M. Nonproteinaceous Effectors: The Terra Incognita of Plant–Fungal Interactions. New Phytol. 2019, 223, 590–596. [Google Scholar] [CrossRef] [Green Version]
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and How to Kill a Plant Cell: Infection Strategies of Plant Pathogenic Fungi. J. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef]
- Mooney, B.C.; Mantz, M.; Graciet, E.; Huesgen, P.F. Cutting the Line: Manipulation of Plant Immunity by Bacterial Type III Effector Proteases. J. Exp. Bot. 2021, 72, 3395–3409. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.J.; Chau-Ly, I.J.; Lewis, J.D. What the Wild Things Do: Mechanisms of Plant Host Manipulation by Bacterial Type III-Secreted Effector Proteins. Microorganisms 2021, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-P.; Li, J.-J.; Dhar, N.; Li, J.-P.; Chen, J.-Y.; Jian, W.; Dai, X.-F.; Yang, X.-Y. Lysin Motif (LysM) Proteins: Interlinking Manipulation of Plant Immunity and Fungi. Int. J. Mol. Sci. 2021, 22, 3114. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.G.; Deb, D.; Fedkenheuer, K.; McDowell, J.M. Recent Progress in RXLR Effector Research. Mol. Plant Microbe Interact. 2015, 28, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chepsergon, J.; Motaung, T.E.; Moleleki, L.N. “Core” RxLR Effectors in Phytopathogenic Oomycetes: A Promising Way to Breeding for Durable Resistance in Plants? Virulence 2021, 12, 1921–1935. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Casteel, C.L. Effector-Mediated Plant–Virus–Vector Interactions. Plant Cell 2022, 34, 1514–1531. [Google Scholar] [CrossRef]
- Tariqjaveed, M.; Mateen, A.; Wang, S.; Qiu, S.; Zheng, X.; Zhang, J.; Bhadauria, V.; Sun, W. Versatile Effectors of Phytopathogenic Fungi Target Host Immunity. J. Integr. Plant Biol. 2021, 63, 1856–1873. [Google Scholar] [CrossRef]
- Pradhan, A.; Ghosh, S.; Sahoo, D.; Jha, G. Fungal Effectors, the Double Edge Sword of Phytopathogens. Curr. Genet. 2021, 67, 27–40. [Google Scholar] [CrossRef]
- Flor, H.H. Current Status of the Gene-For-Gene Concept. Annu. Rev. Phytopathol. 1971, 9, 275–296. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D.G. Plant Pathogens and Integrated Defence Responses to Infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef]
- van der Hoorn, R.A.L.; Kamoun, S. From Guard to Decoy: A New Model for Perception of Plant Pathogen Effectors. Plant Cell 2008, 20, 2009–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Zhu, L.; Song, T.; Wang, Y.; Zhang, Q.; Xia, Y.; Qiu, M.; Lin, Y.; Li, H.; Kong, L.; et al. A Paralogous Decoy Protects Phytophthora sojae Apoplastic Effector PsXEG1 from a Host Inhibitor. Science 2017, 355, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Thordal-Christensen, H. A Holistic View on Plant Effector-Triggered Immunity Presented as an Iceberg Model. Cell. Mol. Life Sci. 2020, 77, 3963–3976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants Have a Sensitive Perception System for the Most Conserved Domain of Bacterial Flagellin: Plants Perceive a Conserved Domain of Bacterial Flagellin. Plant J. 1999, 18, 265–276. [Google Scholar] [CrossRef]
- Naito, K.; Taguchi, F.; Suzuki, T.; Inagaki, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. Amino Acid Sequence of Bacterial Microbe-Associated Molecular Pattern Flg22 Is Required for Virulence. Mol. Plant Microbe Interact. 2008, 21, 1165–1174. [Google Scholar] [CrossRef] [Green Version]
- Nicaise, V.; Roux, M.; Zipfel, C. Recent Advances in PAMP-Triggered Immunity against Bacteria: Pattern Recognition Receptors Watch over and Raise the Alarm. Plant Physiol. 2009, 150, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.G.; Felix, G.; Boller, T. Bacterial Disease Resistance in Arabidopsis through Flagellin Perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef]
- Kunze, G.; Zipfel, C.; Robatzek, S.; Niehaus, K.; Boller, T.; Felix, G. The N Terminus of Bacterial Elongation Factor Tu Elicits Innate Immunity in Arabidopsis Plants. Plant Cell 2004, 16, 3496–3507. [Google Scholar] [CrossRef] [Green Version]
- Aslam, S.N.; Newman, M.-A.; Erbs, G.; Morrissey, K.L.; Chinchilla, D.; Boller, T.; Jensen, T.T.; De Castro, C.; Ierano, T.; Molinaro, A.; et al. Bacterial Polysaccharides Suppress Induced Innate Immunity by Calcium Chelation. Curr. Biol. 2008, 18, 1078–1083. [Google Scholar] [CrossRef]
- Bedini, E.; De Castro, C.; Erbs, G.; Mangoni, L.; Dow, J.M.; Newman, M.-A.; Parrilli, M.; Unverzagt, C. Structure-Dependent Modulation of a Pathogen Response in Plants by Synthetic O-Antigen Polysaccharides. J. Am. Chem. Soc. 2005, 127, 2414–2416. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Kullberg, B.J.; van der Meer, J.W.; Gow, N.A.; Netea, M.G. Host–Microbe Interactions: Innate Pattern Recognition of Fungal Pathogens. Curr. Opin. Microbiol. 2008, 11, 305–312. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, R.; Bolton, M.D.; Thomma, B.P. How Filamentous Pathogens Co-Opt Plants: The Ins and Outs of Fungal Effectors. Curr. Opin. Plant Biol. 2011, 14, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Jay, F.; Nomura, K.; He, S.Y.; Voinnet, O. Suppression of the MicroRNA Pathway by Bacterial Effector Proteins. Science 2008, 321, 964–967. [Google Scholar] [CrossRef] [Green Version]
- Vleeshouwers, V.G.A.A.; Oliver, R.P. Effectors as Tools in Disease Resistance Breeding Against Biotrophic, Hemibiotrophic, and Necrotrophic Plant Pathogens. Mol. Plant Microbe Interact. 2014, 27, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landete, J.M. Effector Molecules and Regulatory Proteins: Applications. Trends Biotechnol. 2016, 34, 777–780. [Google Scholar] [CrossRef]
- Khan, M.; Seto, D.; Subramaniam, R.; Desveaux, D. Oh, the Places They’ll Go! A Survey of Phytopathogen Effectors and Their Host Targets. Plant J. 2018, 93, 651–663. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant–Pathogen Interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The Plant Hypersensitive Response: Concepts, Control and Consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [Green Version]
- Ngou, B.; Ahn, H.-K.; Ding, P.; Jones, J.D.G. Mutual Potentiation of Plant Immunity by Cell-Surface and Intracellular Receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; He, Y.; Zhou, J.-M.; Xin, X.-F. Pattern-Recognition Receptors Are Required for NLR-Mediated Plant Immunity. Nature 2020, 592, 105–109. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ahn, H.-K.; Ding, P.; Redkar, A.; Brown, H.; Ma, Y.; Youles, M.; Tomlinson, L.; Jones, J.D.G. Estradiol-Inducible AvrRps4 Expression Reveals Distinct Properties of TIR-NLR-Mediated Effector-Triggered Immunity. J. Exp. Bot. 2020, 71, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, L.; Lanver, D.; Schweizer, G.; Tanaka, S.; Liang, L.; Tollot, M.; Zuccaro, A.; Reissmann, S.; Kahmann, R. Fungal Effectors and Plant Susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Laluk, K.; Mengiste, T. Necrotroph Attacks on Plants: Wanton Destruction or Covert Extortion? Arab. Book 2010, 8, e0136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, R.G.T.; Howlett, B.J. Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi. PLoS Pathog. 2012, 8, e1002515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riou, C.; Freyssinet, G.; Fevre, M. Production of Cell Wall-Degrading Enzymes by the Phytopathogenic Fungus Sclerotinia sclerotiorum. Appl. Environ. Microbiol. 1991, 57, 1478–1484. [Google Scholar] [CrossRef] [Green Version]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant Stomata Function in Innate Immunity against Bacterial Invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [Green Version]
- Toruño, T.Y.; Stergiopoulos, I.; Coaker, G. Plant-Pathogen Effectors: Cellular Probes Interfering with Plant Defenses in Spatial and Temporal Manners. Annu. Rev. Phytopathol. 2016, 54, 419–441. [Google Scholar] [CrossRef] [Green Version]
- Melotto, M.; Zhang, L.; Oblessuc, P.R.; He, S.Y. Stomatal Defense a Decade Later. Plant Physiol. 2017, 174, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ Family of Repressors Is the Missing Link in Jasmonate Signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, Y.; Yang, Y.; Du, M.; Zhang, X.; Guo, Y.; Li, C.; Zhou, J.-M. An Arabidopsis Plasma Membrane Proton ATPase Modulates JA Signaling and Is Exploited by the Pseudomonas syringae Effector Protein AvrB for Stomatal Invasion. Plant Cell 2015, 27, 2032–2041. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Wang, Y.; Xue, L.; Chu, J.; Yan, C.; Fu, J.; Chen, M.; Innes, R.W.; Zhou, J.-M. Pseudomonas syringae Effector Protein AvrB Perturbs Arabidopsis Hormone Signaling by Activating MAP Kinase 4. Cell Host Microbe 2010, 7, 164–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez-Ibanez, S.; Boter, M.; Fernández-Barbero, G.; Chini, A.; Rathjen, J.P.; Solano, R. The Bacterial Effector HopX1 Targets JAZ Transcriptional Repressors to Activate Jasmonate Signaling and Promote Infection in Arabidopsis. PLoS Biol. 2014, 12, e1001792. [Google Scholar] [CrossRef] [Green Version]
- Raffeiner, M.; Üstün, S.; Guerra, T.; Spinti, D.; Fitzner, M.; Sonnewald, S.; Baldermann, S.; Börnke, F. The Xanthomonas Type-III Effector XopS Stabilizes Ca WRKY40a to Regulate Defense Responses and Stomatal Immunity in Pepper (Capsicum annuum). Plant Cell 2022, 34, 1684–1708. [Google Scholar] [CrossRef] [PubMed]
