Comparative Assessment of the Structural Features of Originator Recombinant Human Follitropin Alfa Versus Recombinant Human Follitropin Alfa Biosimilar Preparations Approved in Non-European Regions
Abstract
:1. Introduction
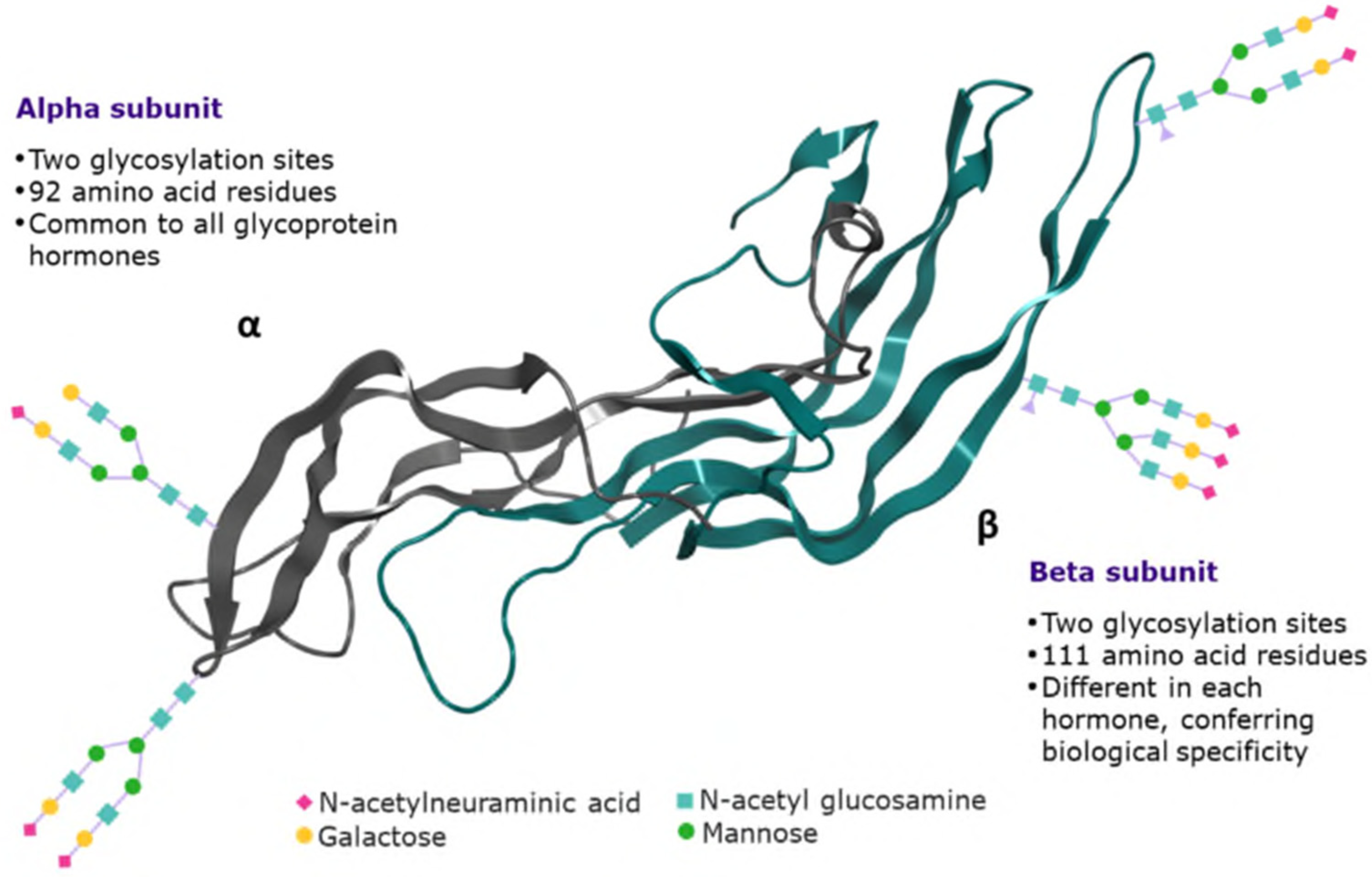
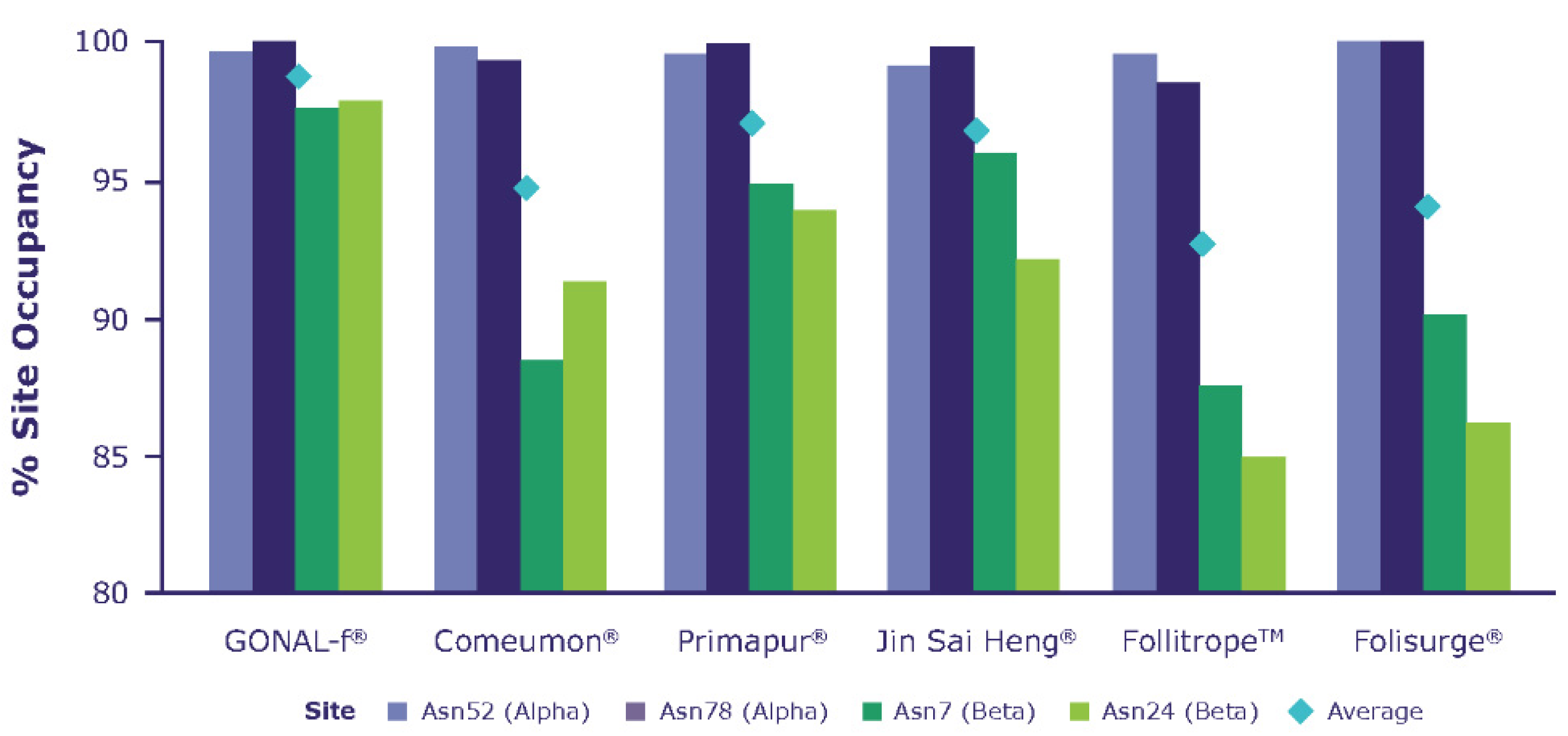
| Macroheterogeneity relates to the presence or absence of a oligosaccharide chain at any of the two known N-glycosylation sites (site occupancy) [17] in the beta subunit. In vivo, the macroheterogeneity of circulating hFSH is dynamic and may have a physiologic role. In women of reproductive age, more glycosylated (fully glycosylated) and acidic glycoforms (with prolonged in vivo half-life due to reduced renal clearance) are secreted during the early and mid-follicular phases, compared with less sialylated, glycosylated (hypo-glycosylated) glycoforms, which are more predominant before ovulation [32,33]. Highly acidic isoforms are more predominant after the menopause than during the fertile lifespan [34]. Furthermore, tri-glycosylated hFSH (hFSH18/21) is more abundant in young women, whereas tetra- glycosylated (hFSH24) and highly sialylated forms are more abundant in peri/postmenopausal women [32,35]. Glycosylation at αAsn 52 is essental for FSH bioactivity, as it has an important role in the assembly of the functional FSH heterodimer and its subsequent stability; it also has a pivotal role in FSH receptor (FSHR) activation