- Roussin-Léveillée, C.; Lajeunesse, G.; St-Amand, M.; Veerapen, V.P.; Silva-Martins, G.; Nomura, K.; Brassard, S.; Bolaji, A.; He, S.Y.; Moffett, P. Evolutionarily Conserved Bacterial Effectors Hijack Abscisic Acid Signaling to Induce an Aqueous Environment in the Apoplast. Cell Host Microbe 2022, 30, 489–501.e4. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant Cell Wall–Degrading Enzymes and Their Secretion in Plant-Pathogenic Fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Gibson, D.M.; King, B.C.; Hayes, M.L.; Bergstrom, G.C. Plant Pathogens as a Source of Diverse Enzymes for Lignocellulose Digestion. Curr. Opin. Microbiol. 2011, 14, 264–270. [Google Scholar] [CrossRef]
- Kema, G.H.J.; van der Lee, T.A.J.; Mendes, O.; Verstappen, E.C.P.; Lankhorst, R.K.; Sandbrink, H.; van der Burgt, A.; Zwiers, L.-H.; Csukai, M.; Waalwijk, C. Large-Scale Gene Discovery in the Septoria Tritici Blotch Fungus Mycosphaerella graminicola with a Focus on In Planta Expression. Mol. Plant Microbe Interact. 2008, 21, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Acero, F.J.; Colby, T.; Harzen, A.; Carbú, M.; Wieneke, U.; Cantoral, J.M.; Schmidt, J. 2-DE Proteomic Approach to the Botrytis Cinerea Secretome Induced with Different Carbon Sources and Plant-Based Elicitors. Proteomics 2010, 10, 2270–2280. [Google Scholar] [CrossRef]
- Espino, J.J.; Brito, N.; Noda, J.; González, C. Botrytis Cinerea Endo-ß-1,4-Glucanase Cel5A Is Expressed during Infection but Is Not Required for Pathogenesis. Physiol. Mol. Plant Pathol. 2005, 66, 213–221. [Google Scholar] [CrossRef]
- Brito, N.; Espino, J.J.; González, C. The Endo-β-1,4-Xylanase Xyn11A Is Required for Virulence in Botrytis cinerea. Mol. Plant Microbe Interact. 2006, 19, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Tzima, A.K.; Paplomatas, E.J.; Rauyaree, P.; Ospina-Giraldo, M.D.; Kang, S. VdSNF1, the Sucrose Nonfermenting Protein Kinase Gene of Verticillium dahliae, Is Required for Virulence and Expression of Genes Involved in Cell-Wall Degradation. Mol. Plant Microbe Interact. 2011, 24, 129–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.-H. Molecular and Evolutionary Mechanisms of Cuticular Wax for Plant Drought Tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Ziv, C.; Zhao, Z.; Gao, Y.G.; Xia, Y. Multifunctional Roles of Plant Cuticle During Plant-Pathogen Interactions. Front. Plant Sci. 2018, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Li, D.-W.; Zheng, L.; Huang, J. CglCUT1 Gene Required for Cutinase Activity and Pathogenicity of Colletotrichum Gloeosporioides Causing Anthracnose of Camellia Oleifera. Eur. J. Plant Pathol. 2017, 147, 103–114. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Itoh, K.; Van Vu, B.; Tosa, Y.; Nakayashiki, H. Simultaneous Silencing of Endo-β-1,4 Xylanase Genes Reveals Their Roles in the Virulence of Magnaporthe oryzae: RNAi Knock-down of Endoxylanase Families. Mol. Microbiol. 2011, 81, 1008–1019. [Google Scholar] [CrossRef]
- Van Vu, B.; Itoh, K.; Nguyen, Q.B.; Tosa, Y.; Nakayashiki, H. Cellulases Belonging to Glycoside Hydrolase Families 6 and 7 Contribute to the Virulence of Magnaporthe oryzae. Mol. Plant Microbe Interact. 2012, 25, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Ökmen, B.; Bachmann, D.; Wit, P.J.G.M. A Conserved GH17 Glycosyl Hydrolase from Plant Pathogenic Dothideomycetes Releases a DAMP Causing Cell Death in Tomato. Mol. Plant Pathol. 2019, 20, 1710–1721. [Google Scholar] [CrossRef]
- Yang, C.; Liang, Y.; Qiu, D.; Zeng, H.; Yuan, J.; Yang, X. Lignin Metabolism Involves Botrytis Cinerea BcGs1- Induced Defense Response in Tomato. BMC Plant Biol. 2018, 18, 103. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Blekemolen, M.C.; Tintor, N.; Cornelissen, B.J.C.; Takken, F.L.W. The Fusarium Oxysporum Avr2-Six5 Effector Pair Alters Plasmodesmatal Exclusion Selectivity to Facilitate Cell-to-Cell Movement of Avr2. Mol. Plant 2018, 11, 691–705. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, L.; Yan, D. Plasmodesmata-Involved Battle Against Pathogens and Potential Strategies for Strengthening Hosts. Front. Plant Sci. 2021, 12, 644870. [Google Scholar] [CrossRef]
- Iswanto, A.B.B.; Vu, M.H.; Pike, S.; Lee, J.; Kang, H.; Son, G.H.; Kim, J.; Kim, S.H. Pathogen Effectors: What Do They Do at Plasmodesmata? Mol. Plant. Pathol. 2021, 23, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Madina, M.H.; Plourde, M.B.; dos Santos, K.C.G.; Huang, X.; Zhang, Y.; Laliberté, J.-F.; Germain, H. The Fungal Effector Mlp37347 Alters Plasmodesmata Fluxes and Enhances Susceptibility to Pathogen. Microorganisms 2021, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Tomczynska, I.; Stumpe, M.; Doan, T.G.; Mauch, F. A Phytophthora Effector Protein Promotes Symplastic Cell-to-cell Trafficking by Physical Interaction with Plasmodesmata-localised Callose Synthases. New Phytol. 2020, 227, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Waigmann, E.; Zambryski, P. Tobacco Mosaic Virus Movemerit Protein-Mediated Protein Transport between Trichome Cells. Plant Cell 1995, 7, 2069–7209. [Google Scholar] [PubMed] [Green Version]
- Aung, K.; Kim, P.; Li, Z.; Joe, A.; Kvitko, B.; Alfano, J.R.; He, S.Y. Pathogenic Bacteria Target Plant Plasmodesmata to Colonize and Invade Surrounding Tissues [CC-BY]. Plant Cell 2020, 32, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Variz, H.; Chen, Y.; Liu, S.-L.; Aung, K. Plasmodesmata-Dependent Intercellular Movement of Bacterial Effectors. Front. Plant Sci. 2021, 12, 640277. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-J.; Zhang, T.; Liu, Z.-H.; Chen, X.; Guo, H.-S.; Ju, B.-H.; Zhang, Y.-Y.; Li, G.-Z.; Zhou, Q.-H.; Qin, Y.-M.; et al. Phytosphinganine Affects Plasmodesmata Permeability via Facilitating PDLP5-Stimulated Callose Accumulation in Arabidopsis. Mol. Plant 2020, 13, 128–143. [Google Scholar] [CrossRef]
- Bonfante, P. At the Interface Between Mycorrhizal Fungi and Plants: The Structural Organization of Cell Wall, Plasma Membrane and Cytoskeleton. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 45–61. ISBN 978-3-642-08310-5. [Google Scholar]
- Schmidt, S.M.; Panstruga, R. Cytoskeleton Functions in Plant–Microbe Interactions. Physiol. Mol. Plant Pathol. 2007, 71, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Jelenska, J.; Cecchini, N.M.; Li, Y.; Lee, M.W.; Kovar, D.R.; Greenberg, J.T. HopW1 from Pseudomonas syringae Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis. PLoS Pathog. 2014, 10, e1004232. [Google Scholar] [CrossRef]
- Nottensteiner, M.; Zechmann, B.; McCollum, C.; Hückelhoven, R. A Barley Powdery Mildew Fungus Non-Autonomous Retrotransposon Encodes a Peptide That Supports Penetration Success on Barley. J. Exp. Bot. 2018, 69, 3745–3758. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Zhu, X.; Li, C.; Ma, Z.; Han, X.; Luo, Y.; Yang, L.; Yu, J.; Miao, Y. Xanthomonas Effector XopR Hijacks Host Actin Cytoskeleton via Complex Coacervation. Nat. Commun. 2021, 12, 4064. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kahmann, R. Cell Wall–Associated Effectors of Plant-Colonizing Fungi. Mycologia 2021, 113, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J.; Ebbole, D.J.; Hamer, J.E. Identification and Characterization of MPG1, a Gene Involved in Pathogenicity from the Rice Blast Fungus Magnaporthe Grisea. Plant Cell 1993, 5, 1575–1590. [Google Scholar] [PubMed] [Green Version]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latgé, J.-P. Hydrophobins—Unique Fungal Proteins. PLoS Pathog. 2012, 8, e1002700. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, N.; Baker, M.O.D.G.; Ball, S.R.; Steain, M.; Pham, C.L.L.; Sunde, M. Microbial Functional Amyloids Serve Diverse Purposes for Structure, Adhesion and Defence. Biophys. Rev. 2019, 11, 287–302. [Google Scholar] [CrossRef]
- Wösten, H.A.B. Hydrophobins: Multipurpose Proteins. Annu. Rev. Microbiol. 2001, 55, 625–646. [Google Scholar] [CrossRef] [Green Version]
- Soanes, D.M.; Kershaw, M.J.; Cooley, R.N.; Talbot, N.J. Regulation of the MPG1 Hydrophobin Gene in the Rice Blast Fungus Magnaporthe grisea. Mol. Plant Microbe Interact. 2002, 15, 1253–1267. [Google Scholar] [CrossRef] [Green Version]
- Talbot, N.J.; Kershaw, M.J.; Wakley, G.E.; De Vries, O.M.H.; Wessels, J.G.H.; Hamer, J.E. MPG1 Encodes a Fungal Hydrophobin Involved in Surface Interactions during Infection-Related Development of Magnaporthe Grisea. Plant Cell 1996, 8, 985–999. [Google Scholar] [CrossRef]
- Quarantin, A.; Hadeler, B.; Kröger, C.; Schäfer, W.; Favaron, F.; Sella, L.; Martínez-Rocha, A.L. Different Hydrophobins of Fusarium graminearum Are Involved in Hyphal Growth, Attachment, Water-Air Interface Penetration and Plant Infection. Front. Microbiol. 2019, 10, 751. [Google Scholar] [CrossRef] [Green Version]
- Ahn, N.; Kim, S.; Choi, W.; Im, K.-H.; Lee, Y.-H. Extracellular Matrix Protein Gene, EMP1, Is Required for Appressorium Formation and Pathogenieity of the Rice Blast Fungus, Magnaporthe Grisea. Mol. Cells 2004, 17, 166–173. [Google Scholar]
- Peñalva, M.A.; Lucena-Agell, D.; Arst, H.N. Liaison Alcaline: Pals Entice Non-Endosomal ESCRTs to the Plasma Membrane for PH Signaling. Curr. Opin. Microbiol. 2014, 22, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.R.; Segorbe, D.; Prusky, D.; Di Pietro, A. How Alkalinization Drives Fungal Pathogenicity. PLoS Pathog. 2017, 13, e1006621. [Google Scholar] [CrossRef] [PubMed]
- Alkan, N.; Espeso, E.A.; Prusky, D. Virulence Regulation of Phytopathogenic Fungi by PH. Antioxid. Redox Signal. 2013, 19, 1012–1025. [Google Scholar] [CrossRef] [Green Version]
- Vylkova, S. Environmental PH Modulation by Pathogenic Fungi as a Strategy to Conquer the Host. PLoS Pathog. 2017, 13, e1006149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A Peptide Hormone and Its Receptor Protein Kinase Regulate Plant Cell Expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masachis, S.; Segorbe, D.; Turrà, D.; Leon-Ruiz, M.; Fürst, U.; El Ghalid, M.; Leonard, G.; López-Berges, M.S.; Richards, T.A.; Felix, G.; et al. A Fungal Pathogen Secretes Plant Alkalinizing Peptides to Increase Infection. Nat. Microbiol. 2016, 1, 16043. [Google Scholar] [CrossRef]
- Thynne, E.; Saur, I.M.L.; Simbaqueba, J.; Ogilvie, H.A.; Gonzalez-Cendales, Y.; Mead, O.; Taranto, A.; Catanzariti, A.; McDonald, M.C.; Schwessinger, B.; et al. Fungal Phytopathogens Encode Functional Homologues of Plant Rapid Alkalinization Factor (RALF) Peptides. Mol. Plant. Pathol. 2016, 18, 811–824. [Google Scholar] [CrossRef]
- Stegmann, M.; Monaghan, J.; Smakowska-Luzan, E.; Rovenich, H.; Lehner, A.; Holton, N.; Belkhadir, Y.; Zipfel, C. The Receptor Kinase FER Is a RALF-Regulated Scaffold Controlling Plant Immune Signaling. Science 2017, 355, 287–289. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Vallet, A.; Mesters, J.R.; Thomma, B.P.H.J. The Battle for Chitin Recognition in Plant-Microbe Interactions. FEMS Microbiol. Rev. 2015, 39, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Miya, A.; Albert, P.; Shinya, T.; Desaki, Y.; Ichimura, K.; Shirasu, K.; Narusaka, Y.; Kawakami, N.; Kaku, H.; Shibuya, N. CERK1, a LysM Receptor Kinase, Is Essential for Chitin Elicitor Signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 19613–19618. [Google Scholar] [CrossRef] [Green Version]
- Kombrink, A.; Rovenich, H.; Shi-Kunne, X.; Rojas-Padilla, E.; van den Berg, G.C.M.; Domazakis, E.; de Jonge, R.; Valkenburg, D.-J.; Sánchez-Vallet, A.; Seidl, M.F.; et al. Verticillium dahliae LysM Effectors Differentially Contribute to Virulence on Plant Hosts: Verticillium dahliae LysM Effectors. Mol. Plant Pathol. 2017, 18, 596–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volk, H.; Marton, K.; Flajšman, M.; Radišek, S.; Tian, H.; Hein, I.; Podlipnik, Č.; Thomma, B.P.H.J.; Košmelj, K.; Javornik, B.; et al. Chitin-Binding Protein of Verticillium Nonalfalfae Disguises Fungus from Plant Chitinases and Suppresses Chitin-Triggered Host Immunity. Mol. Plant Microbe Interact. 2019, 32, 1378–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Burg, H.A.; Harrison, S.J.; Joosten, M.H.A.J.; Vervoort, J.; de Wit, P.J.G.M. Cladosporium fulvum Avr4 Protects Fungal Cell Walls Against Hydrolysis by Plant Chitinases Accumulating During Infection. Mol. Plant Microbe Interact. 2006, 19, 1420–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Esse, H.P.; Bolton, M.D.; Stergiopoulos, I.; de Wit, P.J.G.M.; Thomma, B.P.H.J. The Chitin-Binding Cladosporium fulvum Effector Protein Avr4 Is a Virulence Factor. Mol. Plant Microbe Interact. 2007, 20, 1092–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurlburt, N.K.; Chen, L.-H.; Stergiopoulos, I.; Fisher, A.J. Structure of the Cladosporium fulvum Avr4 Effector in Complex with (GlcNAc)6 Reveals the Ligand-Binding Mechanism and Uncouples Its Intrinsic Function from Recognition by the Cf-4 Resistance Protein. PLoS Pathog. 2018, 14, e1007263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stergiopoulos, I.; van den Burg, H.A.; Ökmen, B.; Beenen, H.G.; van Liere, S.; Kema, G.H.J.; de Wit, P.J.G.M. Tomato Cf Resistance Proteins Mediate Recognition of Cognate Homologous Effectors from Fungi Pathogenic on Dicots and Monocots. Proc. Natl. Acad. Sci. USA 2010, 107, 7610–7615. [Google Scholar] [CrossRef] [Green Version]
- Kohler, A.C.; Chen, L.-H.; Hurlburt, N.; Salvucci, A.; Schwessinger, B.; Fisher, A.J.; Stergiopoulos, I. Structural Analysis of an Avr4 Effector Ortholog Offers Insight into Chitin Binding and Recognition by the Cf-4 Receptor. Plant Cell 2016, 28, 1945–1965. [Google Scholar] [CrossRef] [Green Version]
- Zeng, T.; Rodriguez-Moreno, L.; Mansurkhodzaev, A.; Wang, P.; Berg, W.; Gasciolli, V.; Cottaz, S.; Fort, S.; Thomma, B.P.H.J.; Bono, J.; et al. A Lysin Motif Effector Subverts Chitin-triggered Immunity to Facilitate Arbuscular Mycorrhizal Symbiosis. New Phytol. 2020, 225, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gao, Y.; Liang, Y.; Dong, Y.; Yang, X.; Yuan, J.; Qiu, D. The Verticillium dahliae SnodProt1-Like Protein VdCP1 Contributes to Virulence and Triggers the Plant Immune System. Front. Plant Sci. 2017, 8, 1880. [Google Scholar] [CrossRef] [Green Version]
- Fujikawa, T.; Sakaguchi, A.; Nishizawa, Y.; Kouzai, Y.; Minami, E.; Yano, S.; Koga, H.; Meshi, T.; Nishimura, M. Surface α-1,3-Glucan Facilitates Fungal Stealth Infection by Interfering with Innate Immunity in Plants. PLoS Pathog. 2012, 8, e1002882. [Google Scholar] [CrossRef]
- Otaka, J.; Seo, S.; Nishimura, M. Lutein, a Natural Carotenoid, Induces α-1,3-Glucan Accumulation on the Cell Wall Surface of Fungal Plant Pathogens. Molecules 2016, 21, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujikawa, T.; Kuga, Y.; Yano, S.; Yoshimi, A.; Tachiki, T.; Abe, K.; Nishimura, M. Dynamics of Cell Wall Components of Magnaporthe Grisea during Infectious Structure Development. Mol. Microbiol. 2009, 73, 553–570. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and Biosynthesis of Cell Wall α-1,3-Glucan in Fungi. J. Fungi 2017, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Gollin, I.; Rössel, N.; Kahmann, R. The Functionally Conserved Effector Sta1 Is a Fungal Cell Wall Protein Required for Virulence in Ustilago maydis. New Phytol. 2020, 227, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, W.; He, S.Y. A Prominent Role of the Flagellin Receptor FLAGELLIN-SENSING2 in Mediating Stomatal Response to Pseudomonas syringae Pv Tomato DC3000 in Arabidopsis. Plant Physiol. 2010, 153, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Gimenez-Ibanez, S.; Hann, D.R.; Ntoukakis, V.; Petutschnig, E.; Lipka, V.; Rathjen, J.P. AvrPtoB Targets the LysM Receptor Kinase CERK1 to Promote Bacterial Virulence on Plants. Curr. Biol. 2009, 19, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Irieda, H.; Inoue, Y.; Mori, M.; Yamada, K.; Oshikawa, Y.; Saitoh, H.; Uemura, A.; Terauchi, R.; Kitakura, S.; Kosaka, A.; et al. Conserved Fungal Effector Suppresses PAMP-Triggered Immunity by Targeting Plant Immune Kinases. Proc. Natl. Acad. Sci. USA 2019, 116, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Rocafort, M.; Fudal, I.; Mesarich, C.H. Apoplastic Effector Proteins of Plant-Associated Fungi and Oomycetes. Curr. Opin. Plant Biol. 2020, 56, 9–19. [Google Scholar] [CrossRef]
- de Jonge, R.; Peter van Esse, H.; Kombrink, A.; Shinya, T.; Desaki, Y.; Bours, R.; van der Krol, S.; Shibuya, N.; Joosten, M.H.A.J.; Thomma, B.P.H.J. Conserved Fungal LysM Effector Ecp6 Prevents Chitin-Triggered Immunity in Plants. Science 2010, 329, 953–955. [Google Scholar] [CrossRef]
- Bolton, M.D.; van Esse, H.P.; Vossen, J.H.; de Jonge, R.; Stergiopoulos, I.; Stulemeijer, I.J.E.; van den Berg, G.C.M.; Borrás-Hidalgo, O.; Dekker, H.L.; de Koster, C.G.; et al. The Novel Cladosporium fulvum Lysin Motif Effector Ecp6 Is a Virulence Factor with Orthologues in Other Fungal Species. Mol. Microbiol. 2008, 69, 119–136. [Google Scholar] [CrossRef]
- de Jonge, R.; Thomma, B.P.H.J. Fungal LysM Effectors: Extinguishers of Host Immunity? Trends Microbiol. 2009, 17, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallet, A.; Saleem-Batcha, R.; Kombrink, A.; Hansen, G.; Valkenburg, D.-J.; Thomma, B.P.; Mesters, J.R. Fungal Effector Ecp6 Outcompetes Host Immune Receptor for Chitin Binding through Intrachain LysM Dimerization. eLife 2013, 2, e00790. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Liu, M.; Wang, Y.; Zou, Y.; You, Y.; Yang, L.; Hu, J.; Zhang, H.; Zheng, X.; et al. Magnaporthe Oryzae Auxiliary Activity Protein MoAa91 Functions as Chitin-Binding Protein To Induce Appressorium Formation on Artificial Inductive Surfaces and Suppress Plant Immunity. mBio 2020, 11, 03304–03319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentlak, T.A.; Kombrink, A.; Shinya, T.; Ryder, L.S.; Otomo, I.; Saitoh, H.; Terauchi, R.; Nishizawa, Y.; Shibuya, N.; Thomma, B.P.H.J.; et al. Effector-Mediated Suppression of Chitin-Triggered Immunity by Magnaporthe oryzae Is Necessary for Rice Blast Disease. Plant Cell 2012, 24, 322–335. [Google Scholar] [CrossRef] [Green Version]
- Takahara, H.; Hacquard, S.; Kombrink, A.; Hughes, H.B.; Halder, V.; Robin, G.P.; Hiruma, K.; Neumann, U.; Shinya, T.; Kombrink, E.; et al. Colletotrichum higginsianum Extracellular LysM Proteins Play Dual Roles in Appressorial Function and Suppression of Chitin-triggered Plant Immunity. New Phytol. 2016, 211, 1323–1337. [Google Scholar] [CrossRef] [Green Version]
- Seidl-Seiboth, V.; Zach, S.; Frischmann, A.; Spadiut, O.; Dietzsch, C.; Herwig, C.; Ruth, C.; Rodler, A.; Jungbauer, A.; Kubicek, C.P. Spore Germination of Trichoderma Atroviride Is Inhibited by Its LysM Protein TAL6. FEBS J. 2013, 280, 1226–1236. [Google Scholar] [CrossRef] [Green Version]
- Romero-Contreras, Y.J.; Ramírez-Valdespino, C.A.; Guzmán-Guzmán, P.; Macías-Segoviano, J.I.; Villagómez-Castro, J.C.; Olmedo-Monfil, V. Tal6 From Trichoderma Atroviride Is a LysM Effector Involved in Mycoparasitism and Plant Association. Front. Microbiol. 2019, 10, 2231. [Google Scholar] [CrossRef]