and signalling, whereby glycoforms with smaller and more compact glycosylation at αAsn 52 can fit into the central cavity of the FSHR more rapidly than bulkier and extended glycans, leading to a more rapid response [17]. |
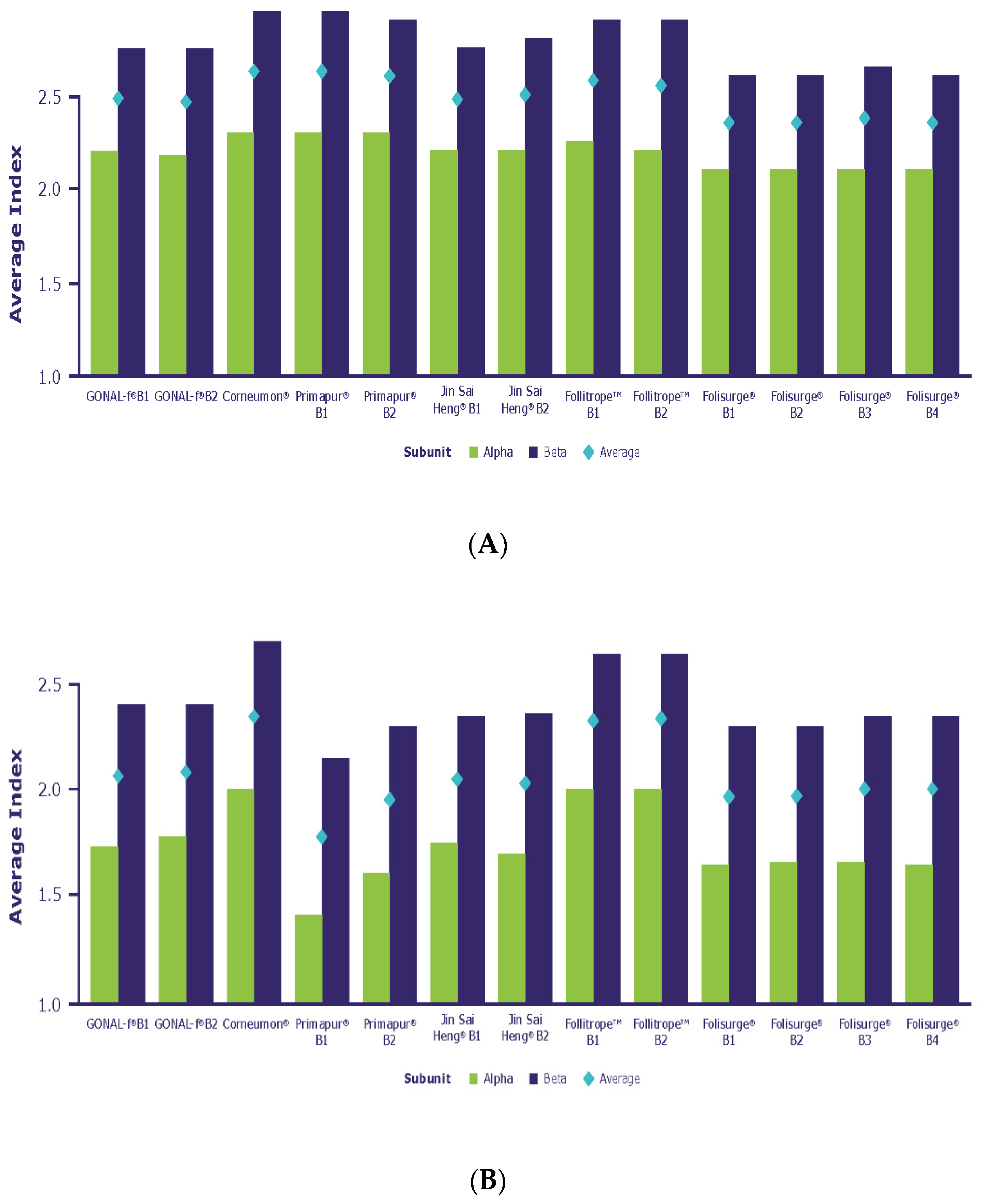
| Microheterogeneity relates to the structural variation in the type of carbohydrates comprising the oligosaccharide chains attached to the protein core and the branching of these chains (antennarity) into bi-, tri- and tetra-antennary structures [36,37,38]. The building block N-acetyl glucosamine (GlcNAc) is linked to asparagine followed by the addition of another GlcNAc, then by one to three Mannose residues that can branch into 1 to 4 antennae. The antennae are then extended by GlcNAc and galactose, the latter of which can be capped by N-acetyl neuraminic acid (sialic acid) [30]. The average number of antennae per glycan is reflected by the A-Index, which is calculated for each N-glycosylation site and as a mean over the entire molecule (see Section 4). |
| Sialylation relates to the inclusion of sialic acid in the glycoprotein antennae. The addition of a sialic acid cap imparts a negative charge on each antenna [30]. The degree of sialic acid capping can vary among glycoforms, whereby variants with a high degree of sialylation are more acidic than those with low sialic acid content [36,37,38]. A high level of sialylation, in combination with the presence of