- Chen, X.-L.; Shi, T.; Yang, J.; Shi, W.; Gao, X.; Chen, D.; Xu, X.; Xu, J.-R.; Talbot, N.J.; Peng, Y.-L. N-Glycosylation of Effector Proteins by an a-1,3- Mannosyltransferase Is Required for the Rice Blast Fungus to Evade Host Innate ImmunityW OPEN. Plant Cell 2014, 26, 1360–1376. [Google Scholar] [CrossRef] [Green Version]
- Fiorin, G.L.; Sanchéz-Vallet, A.; de Toledo Thomazella, D.P.; do Prado, P.F.V.; do Nascimento, L.C.; de Oliveira Figueira, A.V.; Thomma, B.P.H.J.; Pereira, G.A.G.; Teixeira, P.J.P.L. Suppression of Plant Immunity by Fungal Chitinase-like Effectors. Curr. Biol. 2018, 28, 3023–3030.e5. [Google Scholar] [CrossRef] [Green Version]
- Han, L.-B.; Li, Y.-B.; Wang, F.-X.; Wang, W.-Y.; Liu, J.; Wu, J.-H.; Zhong, N.-Q.; Wu, S.-J.; Jiao, G.-L.; Wang, H.-Y.; et al. The Cotton Apoplastic Protein CRR1 Stabilizes Chitinase 28 to Facilitate Defense against the Fungal Pathogen Verticillium dahliae. Plant Cell 2019, 31, 520–536. [Google Scholar] [CrossRef]
- Jashni, M.K.; Mehrabi, R.; Collemare, J.; Mesarich, C.H.; de Wit, P.J.G.M. The Battle in the Apoplast: Further Insights into the Roles of Proteases and Their Inhibitors in Plant–Pathogen Interactions. Front. Plant Sci. 2015, 6, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Song, L.; Peng, C.; Liu, X.; Liu, L.; Zhang, Y.; Wang, W.; Zhou, J.; Wang, S.; Ebbole, D.; et al. A Magnaporthe Chitinase Interacts with a Rice Jacalin-Related Lectin to Promote Host Colonization. Plant Physiol. 2019, 179, 1416–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Yu, Y.; Huang, J.; Meng, F.; Pang, J.; Zhao, Q.; Islam, M.A.; Xu, N.; Tian, Y.; Liu, J. Binding of the Magnaporthe oryzae Chitinase MoChia1 by a Rice Tetratricopeptide Repeat Protein Allows Free Chitin to Trigger Immune Responses. Plant Cell 2019, 31, 172–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, B.-Q.; Wang, F.-Z.; Li, J.-F. Hide-and-Seek: Chitin-Triggered Plant Immunity and Fungal Counterstrategies. Trends Plant Sci. 2020, 25, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, B.-S.; Zhao, J.-H.; Huang, J.-F.; Jia, P.-S.; Wang, S.; Zhang, J.; Zhou, J.-M.; Guo, H.-S. Deacetylation of Chitin Oligomers Increases Virulence in Soil-Borne Fungal Pathogens. Nat. Plants 2019, 5, 1167–1176. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple Effects of Chitosan on Plant Systems: Solid Science or Hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef]
- Cord-Landwehr, S.; Melcher, R.L.J.; Kolkenbrock, S.; Moerschbacher, B.M. A Chitin Deacetylase from the Endophytic Fungus Pestalotiopsis Sp. Efficiently Inactivates the Elicitor Activity of Chitin Oligomers in Rice Cells. Sci. Rep. 2016, 6, 38018. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, J.; Zhao, J.; Xu, J.; Sun, S.; Zhang, H.; Wu, J.; Tang, C.; Kang, Z.; Wang, X. A Polysaccharide Deacetylase from Puccinia striiformis f. Sp. Tritici Is an Important Pathogenicity Gene That Suppresses Plant Immunity. Plant Biotechnol. J. 2020, 18, 1830–1842. [Google Scholar] [CrossRef] [Green Version]
- El Gueddari, N.E.; Rauchhaus, U.; Moerschbacher, B.M.; Deising, H.B. Developmentally Regulated Conversion of Surface-Exposed Chitin to Chitosan in Cell Walls of Plant Pathogenic Fungi. New Phytol. 2002, 156, 103–112. [Google Scholar] [CrossRef]
- Geoghegan, I.; Steinberg, G.; Gurr, S. The Role of the Fungal Cell Wall in the Infection of Plants. Trends Microbiol. 2017, 25, 957–967. [Google Scholar] [CrossRef]
- Jian, J.; Liang, X. One Small RNA of Fusarium graminearum Targets and Silences CEBiP Gene in Common Wheat. Microorganisms 2019, 7, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, A.; Gao, H.; Zhang, N.; Zheng, X.; Qiu, S.; Li, Y.; Zhou, S.; Cui, F.; Sun, W. A Novel Effector Gene SCRE2 Contributes to Full Virulence of Ustilaginoidea Virens to Rice. Front. Microbiol. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, X.; Wang, Y.; Hua, K.; Xing, F.; Zheng, Y.; Liu, W.; Sun, W.; Wei, S. The Effector AGLIP1 in Rhizoctonia Solani AG1 IA Triggers Cell Death in Plants and Promotes Disease Development Through Inhibiting PAMP-Triggered Immunity in Arabidopsis Thaliana. Front. Microbiol. 2019, 10, 2228. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A. Saponins and Plant Defence—A Soap Story. Trends Plant Sci. 1996, 1, 4–9. [Google Scholar] [CrossRef]
- Selitrennikoff, C.P. Antifungal Proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef] [Green Version]
- Ökmen, B.; Etalo, D.W.; Joosten, M.H.A.J.; Bouwmeester, H.J.; Vos, R.C.H.; Collemare, J.; Wit, P.J.G.M. Detoxification of A-tomatine by Cladosporium fulvum Is Required for Full Virulence on Tomato. New Phytol. 2013, 198, 1203–1214. [Google Scholar] [CrossRef]
- Ma, L.-S.; Wang, L.; Trippel, C.; Mendoza-Mendoza, A.; Ullmann, S.; Moretti, M.; Carsten, A.; Kahnt, J.; Reissmann, S.; Zechmann, B.; et al. The Ustilago maydis Repetitive Effector Rsp3 Blocks the Antifungal Activity of Mannose-Binding Maize Proteins. Nat. Commun. 2018, 9, 1711. [Google Scholar] [CrossRef] [Green Version]
- Bonas, U.; Stall, R.E.; Staskawicz, B. Genetic and Structural Characterization of the Avirulence Gene AvrBs3 from Xanthomonas campestris Pv. Vesicatoria. Mol. Gen. Genet. 1989, 218, 127–136. [Google Scholar] [CrossRef]
- Schornack, S.; Minsavage, G.V.; Stall, R.E.; Jones, J.B.; Lahaye, T. Characterization of AvrHah1, a Novel AvrBs3-like Effector from Xanthomonas gardneri with Virulence and Avirulence Activity. New Phytol. 2008, 179, 546–556. [Google Scholar] [CrossRef]
- Zlobin, N.; Lebedeva, M.; Monakhova, Y.; Ustinova, V.; Taranov, V. An ERF121 Transcription Factor from Brassica Oleracea Is a Target for the Conserved TAL-effectors from Different Xanthomonas campestris Pv. Campestris Strains. Mol. Plant Pathol. 2021, 22, 618–624. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.; Li, Y.; Zhang, Q.; Ding, Y.; Zhang, Y. ChIP-Seq Reveals Broad Roles of SARD1 and CBP60g in Regulating Plant Immunity. Nat. Commun. 2015, 6, 10159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Wang, K.; Sun, L.; Xing, H.; Wang, S.; Li, L.; Chen, S.; Guo, H.-S.; Zhang, J. The Plant-Specific Transcription Factors CBP60g and SARD1 Are Targeted by a Verticillium Secretory Protein VdSCP41 to Modulate Immunity. eLife 2018, 7, e34902. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Link, T.I.; Müller, M.; Hirschburger, D.; Pudake, R.N.; Pedley, K.F.; Braun, E.; Voegele, R.T.; Baum, T.J.; Whitham, S.A. A Small Cysteine-Rich Protein from the Asian Soybean Rust Fungus, Phakopsora Pachyrhizi, Suppresses Plant Immunity. PLoS Pathog. 2016, 12, e1005827. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Kumari, P.; Lapin, D.; Griebel, T.; Hickman, R.; Guo, W.; Zhang, R.; Parker, J.E.; Beynon, J.; Denby, K.; et al. Downy Mildew Effector HaRxL21 Interacts with the Transcriptional Repressor TOPLESS to Promote Pathogen Susceptibility. PLoS Pathog. 2020, 16, e1008835. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, C.-Y.; Park, S.-Y.; Kim, K.-T.; Jeon, J.; Chung, H.; Choi, G.; Kwon, S.; Choi, J.; Jeon, J.; et al. Two Nuclear Effectors of the Rice Blast Fungus Modulate Host Immunity via Transcriptional Reprogramming. Nat. Commun. 2020, 11, 5845. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Huang, M.; Hu, X.; Zhang, Y.; Wang, Y.; Li, P.; Chen, S.; Zhang, D.; Cao, S.; Zhu, W.; et al. A Ralstonia solanacearum Effector Targets TGA Transcription Factors to Subvert Salicylic Acid Signaling. Plant Cell 2022, 34, 1666–1683. [Google Scholar] [CrossRef]
- Tang, C.; Xu, Q.; Zhao, J.; Yue, M.; Wang, J.; Wang, X.; Kang, Z.; Wang, X. A Rust Fungus Effector Directly Binds Plant Pre-mRNA Splice Site to Reprogram Alternative Splicing and Suppress Host Immunity. Plant Biotechnol. J. 2022, 20, 1167–1181. [Google Scholar] [CrossRef]
- Pennington, H.G.; Jones, R.; Kwon, S.; Bonciani, G.; Thieron, H.; Chandler, T.; Luong, P.; Morgan, S.N.; Przydacz, M.; Bozkurt, T.; et al. The Fungal Ribonuclease-like Effector Protein CSEP0064/BEC1054 Represses Plant Immunity and Interferes with Degradation of Host Ribosomal RNA. PLoS Pathog. 2019, 15, e1007620. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Tian, M.; Dai, K.; Zheng, W.; Liu, Z.; Yang, S.; Liu, X.; Shi, D.; Zhang, H.; et al. Fg12 Ribonuclease Secretion Contributes to Fusarium graminearum Virulence and Induces Plant Cell Death. J. Integr. Plant Biol. 2021, 63, 365–377. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Wang, D.; Zhang, D.; Song, J.; Kong, Z.; Wang, B.; Hu, X.; Klosterman, S.J.; Subbarao, K.V.; et al. A Secreted Ribonuclease Effector from Verticillium dahliae Localizes in the Plant Nucleus to Modulate Host Immunity. Mol. Plant Pathol. 2022; in print. [Google Scholar] [CrossRef]
- Kettles, G.J.; Bayon, C.; Sparks, C.A.; Canning, G.; Kanyuka, K.; Rudd, J.J. Characterization of an Antimicrobial and Phytotoxic Ribonuclease Secreted by the Fungal Wheat Pathogen Zymoseptoria tritici. New Phytol. 2018, 217, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Kumakura, N.; Singkaravanit-Ogawa, S.; Gan, P.; Tsushima, A.; Ishihama, N.; Watanabe, S.; Seo, M.; Iwasaki, S.; Narusaka, M.; Narusaka, Y.; et al. Guanosine-Specific Single-Stranded Ribonuclease Effectors of a Phytopathogenic Fungus Potentiate Host Immune Responses. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cohen-Kaplan, V.; Livneh, I.; Avni, N.; Cohen-Rosenzweig, C.; Ciechanover, A. The Ubiquitin-Proteasome System and Autophagy: Coordinated and Independent Activities. Int. J. Biochem. Cell Biol. 2016, 79, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Langin, G.; Gouguet, P.; Üstün, S. Microbial Effector Proteins—A Journey through the Proteolytic Landscape. Trends Microbiol. 2020, 28, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Banfield, M.J. Perturbation of Host Ubiquitin Systems by Plant Pathogen/Pest Effector Proteins. Cell Microbiol. 2015, 17, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ma, C.; Zhang, M.; Yang, L.; Chen, W. ATG5 Is Required to Limit Cell Death Induced by Pseudomonas syringae in Arabidopsis and May Be Mediated by the Salicylic Acid Pathway. Acta Physiol. Plant 2015, 37, 1731. [Google Scholar] [CrossRef]