bulkier glycans, may contribute to a longer half-life through reduced glomerular filtration and, therefore, higher net in vivo potency. CHO cells, in which r-hFSH is produced, do not have the ability to synthesize sialic acid attached in the position α2-6, so only α2-3 sialic acid is found in reference r-hFSH-alfa and biosimilar preparations [30]. The average number of sialic acid moieties per glycan is reflected by the S-Index, which is calculated for each N-glycosylation site and as a mean over the entire molecule (see Section 4). The NGNA index reflects the content of glycans with N-glyconeuramic acid (NGNA) moieties. NGNA may be linked to immunogenic reactions, as humans do not produce CMP-N-acetylneuraminic acid hydroxylase, the enzyme responsible for this glycan modification [38]. Anti-NGNA activity has been reported in 85% of healthy humans, suggesting its potential for eliciting an immune response in humans [39]. The NGNA index is calculated for each N-glycosylation site and as a mean over the entire molecule (see Section 4). |
| O-acetylation: The nine-carbon backbone of sialic acids can undergo extensive enzymatic modification in nature, and O-acetylation at the C-4/7/8/9 positions in particular is widely observed [40]. O-acetylation increases the hydrophobic character of sialylated glycans and can change the biophysical properties of the glycoprotein, potentially leading to changes in activity and glycan antigen recognition [40]. |
| Post-translational modifications can include the oxidation of methionine residues. The methionine residues in FSH are not directly located in regions that are critical for binding to the FSHR: methionine 29 is involved in α–β subunit heterodimerization; methionine 47 is located close to the FSHR binding site, but is not directly involved in ligand–receptor interaction; methionine 71 is located close to the heterodimerization site but is not directly involved in heterodimerization; and methionine 109 is located in the non-structural C-terminal region. However, the oxidation of these residues may lead to conformational changes, with the potential for indirect effects on biological activity, pharmacokinetics or protein aggregation, and the alteration of the immunogenicity of therapeutic proteins [41]. In contrast to the post-translational modifications in the oligosaccharide chains attached to the protein core, other modifications, such as the conversion of asparagine to succinimide and N-terminal clipping, are not known to have an impact on biological activity. |
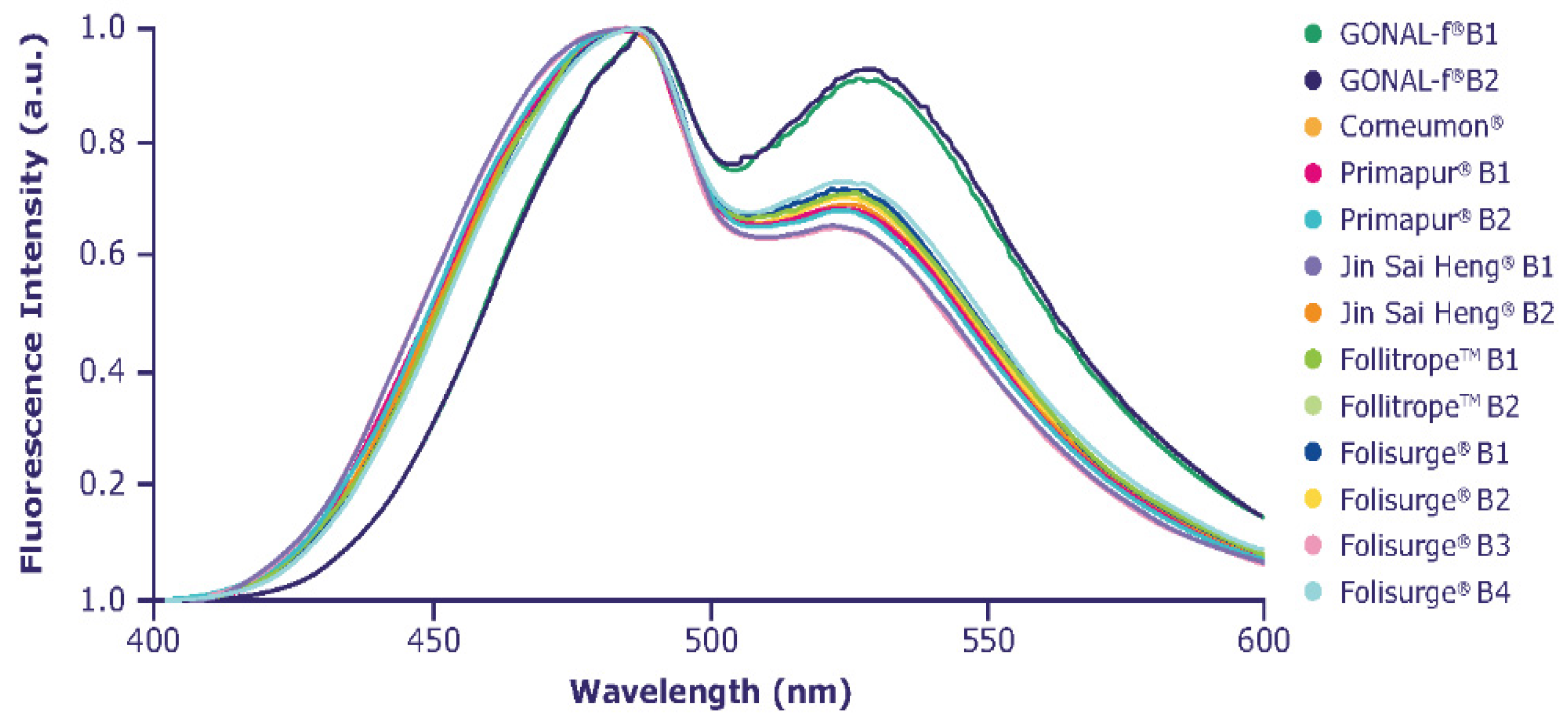
| Higher-order structure refers to the self-assembly into either the seconday-, tertiary- and quarternary-order structure of a protein. Higher-order structure is responsible for the correct folding and three-dimensional shape of a protein and is strongly dependent on the protein environment; therefore, different formulations can bear conformational differences compared with the reference preparation, which can have an impact on the activity of the molecule. |