- Dagdas, Y.F.; Belhaj, K.; Maqbool, A.; Chaparro-Garcia, A.; Pandey, P.; Petre, B.; Tabassum, N.; Cruz-Mireles, N.; Hughes, R.K.; Sklenar, J.; et al. An Effector of the Irish Potato Famine Pathogen Antagonizes a Host Autophagy Cargo Receptor. eLife 2016, 5, e10856. [Google Scholar] [CrossRef]
- Lal, N.K.; Thanasuwat, B.; Huang, P.; Cavanaugh, K.A.; Carter, A.; Michelmore, R.W.; Dinesh-Kumar, S.P. Phytopathogen Effectors Use Multiple Mechanisms to Manipulate Plant Autophagy. Cell Host Microbe 2020, 28, 558–571.e6. [Google Scholar] [CrossRef]
- Park, C.H.; Shirsekar, G.; Bellizzi, M.; Chen, S.; Songkumarn, P.; Xie, X.; Shi, X.; Ning, Y.; Zhou, B.; Suttiviriya, P.; et al. The E3 Ligase APIP10 Connects the Effector AvrPiz-t to the NLR Receptor Piz-t in Rice. PLoS Pathog. 2016, 12, e1005529. [Google Scholar] [CrossRef] [Green Version]
- Park, C.-H.; Chen, S.; Shirsekar, G.; Zhou, B.; Khang, C.H.; Songkumarn, P.; Afzal, A.J.; Ning, Y.; Wang, R.; Bellizzi, M.; et al. The Magnaporthe oryzae Effector AvrPiz-t Targets the RING E3 Ubiquitin Ligase APIP6 to Suppress Pathogen-Associated Molecular Pattern–Triggered Immunity in Rice. Plant Cell 2012, 24, 4748–4762. [Google Scholar] [CrossRef] [Green Version]
- Üstün, S.; König, P.; Guttman, D.S.; Börnke, F. HopZ4 from Pseudomonas syringae, a Member of the HopZ Type III Effector Family from the YopJ Superfamily, Inhibits the Proteasome in Plants. Mol. Plant Microbe Interact. 2014, 27, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Brefort, T.; Neidig, N.; Djamei, A.; Kahnt, J.; Vermerris, W.; Koenig, S.; Feussner, K.; Feussner, I.; Kahmann, R. A Secreted Ustilago maydis Effector Promotes Virulence by Targeting Anthocyanin Biosynthesis in Maize. eLife 2014, 3, e01355. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Schweizer, G.; Rössel, N.; Fukada, F.; Thines, M.; Kahmann, R. Neofunctionalization of the Secreted Tin2 Effector in the Fungal Pathogen Ustilago maydis. Nat. Microbiol. 2019, 4, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Jianguang, J.; Kahla, A.; Arunachalam, C.; Scofield, S.R.; Bowden, S.; Wallington, E.; Doohan, F.M. TaFROG Encodes a Pooideae Orphan Protein That Interacts with SnRK1 and Enhances Resistance to the Mycotoxigenic Fungus Fusarium Graminearum1 [OPEN]. Plant Physiol. 2015, 169, 2895–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Hei, R.; Yang, Y.; Zhang, S.; Wang, Q.; Wang, W.; Zhang, Q.; Yan, M.; Zhu, G.; Huang, P.; et al. An Orphan Protein of Fusarium graminearum Modulates Host Immunity by Mediating Proteasomal Degradation of TaSnRK1α. Nat. Commun. 2020, 11, 4382. [Google Scholar] [CrossRef]
- Karki, S.J.; Reilly, A.; Zhou, B.; Mascarello, M.; Burke, J.; Doohan, F.; Douchkov, D.; Schweizer, P.; Feechan, A. A Small Secreted Protein from Zymoseptoria tritici Interacts with a Wheat E3 Ubiquitin Ligase to Promote Disease. J. Exp. Bot. 2021, 72, 733–746. [Google Scholar] [CrossRef]
- Jelenska, J.; Yao, N.; Vinatzer, B.A.; Wright, C.M.; Brodsky, J.L.; Greenberg, J.T. A J Domain Virulence Effector of Pseudomonas syringae Remodels Host Chloroplasts and Suppresses Defenses. Curr. Biol. 2007, 17, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Jelenska, J.; van Hal, J.A.; Greenberg, J.T. Pseudomonas syringae Hijacks Plant Stress Chaperone Machinery for Virulence. Proc Natl. Acad. Sci. USA 2010, 107, 13177–13182. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Tang, C.; Wang, X.; Sun, S.; Zhao, J.; Kang, Z.; Wang, X. An Effector Protein of the Wheat Stripe Rust Fungus Targets Chloroplasts and Suppresses Chloroplast Function. Nat. Commun. 2019, 10, 5571. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Wei, W.; Fu, Y.; Cheng, J.; Xie, J.; Li, G.; Yi, X.; Kang, Z.; Dickman, M.B.; Jiang, D. A Secretory Protein of Necrotrophic Fungus Sclerotinia sclerotiorum That Suppresses Host Resistance. PLoS ONE 2013, 8, e53901. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Yang, G.; Ma, M.; Liu, X.; Li, B.; Xie, J.; Fu, Y.; Chen, T.; Yu, Y.; Chen, W.; et al. An Effector of a Necrotrophic Fungal Pathogen Targets the Calcium-sensing Receptor in Chloroplasts to Inhibit Host Resistance. Mol. Plant Pathol. 2020, 21, 686–701. [Google Scholar] [CrossRef] [Green Version]
- Jing, M.; Guo, B.; Li, H.; Yang, B.; Wang, H.; Kong, G.; Zhao, Y.; Xu, H.; Wang, Y.; Ye, W.; et al. A Phytophthora sojae Effector Suppresses Endoplasmic Reticulum Stress-Mediated Immunity by Stabilizing Plant Binding Immunoglobulin Proteins. Nat. Commun. 2016, 7, 11685. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhong, X.; Shi, Y.; Liu, Z.; Jiang, N.; Liu, J.; Ding, B.; Li, Z.; Kang, H.; Ning, Y.; et al. A Fungal Effector Targets a Heat Shock–Dynamin Protein Complex to Modulate Mitochondrial Dynamics and Reduce Plant Immunity. Sci. Adv. 2020, 6, eabb7719. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Long, Y.; He, F.; Zhang, C.; Wang, R.; Zhang, T.; Wu, W.; Hao, Z.; Wang, Y.; Wang, G.-L.; et al. The Fungal Pathogen Magnaporthe oryzae Suppresses Innate Immunity by Modulating a Host Potassium Channel. PLoS Pathog. 2018, 14, e1006878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Klejnot, J.; Zhao, X.; Shalitin, D.; Maymon, M.; Yang, H.; Lee, J.; Liu, X.; Lopez, J.; Lin, C. Arabidopsis Cryptochrome 2 Completes Its Posttranslational Life Cycle in the Nucleus. Plant Cell 2007, 19, 3146–3156. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.-C.; Meng, Y.-Y.; Yu, X.-H.; Zhang, Z.-L.; Feng, D.-S.; Sun, S.-F.; Liu, B.; Lin, C.-T. A Study of the Blue-Light-Dependent Phosphorylation, Degradation, and Photobody Formation of Arabidopsis CRY2. Mol. Plant 2012, 5, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhu, T.; Han, L.; Liu, M.; Xu, M.; Liu, Y.; Han, D.; Qiu, D.; Gong, Q.; Liu, X. The Asparagine-Rich Protein NRP Interacts with the Verticillium Effector PevD1 and Regulates the Subcellular Localization of Cryptochrome 2. J. Exp. Bot. 2017, 68, 3427–3440. [Google Scholar] [CrossRef]
- Boevink, P.C.; Wang, X.; McLellan, H.; He, Q.; Naqvi, S.; Armstrong, M.R.; Zhang, W.; Hein, I.; Gilroy, E.M.; Tian, Z.; et al. A Phytophthora Infestans RXLR Effector Targets Plant PP1c Isoforms That Promote Late Blight Disease. Nat. Commun. 2016, 7, 10311. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Guo, J.; Liu, P.; He, F.; Wan, C.; Islam, M.A.; Tyler, B.M.; Kang, Z.; Guo, J. Stripe Rust Effector PstGSRE1 Disrupts Nuclear Localization of ROS-Promoting Transcription Factor TaLOL2 to Defeat ROS-Induced Defense in Wheat. Mol. Plant 2019, 12, 1624–1638. [Google Scholar] [CrossRef]
- Singh, Y.; Nair, A.M.; Verma, P.K. Surviving the Odds: From Perception to Survival of Fungal Phytopathogens under Host-Generated Oxidative Burst. Plant Commun. 2021, 2, 100142. [Google Scholar] [CrossRef]
- Kwon, S.; Rupp, O.; Brachmann, A.; Blum, C.F.; Kraege, A.; Goesmann, A.; Feldbrügge, M. MRNA Inventory of Extracellular Vesicles from Ustilago maydis. J. Fungi 2021, 7, 562. [Google Scholar] [CrossRef]
- Nomura, K.; Mecey, C.; Lee, Y.-N.; Imboden, L.A.; Chang, J.H.; He, S.Y. Effector-Triggered Immunity Blocks Pathogen Degradation of an Immunity-Associated Vesicle Traffic Regulator in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 10774–10779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D.G. A Plant MiRNA Contributes to Antibacterial Resistance by Repressing Auxin Signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Hu, J.; Oh, Y.; Park, J.; Choi, J.; Lee, Y.-H.; Dean, R.A.; Mitchell, T.K. Combining ChIP-Chip and Expression Profiling to Model the MoCRZ1 Mediated Circuit for Ca2+/Calcineurin Signaling in the Rice Blast Fungus. PLoS Pathog. 2010, 6, e1000909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, S.M.; Kuhn, H.; Micali, C.; Liller, C.; Kwaaitaal, M.; Panstruga, R. Interaction of a Blumeria Graminis f. Sp. Hordei Effector Candidate with a Barley ARF-GAP Suggests That Host Vesicle Trafficking Is a Fungal Pathogenicity Target. Mol. Plant Pathol. 2014, 15, 535–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomczynska, I.; Stumpe, M.; Mauch, F. A Conserved RxLR Effector Interacts with Host RABA-Type GTPases to Inhibit Vesicle-Mediated Secretion of Antimicrobial Proteins. Plant J. 2018, 95, 187–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petre, B.; Contreras, M.P.; Bozkurt, T.O.; Schattat, M.H.; Sklenar, J.; Schornack, S.; Abd-El-Haliem, A.; Castells-Graells, R.; Lozano-Duran, R.; Dagdas, Y.F.; et al. Host-Interactor Screens of Phytophthora Infestans RXLR Proteins Reveal Vesicle Trafficking as a Major Effector-Targeted Process. Plant Cell 2021, 33, 25. [Google Scholar] [CrossRef]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic Priming by a Secreted Fungal Effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef]