2. Results
2.1. Macroheterogeneity (Degree of N-Glycosylation Site Occupancy)
2.2. Microheterogeneity (Degree of Antennarity, Sialylation, NGNA, and O-Acetylation)
2.2.1. Antennarity
2.2.2. Sialylation
2.2.3. NGNA Content and O-Acetylation
2.3. Post-Translational Modifications
2.4. Higher-Order Structure
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Materials
4.3. Peptide Mapping for Assessment of Post-Translational Modifications and of Macroheterogeneity (N-Glycosylation Site Occupancy)
4.4. Glycopeptide Mapping for Assessment of Microheterogeneity (Indices for Antennarity, Sialylation, Acetylation and NGNA (N-Glycolyl Neuraminic Acid))
4.5. Fluorescence Spectroscopy for the Assessment of Higher-Order Glycoprotein Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marques, P.; Skorupskaite, K.; Rozario, K.; Anderson, R.; George, J. Physiology of GnRH and Gonadotropin Secretion. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com: South Dartmouth, MA, USA, 2022. [Google Scholar]
- McGee, E.; Hsueh, A. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [PubMed] [Green Version]
- Bergandi, L.; Canosa, S.; Carosso, A.R.; Paschero, C.; Gennarelli, G.; Silvagno, F.; Benedetto, C.; Revelli, A. Human Recombinant FSH and Its Biosimilars: Clinical Efficacy, Safety, and Cost-Effectiveness in Controlled Ovarian Stimulation for In Vitro Fertilization. Pharmaceuticals 2020, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Alam, V.; D’Hooghe, T.; Sunkara, S.K. The Development of Gonadotropins for Clinical Use in the Treatment of Infertility. Front. Endocrinol. 2019, 10, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. GONAL-f® Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/gonal-f-epar-product-information_en.pdf (accessed on 14 June 2022).
- Ulloa-Aguirre, A.; Lira-Albarrán, S. Clinical Applications of Gonadotropins in the Male. Prog. Mol. Biol. Transl. Sci. 2016, 143, 121–174. [Google Scholar]
- Velthuis, E.; Hubbard, J.; Longobardi, S.; D’Hooghe, T. The Frequency of Ovarian Hyperstimulation Syndrome and Thromboembolism with Originator Recombinant Human Follitropin Alfa (GONAL-f) for Medically Assisted Reproduction: A Systematic Review. Adv. Ther. 2021, 37, 4831–4847. [Google Scholar] [CrossRef]
- Bosch, E.; Havelock, J.; Martin, F.S.; Rasmussen, B.B.; Klein, B.M.; Mannaerts, B.; Arce, J.C.; ESTHER-2 Study Group. Follitropin delta in repeated ovarian stimulation for IVF: A controlled, assessor-blind Phase 3 safety trial. Reprod. BioMed. Online 2019, 38, 195–205. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Biosimilars in the EU: Information Guide for Healthcare Professionals. Available online: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf (accessed on 14 June 2022).
- Kang, H.N.; Thorpe, R.; Knezevic, I.; Casas Levano, M.; Chilufya, M.B.; Chirachanakul, P.; Chua, H.M.; Dalili, D.; Foo, F.; Gao, K.; et al. Regulatory challenges with biosimilars: An update from 20 countries. Ann. N. Y. Acad. Sci. 2021, 1491, 42–59. [Google Scholar] [CrossRef]
- European Medicines Agency. European Public Assessment Report (EPAR): Ovaleap® (Follitropin Alfa). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ovaleap (accessed on 14 June 2022).
- European Medicines Agency. European Public Assessment Report (EPAR): Bemfola® (Follitropin Alfa). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/bemfola (accessed on 14 June 2022).
- European Medicines Agency. Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Non-Clinical and Clinical Issues. 18 December 2014. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf (accessed on 14 June 2022).
- International Conference on Harmonisation. ICH Q5E Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process: Comparability of Biotechnological/Biological Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-5-e-comparability-biotechnological/biological-products-step-5_en.pdf (accessed on 14 June 2022).
- Hye-Na, K.; Thorpe, R.; Knezevica, I. The regulatory landscape of biosimilars: WHO efforts and progress made from 2009 to 2019. Biologicals 2020, 65, 1–9. [Google Scholar]
- Grampp, G.; Ramanan, S. The Diversity of Biosimilar Design and Development: Implications for Policies and Stakeholders. BioDrugs 2015, 29, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Mastrangeli, R.; Satwekar, A.; Cutillo, F.; Ciampolillo, C.; Palinsky, W.; Longobardi, S. In-vivo biological activity and glycosylation analysis of a biosimilar recombinant human follicle-stimulating hormone product (Bemfola) compared with its reference medicinal product (GONAL-f). PLoS ONE 2017, 12, e0184139. [Google Scholar] [CrossRef] [Green Version]
- de Mora, F.; Fauser, B.C.J.M. Biosimilars to recombinant human FSH medicines: Comparable efficacy and safety to the original biologic. Reprod. BioMed. Online 2017, 35, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Guideline on Non-Clinical and Clinical Development of Similar Biological Medicinal Products Containing Recombinant Human Follicle Stimulating Hormone (r-hFSH). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-clinical-development-similar-biological-medicinal-products-containing_en.pdf (accessed on 14 June 2022).
- Rettenbacher, M.; Andersen, A.N.; Garcia-Velasco, J.A.; Sator, M.; Barri, P.; Lindenberg, S.; van der Ven, K.; Khalaf, Y.; Bentin-Ley, U.; Obruca, A.; et al. A multi-centre phase 3 study comparing efficacy and safety of Bemfola® versus Gonal-f® in women undergoing ovarian stimulation for IVF. Reprod. BioMed. Online 2015, 30, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strowitzki, T.; Kuczynski, W.; Mueller, A.; Bias, P. Randomized, active-controlled, comparative phase 3 efficacy and safety equivalence trial of Ovaleap® (recombinant human follicle-stimulating hormone) in infertile women using assisted reproduction technology (ART). Reprod. Biol. Endocrinol. 2016, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braam, S.C.; de Bruin, J.P.; Buisman, E.; Brandes, M.; Nelen, W.; Smeenk, J.M.J.; van der Steeg, J.W.; Mol, B.W.J.; Hamilton, C. Treatment strategies and cumulative live birth rates in WHO-II ovulation disorders. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 84–89. [Google Scholar] [CrossRef]