- Patkar, R.N.; Benke, P.I.; Qu, Z.; Constance Chen, Y.Y.; Yang, F.; Swarup, S.; Naqvi, N.I. A Fungal Monooxygenase-Derived Jasmonate Attenuates Host Innate Immunity. Nat. Chem. Biol. 2015, 11, 733–740. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of Phytohormone Pathways by Pathogen Effectors [OPEN]. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.-W.; Ma, W. Phytohormone Pathways as Targets of Pathogens to Facilitate Infection. Plant Mol. Biol. 2016, 91, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Kahmann, R. Manipulation of Phytohormone Pathways by Effectors of Filamentous Plant Pathogens. Front. Plant Sci. 2019, 10, 822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of Jasmonate and Salicylate Signal Crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.M.; Weaver, L.M. The Shikimate Pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate Synthase Is Required to Synthesize Salicylic Acid for Plant Defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Garcion, C.; Lohmann, A.; Lamodière, E.; Catinot, J.; Buchala, A.; Doermann, P.; Métraux, J.-P. Characterization and Biological Function of the ISOCHORISMATE SYNTHASE2 Gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Strawn, M.A.; Marr, S.K.; Inoue, K.; Inada, N.; Zubieta, C.; Wildermuth, M.C. Arabidopsis Isochorismate Synthase Functional in Pathogen-Induced Salicylate Biosynthesis Exhibits Properties Consistent with a Role in Diverse Stress Responses. J. Biol. Chem. 2007, 282, 5919–5933. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid Biosynthesis and Metabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef] [Green Version]
- Waheed, S.; Anwar, M.; Saleem, M.A.; Wu, J.; Tayyab, M.; Hu, Z. The Critical Role of Small RNAs in Regulating Plant Innate Immunity. Biomolecules 2021, 11, 184. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Qi, G.; Chen, J.; Chang, M.; Chen, H.; Hall, K.; Korin, J.; Liu, F.; Wang, D.; Fu, Z.Q. Pandemonium Breaks Out: Disruption of Salicylic Acid-Mediated Defense by Plant Pathogens. Mol. Plant 2018, 11, 1427–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauters, L.; Stojilković, B.; Gheysen, G. Pathogens Pulling the Strings: Effectors Manipulating Salicylic Acid and Phenylpropanoid Biosynthesis in Plants. Mol. Plant Pathol. 2021, 22, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.M.; Milling, A.; Mitra, R.M.; Hogan, C.S.; Ailloud, F.; Prior, P.; Allen, C. Ralstonia solanacearum Requires PopS, an Ancient AvrE-Family Effector, for Virulence and To Overcome Salicylic Acid-Mediated Defenses during Tomato Pathogenesis. mBio 2013, 4, e00875-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabbage, M.; Williams, B.; Dickman, M.B. Cell Death Control: The Interplay of Apoptosis and Autophagy in the Pathogenicity of Sclerotinia sclerotiorum. PLoS Pathog. 2013, 9, e1003287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X. NPR1, All Things Considered. Curr. Opin. Plant Biol. 2004, 7, 547–552. [Google Scholar] [CrossRef]
- Wang, D.; Amornsiripanitch, N.; Dong, X. A Genomic Approach to Identify Regulatory Nodes in the Transcriptional Network of Systemic Acquired Resistance in Plants. PLoS Pathog. 2006, 2, e123. [Google Scholar] [CrossRef] [Green Version]
- Saijo, Y.; Loo, E.P. Plant Immunity in Signal Integration between Biotic and Abiotic Stress Responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, B.; Li, K.; Kang, Z.; Cantu, D.; Dubcovsky, J. A Conserved Puccinia striiformis Protein Interacts with Wheat NPR1 and Reduces Induction of Pathogenesis—Related Genes in Response to Pathogens. Mol. Plant Microbe Interact. 2016, 29, 977–989. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Chen, J.; Li, M.; Chang, M.; Xu, K.; Shang, Z.; Zhao, Y.; Palmer, I.; Zhang, Y.; McGill, J.; et al. A Bacterial Type III Effector Targets the Master Regulator of Salicylic Acid Signaling, NPR1, to Subvert Plant Immunity. Cell Host Microbe 2017, 22, 777–788.e7. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chen, Y.; Wang, J.; Zou, F.; Jia, Y.; Shen, D.; Zhang, Q.; Jing, M.; Dou, D.; Zhang, M. A Phytophthora Capsici Virulence Effector Associates with NPR1 and Suppresses Plant Immune Responses. Phytopathol. Res. 2019, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Riemann, M.; Haga, K.; Shimizu, T.; Okada, K.; Ando, S.; Mochizuki, S.; Nishizawa, Y.; Yamanouchi, U.; Nick, P.; Yano, M.; et al. Identification of Rice Allene Oxide Cyclase Mutants and the Function of Jasmonate for Defence against Magnaporthe oryzae. Plant J. 2013, 74, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Lemarié, S.; Robert-Seilaniantz, A.; Lariagon, C.; Lemoine, J.; Marnet, N.; Jubault, M.; Manzanares-Dauleux, M.J.; Gravot, A. Both the Jasmonic Acid and the Salicylic Acid Pathways Contribute to Resistance to the Biotrophic Clubroot Agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol. 2015, 25, 2158–2168. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Yao, J.; Ma, K.-W.; Zhou, H.; Song, J.; He, S.Y.; Ma, W. Bacterial Effector Activates Jasmonate Signaling by Directly Targeting JAZ Transcriptional Repressors. PLoS Pathog. 2013, 9, e1003715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Teixeira, P.J.P.L.; Biswas, S.; Finkel, O.M.; He, Y.; Salas-Gonzalez, I.; English, M.E.; Epple, P.; Mieczkowski, P.; Dangl, J.L. Pseudomonas syringae Type III Effector HopBB1 Promotes Host Transcriptional Repressor Degradation to Regulate Phytohormone Responses and Virulence. Cell Host Microbe 2017, 21, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.J.; Yoon, A.J.; Faull, K.F.; Diener, A.C. Host Perception of Jasmonates Promotes Infection by Fusarium oxysporum Formae Speciales That Produce Isoleucine- and Leucine-Conjugated Jasmonates: Infection by JA-Ile-Producing F. oxysporum. Mol. Plant Pathol. 2014, 15, 589–600. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Gardiner, D.M.; Kazan, K.; Manners, J.M. A Highly Conserved Effector in Fusarium oxysporum Is Required for Full Virulence on Arabidopsis. Mol. Plant Microbe Interact. 2012, 25, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Chini, A.; Cimmino, A.; Masi, M.; Reveglia, P.; Nocera, P.; Solano, R.; Evidente, A. The Fungal Phytotoxin Lasiojasmonate A Activates the Plant Jasmonic Acid Pathway. J. Exp. Bot. 2018, 69, 3095–3102. [Google Scholar] [CrossRef] [Green Version]
- Darino, M.; Chia, K.; Marques, J.; Aleksza, D.; Soto-Jiménez, L.M.; Saado, I.; Uhse, S.; Borg, M.; Betz, R.; Bindics, J.; et al. Ustilago maydis Effector Jsi1 Interacts with Topless Corepressor, Hijacking Plant Jasmonate/Ethylene Signaling. New Phytol. 2021, 229, 3393–3407. [Google Scholar] [CrossRef]
- Hoffman, T.; Schmidt, J.S.; Zheng, X.; Bent, A.F. Isolation of Ethylene-Insensitive Soybean Mutants That Are Altered in Pathogen Susceptibility and Gene-for-Gene Disease Resistance. Plant Physiol. 1999, 119, 935–949. [Google Scholar] [CrossRef] [Green Version]
- Thomma, B.P.H.J.; Eggermont, K.; Tierens, K.F.M.-J.; Broekaert, W.F. Requirement of Functional Ethylene-Insensitive 2 Gene for Efficient Resistance of Arabidopsis to Infection by Botrytis Cinerea. Plant Physiol. 1999, 121, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrocal-Lobo, M.; Molina, A.; Solano, R. Constitutive Expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis Confers Resistance to Several Necrotrophic Fungi. Plant J. 2002, 29, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, W.; Cao, J.; Meng, F.; Yu, Y.; Huang, J.; Jiang, L.; Liu, M.; Zhang, Z.; Chen, X.; et al. Activation of Ethylene Signaling Pathways Enhances Disease Resistance by Regulating ROS and Phytoalexin Production in Rice. Plant J. 2017, 89, 338–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christians, M.J.; Gingerich, D.J.; Hansen, M.; Binder, B.M.; Kieber, J.J.; Vierstra, R.D. The BTB Ubiquitin Ligases ETO1, EOL1 and EOL2 Act Collectively to Regulate Ethylene Biosynthesis in Arabidopsis by Controlling Type-2 ACC Synthase Levels. Plant J. 2009, 57, 332–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skottke, K.R.; Yoon, G.M.; Kieber, J.J.; DeLong, A. Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms. PLoS Genet. 2011, 7, e1001370. [Google Scholar] [CrossRef]
- Li, G.; Meng, X.; Wang, R.; Mao, G.; Han, L.; Liu, Y.; Zhang, S. Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis. PLoS Genet. 2012, 8, e1002767. [Google Scholar] [CrossRef]
- Helliwell, E.E.; Wang, Q.; Yang, Y. Ethylene Biosynthesis and Signaling Is Required for Rice Immune Response and Basal Resistance Against Magnaporthe oryzae Infection. Mol. Plant Microbe Interact. 2016, 29, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Washington, E.J.; Mukhtar, M.S.; Finkel, O.M.; Wan, L.; Banfield, M.J.; Kieber, J.J.; Dangl, J.L. Pseudomonas syringae Type III Effector HopAF1 Suppresses Plant Immunity by Targeting Methionine Recycling to Block Ethylene Induction. Proc. Natl. Acad. Sci. USA 2016, 113, E3577–E3586. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wang, Y.; Guo, B.; Jing, M.; Zhou, H.; Li, Y.; Wang, H.; Huang, J.; Wang, Y.; Ye, W.; et al. The Phytophthora sojae RXLR Effector Avh238 Destabilizes Soybean Type2 Gm ACS s to Suppress Ethylene Biosynthesis and Promote Infection. New Phytol. 2019, 222, 425–437. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; Spíchal, L.; Novák, O.; Strnad, M.; Asano, T.; Kikuchi, S.; Höfte, M.; De Vleesschauwer, D. Silicon Induces Resistance to the Brown Spot Fungus Cochliobolus miyabeanus by Preventing the Pathogen from Hijacking the Rice Ethylene Pathway. New Phytol. 2015, 206, 761–773. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by Small-Molecule Hormones in Plant Immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, K.; Katagiri, F. Comparing Signaling Mechanisms Engaged in Pattern-Triggered and Effector-Triggered Immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of Hormone Signaling Networks in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef]
- Evangelisti, E.; Govetto, B.; Minet-Kebdani, N.; Kuhn, M.; Attard, A.; Ponchet, M.; Panabières, F.; Gourgues, M. The Phytophthora Parasitica RXLR Effector Penetration-Specific Effector 1 Favours Arabidopsis thaliana Infection by Interfering with Auxin Physiology. New Phytol. 2013, 199, 476–489. [Google Scholar] [CrossRef]
- Cui, F.; Wu, S.; Sun, W.; Coaker, G.; Kunkel, B.; He, P.; Shan, L. The Pseudomonas syringae Type III Effector AvrRpt2 Promotes Pathogen Virulence via Stimulating Arabidopsis Auxin/Indole Acetic Acid Protein Turnover. Plant Physiol. 2013, 162, 1018–1029. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Agnew, J.L.; Cohen, J.D.; He, P.; Shan, L.; Sheen, J.; Kunkel, B.N. Pseudomonas syringae Type III Effector AvrRpt2 Alters Arabidopsis Thaliana Auxin Physiology. Proc. Natl. Acad. Sci. USA 2007, 104, 20131–20136. [Google Scholar] [CrossRef] [Green Version]
- Hann, D.R.; Domínguez-Ferreras, A.; Motyka, V.; Dobrev, P.I.; Schornack, S.; Jehle, A.; Felix, G.; Chinchilla, D.; Rathjen, J.P.; Boller, T. The P Seudomonas Type III Effector HopQ1 Activates Cytokinin Signaling and Interferes with Plant Innate Immunity. New Phytol. 2014, 201, 585–598. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; He, Q.; Li, S.; Liu, W.; Lin, C.; Miao, W. A Candidate Secreted Effector Protein of Rubber Tree Powdery Mildew Fungus Contributes to Infection by Regulating Plant ABA Biosynthesis. Front. Microbiol. 2020, 11, 591387. [Google Scholar] [CrossRef]
- Weiss, D.; Ori, N. Mechanisms of Cross Talk between Gibberellin and Other Hormones. Plant Physiol. 2007, 144, 1240–1246. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Huh, S.U.; Kojima, M.; Sakakibara, H.; Paek, K.-H.; Hwang, I. The Cytokinin-Activated Transcription Factor ARR2 Promotes Plant Immunity via TGA3/NPR1-Dependent Salicylic Acid Signaling in Arabidopsis. Dev. Cell 2010, 19, 284–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.-J.; Shimono, M.; Sugano, S.; Kojima, M.; Yazawa, K.; Yoshida, R.; Inoue, H.; Hayashi, N.; Sakakibara, H.; Takatsuji, H. Abscisic Acid Interacts Antagonistically with Salicylic Acid Signaling Pathway in Rice—Magnaporthe grisea Interaction. Mol. Plant Microbe Interact. 2010, 23, 791–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argueso, C.T.; Ferreira, F.J.; Epple, P.; To, J.P.C.; Hutchison, C.E.; Schaller, G.E.; Dangl, J.L.; Kieber, J.J. Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity. PLoS Genet. 2012, 8, e1002448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseem, M. Cytokinins for Immunity beyond Growth, Galls and Green Islands. Trends Plant Sci. 2014, 4, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Kaltdorf, M.; Dandekar, T. The Nexus between Growth and Defence Signalling: Auxin and Cytokinin Modulate Plant Immune Response Pathways. J. Exp. Bot. 2015, 66, 4885–4896. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, B.N.; Brooks, D.M. Cross Talk between Signaling Pathways in Pathogen Defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Takahashi, H.; Kanayama, Y.; Zheng, M.S.; Kusano, T.; Hase, S.; Ikegami, M.; Shah, J. Antagonistic Interactions between the SA and JA Signaling Pathways in Arabidopsis Modulate Expression of Defense Genes and Gene-for-Gene Resistance to Cucumber Mosaic Virus. Plant Cell Physiol. 2004, 45, 803–809. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. Making Sense of Hormone Crosstalk during Plant Immune Responses. Cell Host Microbe 2008, 3, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Chang, P.F.L.; Liu, D.; Narasimhan, M.L.; Raghothama, K.G.; Hasegawa, P.M.; Bressan, R.A. Plant Defense Genes Are Synergistically Induced by Ethylene and Methyl Jasmonate. Plant Cell 1994, 6, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, O.; Piqueras, R.; Sánchez-Serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 Integrates Signals from Ethylene and Jasmonate Pathways in Plant Defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; An, F.; Feng, Y.; Li, P.; Xue, L.; Mu, A.; Jiang, Z.; Kim, J.-M.; To, T.K.; Li, W.; et al. Derepression of Ethylene-Stabilized Transcription Factors (EIN3/EIL1) Mediates Jasmonate and Ethylene Signaling Synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 12539–12544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazan, K.; Manners, J.M. Linking Development to Defense: Auxin in Plant–Pathogen Interactions. Trends Plant Sci. 2009, 14, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.-F.; He, S.Y. Pseudomonas syringae Pv. Tomato DC3000: A Model Pathogen for Probing Disease Susceptibility and Hormone Signaling in Plants. Annu. Rev. Phytopathol. 2013, 51, 473–498. [Google Scholar] [CrossRef] [PubMed]
- Veselova, S.V.; Nuzhnaya, T.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Khusnutdinova, E.K.; Maksimov, I.V. Ethylene-Cytokinin Interaction Determines Early Defense Response of Wheat against Stagonospora Nodorum Berk. Biomolecules 2021, 11, 174. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Hao, L.; Wang, S.; Wang, S.; Zhang, W.; Xu, C.; Yu, Y.; Li, T. A Novel MiRNA Negatively Regulates Resistance to Glomerella Leaf Spot by Suppressing Expression of an NBS Gene in Apple. Hortic. Res. 2019, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhao, Y.-L.; Zhao, J.-H.; Wang, S.; Jin, Y.; Chen, Z.-Q.; Fang, Y.-Y.; Hua, C.-L.; Ding, S.-W.; Guo, H.-S. Cotton Plants Export MicroRNAs to Inhibit Virulence Gene Expression in a Fungal Pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Song, N.; Zhao, M.; Liu, R.; Feng, H.; Wang, X.; Kang, Z. Puccinia striiformis f. Sp. Tritici Mi croRNA -like RNA 1 (Pst-milR1), an Important Pathogenicity Factor of Pst, Impairs Wheat Resistance to Pst by Suppressing the Wheat Pathogenesis-related 2 Gene. New Phytol. 2017, 215, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Li, Y.; Ding, S.-W. Small RNA-Based Antimicrobial Immunity. Nat. Rev. Immunol. 2019, 19, 31–44. [Google Scholar] [CrossRef]
- Yin, C.; Ramachandran, S.R.; Zhai, Y.; Bu, C.; Pappu, H.R.; Hulbert, S.H. A Novel Fungal Effector from Puccinia Graminis Suppressing RNA Silencing and Plant Defense Responses. New Phytol. 2019, 222, 1561–1572. [Google Scholar] [CrossRef]
- Derbyshire, M.; Mbengue, M.; Barascud, M.; Navaud, O.; Raffaele, S. Small RNAs from the Plant Pathogenic Fungus Sclerotinia sclerotiorum Highlight Host Candidate Genes Associated with Quantitative Disease Resistance. Mol. Plant Pathol. 2019, 20, 1279–1297. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Liu, L.; Xiong, Q.; Flores, C.; Wong, J.; Shi, J.; Wang, X.; Liu, X.; Xiang, Q.; Jiang, S.; et al. Oomycete Pathogens Encode RNA Silencing Suppressors. Nat. Genet. 2013, 45, 330–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Q.; Ye, W.; Choi, D.; Wong, J.; Qiao, Y.; Tao, K.; Wang, Y.; Ma, W. Phytophthora Suppressor of RNA Silencing 2 Is a Conserved RxLR Effector That Promotes Infection in Soybean and Arabidopsis thaliana. Mol. Plant Microbe Interact. 2014, 27, 1379–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Zhai, Y.; Feng, L.; Karimi, H.Z.; Rutter, B.D.; Zeng, L.; Choi, D.S.; Zhang, B.; Gu, W.; Chen, X.; et al. A Phytophthora Effector Suppresses Trans-Kingdom RNAi to Promote Disease Susceptibility. Cell Host Microbe 2019, 25, 153–165.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, W.; Ma, W. Filamentous Pathogen Effectors Interfering with Small RNA Silencing in Plant Hosts. Curr. Opin. Microbiol. 2016, 32, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.A. ROS in Biotic Interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Jwa, N.-S.; Hwang, B.K. Convergent Evolution of Pathogen Effectors toward Reactive Oxygen Species Signaling Networks in Plants. Front. Plant Sci. 2017, 8, 1687. [Google Scholar] [CrossRef] [Green Version]
- Thoma, I.; Loeffler, C.; Sinha, A.K.; Gupta, M.; Krischke, M.; Steffan, B.; Roitsch, T.; Mueller, M.J. Cyclopentenone Isoprostanes Induced by Reactive Oxygen Species Trigger Defense Gene Activation and Phytoalexin Accumulation in Plants. Plant J. 2003, 34, 363–375. [Google Scholar] [CrossRef]
- Bindschedler, L.V.; Dewdney, J.; Blee, K.A.; Stone, J.M.; Asai, T.; Plotnikov, J.; Denoux, C.; Hayes, T.; Gerrish, C.; Davies, D.R.; et al. Peroxidase-Dependent Apoplastic Oxidative Burst in Arabidopsis Required for Pathogen Resistance. Plant J. 2006, 47, 851–863. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the News: Subcellular and Organellar Reactive Oxygen Species Production and Signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [Green Version]
- Janků, M.; Luhová, L.; Petřivalský, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemetsberger, C.; Herrberger, C.; Zechmann, B.; Hillmer, M.; Doehlemann, G. The Ustilago maydis Effector Pep1 Suppresses Plant Immunity by Inhibition of Host Peroxidase Activity. PLoS Pathog. 2012, 8, e1002684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Guan, T.; Zheng, P.; Chen, L.; Yang, Y.; Huai, B.; Li, D.; Chang, Q.; Huang, L.; Kang, Z. An Extracellular Zn-Only Superoxide Dismutase from Puccinia striiformis Confers Enhanced Resistance to Host-Derived Oxidative Stress: Characterization of a Superoxide Dismutase PsSOD1. Environ. Microbiol. 2016, 18, 4118–4135. [Google Scholar] [CrossRef] [PubMed]
- Drincovich, M.F.; Casati, P.; Andreo, C.S. NADP-malic enzyme from plants: A ubiquitous enzyme involved in different metabolic pathways. FEBS Lett. 2001, 490, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Dangol, S.; Chen, Y.; Choi, J.; Cho, Y.-S.; Lee, J.-E.; Choi, M.-O.; Jwa, N.-S. Magnaporthe oryzae Effector AVR-Pii Helps to Establish Compatibility by Inhibition of the Rice NADP-Malic Enzyme Resulting in Disruption of Oxidative Burst and Host Innate Immunity. Mol. Cells 2016, 39, 426–438. [Google Scholar] [CrossRef] [Green Version]
- Dangol, S.; Chen, Y.; Hwang, B.K.; Jwa, N.-S. Iron- and Reactive Oxygen Species-Dependent Ferroptotic Cell Death in Rice-Magnaporthe Oryzae Interactions. Plant Cell 2019, 31, 189–209. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Gueguen-Chaignon, V.; Gonçalves, I.R.; Rascle, C.; Rigault, M.; Dellagi, A.; Loisel, E.; Poussereau, N.; Rodrigue, A.; Terradot, L.; et al. A Secreted Metal-Binding Protein Protects Necrotrophic Phytopathogens from Reactive Oxygen Species. Nat. Commun. 2019, 10, 4853. [Google Scholar] [CrossRef] [Green Version]
- Shidore, T.; Broeckling, C.D.; Kirkwood, J.S.; Long, J.J.; Miao, J.; Zhao, B.; Leach, J.E.; Triplett, L.R. The Effector AvrRxo1 Phosphorylates NAD in Planta. PLoS Pathog. 2017, 13, e1006442. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Huai, B.; Lu, Y.; Cai, K.; Guo, J.; Zhu, X.; Kang, Z.; Guo, J. A Stripe Rust Effector Pst18363 Targets and Stabilises TaNUDX23 That Promotes Stripe Rust Disease. New Phytol. 2020, 225, 880–895. [Google Scholar] [CrossRef]
- Tintor, N.; Paauw, M.; Rep, M.; Takken, F.L.W. The Root-invading Pathogen Fusarium oxysporum Targets Pattern-triggered Immunity Using Both Cytoplasmic and Apoplastic Effectors. New Phytol. 2020, 227, 1479–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Wang, X.; Wang, F.; Zhao, Z.; Li, G.; Zhu, X.; Su, J.; Chen, L. The Fungal Effector Avr-Pita Suppresses Innate Immunity by Increasing COX Activity in Rice Mitochondria. Rice 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Coll, N.S.; Epple, P.; Dangl, J.L. Programmed Cell Death in the Plant Immune System. Cell Death Differ. 2011, 18, 1247–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, K.; Scalschi, L.; Jaiswal, N.; Mengiste, T.; Fried, R.; Sanz, A.B.; Arroyo, J.; Zhu, W.; Masrati, G.; Sharon, A. The Botrytis Cinerea Crh1 Transglycosylase Is a Cytoplasmic Effector Triggering Plant Cell Death and Defense Response. Nat. Commun. 2021, 12, 2166. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Tian, F.; Wamboldt, Y.; Alfano, J.R. The Majority of the Type III Effector Inventory of Pseudomonas syringae Pv. Tomato DC3000 Can Suppress Plant Immunity. Mol. Plant Microbe Interact. 2009, 22, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Peng, H.; Goher, F.; Islam, M.A.; Xu, S.; Guo, J.; Kang, Z.; Guo, J. A Candidate Effector Protein PstCFEM1 Contributes to Virulence of Stripe Rust Fungus and Impairs Wheat Immunity. Stress Biol. 2022. [Google Scholar] [CrossRef]
- Manning, V.A.; Chu, A.L.; Steeves, J.E.; Wolpert, T.J.; Ciuffetti, L.M. A Host-Selective Toxin of Pyrenophora Tritici-Repentis, Ptr ToxA, Induces Photosystem Changes and Reactive Oxygen Species Accumulation in Sensitive Wheat. Mol. Plant Microbe Interact. 2009, 22, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Manning, V.A.; Chu, A.L.; Scofield, S.R.; Ciuffetti, L.M. Intracellular Expression of a Host-selective Toxin, ToxA, in Diverse Plants Phenocopies Silencing of a ToxA-interacting Protein, ToxABP1. New Phytol. 2010, 187, 1034–1047. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Faris, J.D.; Oliver, R.P.; Syme, R.; McDonald, M.C.; McDonald, B.A.; Solomon, P.S.; Lu, S.; Shelver, W.L.; et al. The Cysteine Rich Necrotrophic Effector SnTox1 Produced by Stagonospora Nodorum Triggers Susceptibility of Wheat Lines Harboring Snn1. PLoS Pathog. 2012, 8, e1002467. [Google Scholar] [CrossRef] [Green Version]
- Outram, M.A.; Sung, Y.; Yu, D.; Dagvadorj, B.; Rima, S.A.; Jones, D.A.; Ericsson, D.J.; Sperschneider, J.; Solomon, P.S.; Kobe, B.; et al. The Crystal Structure of SnTox3 from the Necrotrophic Fungus Parastagonospora nodorum Reveals a Unique Effector Fold and Provides Insight into Snn3 Recognition and Pro-domain Protease Processing of Fungal Effectors. New Phytol. 2021, 231, 2282–2296. [Google Scholar] [CrossRef]
- Veselova, S.; Nuzhnaya, T.; Burkhanova, G.; Rumyantsev, S.; Maksimov, I. Reactive Oxygen Species in Host Plant Are Required for an Early Defense Response against Attack of Stagonospora Nodorum Berk. Necrotrophic Effectors SnTox. Plants 2021, 10, 1586. [Google Scholar] [CrossRef]
- Zhu, W.; Ronen, M.; Gur, Y.; Minz-Dub, A.; Masrati, G.; Ben-Tal, N.; Savidor, A.; Sharon, I.; Eizner, E.; Valerius, O.; et al. BcXYG1, a Secreted Xyloglucanase from Botrytis Cinerea, Triggers Both Cell Death and Plant Immune Responses1. Plant Physiol. 2017, 175, 438–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yang, X.; Dong, Y.; Qiu, D. The Botrytis Cinerea Xylanase BcXyl1 Modulates Plant Immunity. Front. Microbiol. 2018, 9, 2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yan, J.; Fu, Z.; Shi, W.; Ninkuu, V.; Li, G.; Yang, X.; Zeng, H. FoEG1, a Secreted Glycoside Hydrolase Family 12 Protein from Fusarium oxysporum, Triggers Cell Death and Modulates Plant Immunity. Mol. Plant Pathol. 2021, 22, 522–538. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Y.; Zhang, Z. Global Characterization of GH10 Family Xylanase Genes in Rhizoctonia Cerealis and Functional Analysis of Xylanase RcXYN1 During Fungus Infection in Wheat. Int. J. Mol. Sci. 2020, 21, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qutob, D.; Kemmerling, B.; Brunner, F.; Küfner, I.; Engelhardt, S.; Gust, A.A.; Luberacki, B.; Seitz, H.U.; Stahl, D.; Rauhut, T.; et al. Phytotoxicity and Innate Immune Responses Induced by Nep1-Like Proteins. Plant Cell 2007, 18, 3721–3744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staats, M.; Van Baarlen, P.; Schouten, A.; Van Kan, J.A.L. Functional Analysis of NLP Genes from Botrytis Elliptica. Mol. Plant Pathol. 2007, 8, 209–214. [Google Scholar] [CrossRef]
- Dallal Bashi, Z.; Hegedus, D.D.; Buchwaldt, L.; Rimmer, S.R.; Borhan, M.H. Expression and Regulation of Sclerotinia sclerotiorum Necrosis and Ethylene-Inducing Peptides (NEPs). Mol. Plant Pathol. 2010, 11, 43–53. [Google Scholar] [CrossRef]
- Santhanam, P.; van Esse, H.P.; Albert, I.; Faino, L.; Nürnberger, T.; Thomma, B.P.H.J. Evidence for Functional Diversification Within a Fungal NEP1-Like Protein Family. Mol. Plant Microbe Interact. 2013, 26, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Kleemann, J.; Rincon-Rivera, L.J.; Takahara, H.; Neumann, U.; van Themaat, E.V.L.; van der Does, H.C.; Hacquard, S.; Stüber, K.; Will, I.; Schmalenbach, W.; et al. Sequential Delivery of Host-Induced Virulence Effectors by Appressoria and Intracellular Hyphae of the Phytopathogen Colletotrichum higginsianum. PLoS Pathog. 2012, 8, e1002643. [Google Scholar] [CrossRef]
- Cabral, A.; Oome, S.; Sander, N.; Küfner, I.; Nürnberger, T.; Van den Ackerveken, G. Nontoxic Nep1-Like Proteins of the Downy Mildew Pathogen Hyaloperonospora arabidopsidis: Repression of Necrosis-Inducing Activity by a Surface-Exposed Region. Mol. Plant Microbe Interact. 2012, 25, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Kanja, C.; Hammond-Kosack, K.E. Proteinaceous Effector Discovery and Characterization in Filamentous Plant Pathogens. Mol. Plant Pathol. 2020, 21, 1353–1376. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, R.; Kiran, K.; Rajarammohan, S.; Dubey, H.; Singh, P.K.; Sharma, Y.; Deshmukh, R.; Sonah, H.; Gupta, N.; Sharma, T.R. Effector Biology of Biotrophic Plant Fungal Pathogens: Current Advances and Future Prospects. Microbiol. Res. 2020, 241, 126567. [Google Scholar] [CrossRef] [PubMed]
- Beckerson, W.C.; Rodríguez de la Vega, R.C.; Hartmann, F.E.; Duhamel, M.; Giraud, T.; Perlin, M.H. Cause and Effectors: Whole-Genome Comparisons Reveal Shared but Rapidly Evolving Effector Sets among Host-Specific Plant-Castrating Fungi. mBio 2019, 10, e02391-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisrimler, C.; Allan, C.; Eccersall, S.; Morris, R.J. Interior Design: How Plant Pathogens Optimize Their Living Conditions. New Phytol. 2021, 229, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of Tradeoffs between Plant Defenses against Pathogens with Different Lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef] [Green Version]

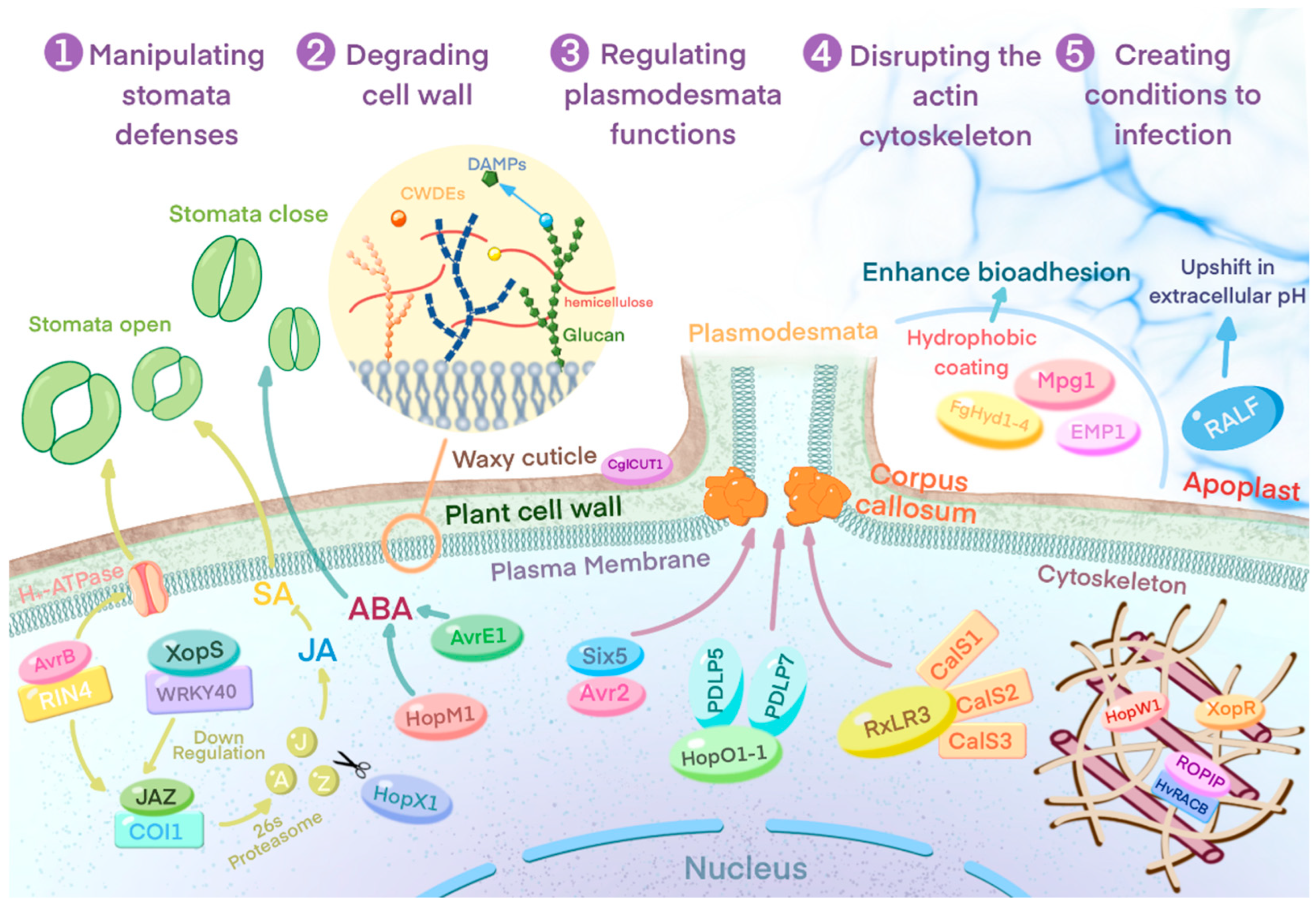

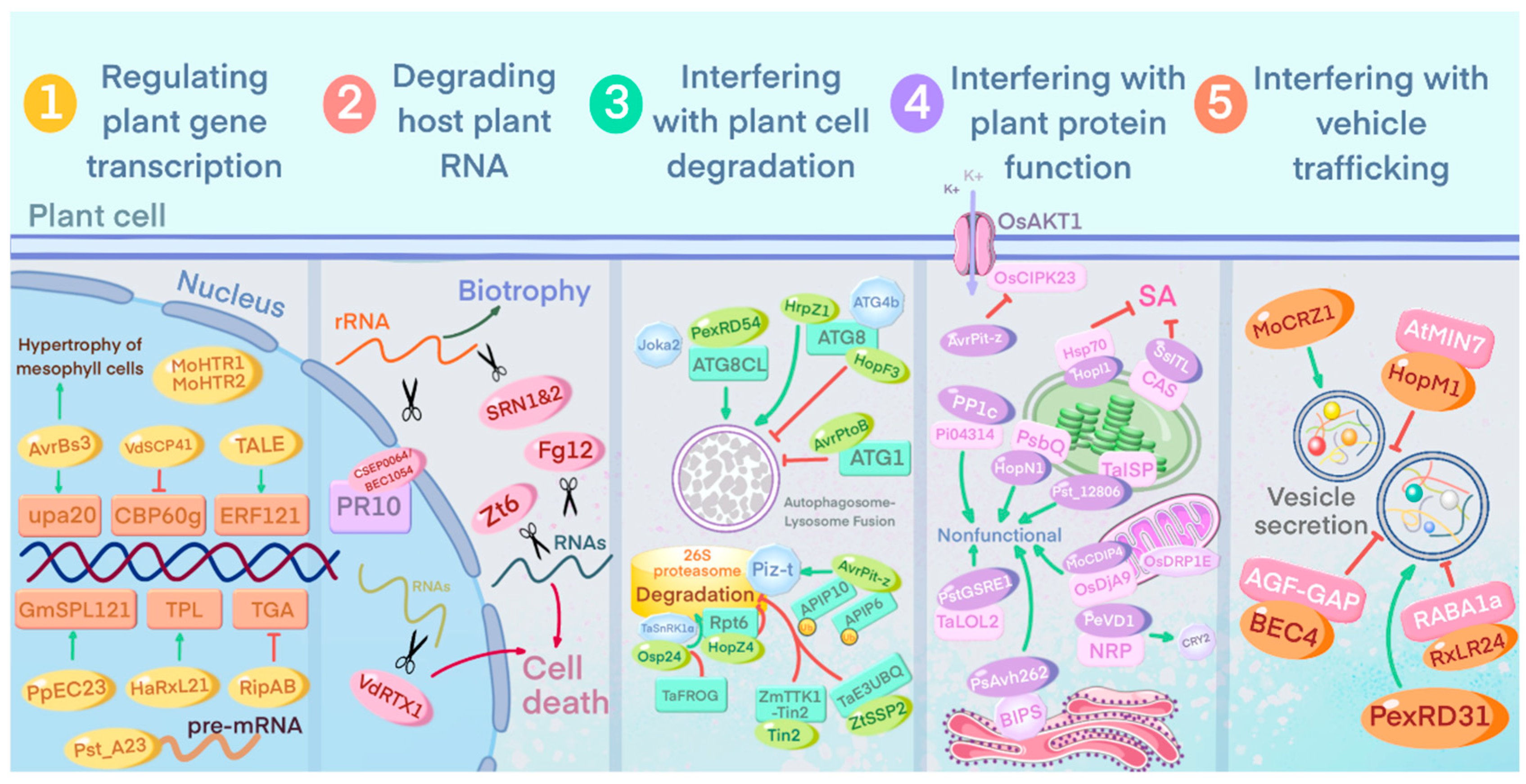
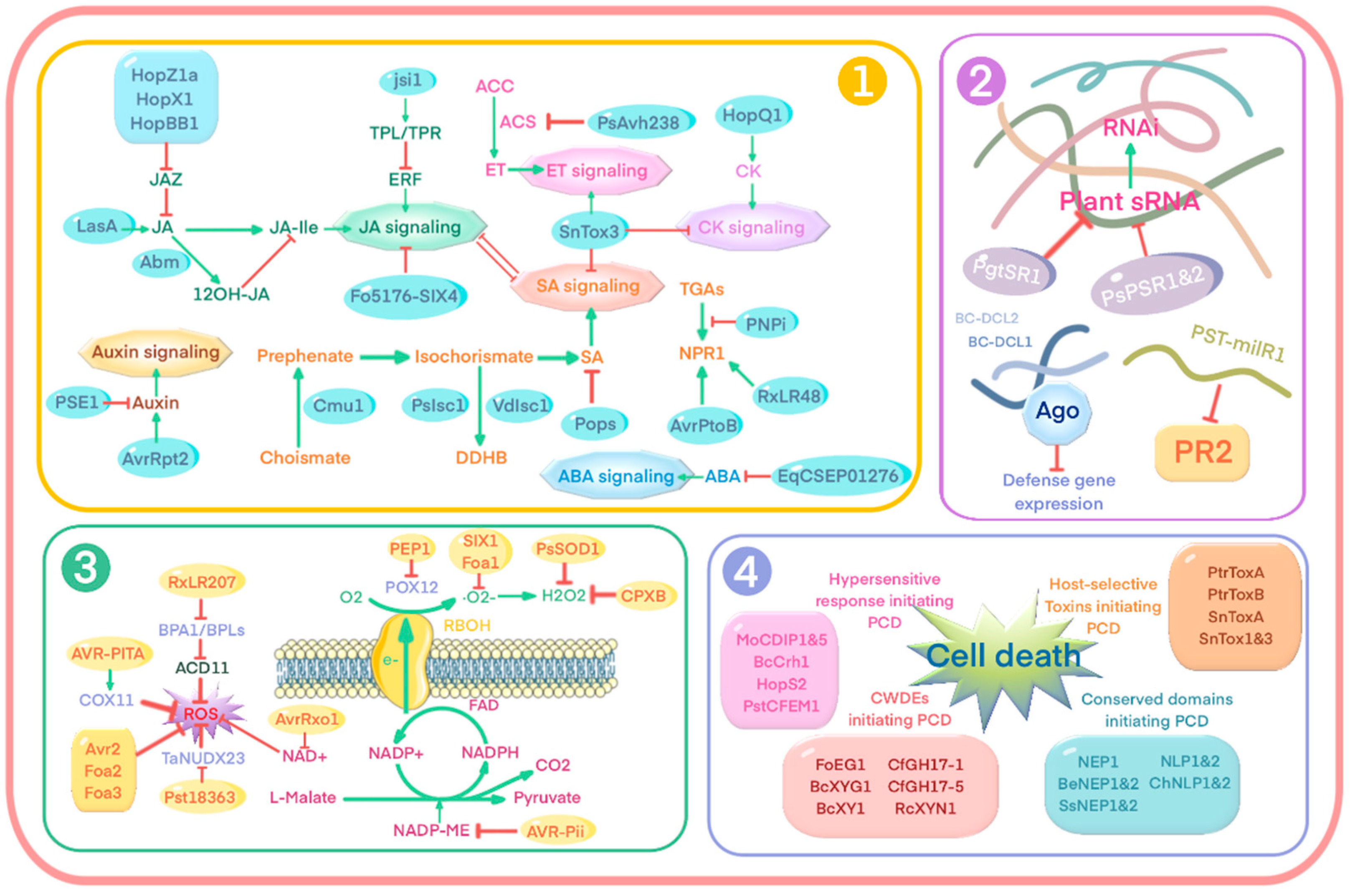
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action Mechanisms of Effectors in Plant-Pathogen Interaction. Int. J. Mol. Sci. 2022, 23, 6758. https://doi.org/10.3390/ijms23126758
Zhang S, Li C, Si J, Han Z, Chen D. Action Mechanisms of Effectors in Plant-Pathogen Interaction. International Journal of Molecular Sciences. 2022; 23(12):6758. https://doi.org/10.3390/ijms23126758
Chicago/Turabian StyleZhang, Shiyi, Cong Li, Jinping Si, Zhigang Han, and Donghong Chen. 2022. "Action Mechanisms of Effectors in Plant-Pathogen Interaction" International Journal of Molecular Sciences 23, no. 12: 6758. https://doi.org/10.3390/ijms23126758
APA StyleZhang, S., Li, C., Si, J., Han, Z., & Chen, D. (2022). Action Mechanisms of Effectors in Plant-Pathogen Interaction. International Journal of Molecular Sciences, 23(12), 6758. https://doi.org/10.3390/ijms23126758