- Germond, M.; Urner, F.; Chanson, A.; Primi, M.P.; Wirthner, D.; Senn, A. What is the most relevant standard of success in assisted reproduction?: The cumulated singleton/twin delivery rates per oocyte pick-up: The CUSIDERA and CUTWIDERA. Hum. Reprod. 2004, 19, 2442–2444. [Google Scholar] [CrossRef] [Green Version]
- Malizia, B.A.; Hacker, M.R.; Penzias, A.S. Cumulative live-birth rates after in vitro fertilization. N. Engl. J. Med. 2009, 360, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Nayudu, P.L.; Vitt, U.A.; Barrios De Tomasi, J.; Pancharatna, K.; Ulloa-Aguirre, A. Intact follicle culture: What it can tell us about the roles of FSH glycoforms during follicle development. Reprod. BioMed. Online 2002, 5, 240–253. [Google Scholar] [CrossRef]
- Selman, H.; Pacchiarotti, A.; El-Danasouri, I. Ovarian stimulation protocols based on follicle-stimulating hormone glycosylation pattern: Impact on oocyte quality and clinical outcome. Fertil. Steril. 2009, 94, 1782–1786. [Google Scholar] [CrossRef]
- Orvieto, R.; Seifer, D.B. Biosimilar FSH preparations- are they identical twins or just siblings? Reprod. Biol. Endocrinol. 2016, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Chua, S.J.; Mol, B.W.; Longobardi, S.; Orvieto, R.; Venetis, C.A.; Lispi, M.; Storr, A.; D’Hooghe, T. Biosimilar recombinant follitropin alfa preparations versus the reference product (Gonal-F(R)) in couples undergoing assisted reproductive technology treatment: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 19, 51. [Google Scholar] [CrossRef]
- Andersen, C.; Ezcurra, D. Human steroidogenesis: Implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod. Biol. Endocrinol. 2014, 12, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, J.A.; Ulloa-Aguirre, A. New Human Follitropin Preparations: How Glycan Structural Differences May Affect Biochemical and Biological Function and Clinical Effect. Front. Endocrinol. 2021, 12, 636038. [Google Scholar] [CrossRef] [PubMed]
- Bousfield, G.R.; Butnev, V.Y.; White, W.K.; Hall, A.S.; Harvey, D.J. Comparison of Follicle-Stimulating Hormone Glycosylation Microheterogenity by Quantitative Negative Mode Nano-Electrospray Mass Spectrometry of Peptide-N Glycanase-Released Oligosaccharides. J. Glycom. Lipidom. 2015, 5, 129. [Google Scholar] [CrossRef] [PubMed]
- Wide, L.; Eriksson, K. Low-glycosylated forms of both FSH and LH play major roles in the natural ovarian stimulation. Ups. J. Med. Sci. 2018, 123, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Zambrano, E.; Olivares, A.; Mendez, J.P.; Guerrero, L.; Díaz-Cueto, L.; Veldhuis, J.D.; Ulloa-Aguirre, A. Dynamics of basal and gonadotropin-releasing hormone-releasable serum follicle-stimulating hormone charge isoform distribution throughout the human menstrual cycle. J. Clin. Endocrinol. Metab. 1995, 80, 1647–1656. [Google Scholar]
- Riccetti, L.; Sperduti, S.; Lazzaretti, C.; Klett, D.; De Pascali, F.; Paradiso, E.; Limoncella, S.; Potì, F.; Tagliavini, S.; Trenti, T.; et al. Glycosylation Pattern and in vitro Bioactivity of Reference Follitropin alfa and Biosimilars. Front. Endocrinol. 2019, 10, 503. [Google Scholar] [CrossRef] [Green Version]
- Ulloa-Aguirre, A.; Timossi, C.; Mendez, J.P. Is there any physiological role for gonadotrophin oligosaccharide heterogeneity in humans? I. Gondatrophins are synthesized and released in multiple molecular forms. A matter of fact. Hum. Reprod. 2001, 16, 599–604. [Google Scholar] [CrossRef]
- Bousfield, G.R.; Harvey, D.J. Follicle-Stimulating Hormone Glycobiology. Endocrinology 2019, 160, 1515–1535. [Google Scholar] [CrossRef]
- Leão Rde, B.; Esteves, S.C. Gonadotropin therapy in assisted reproduction: An evolutionary perspective from biologics to biotech. Clinics 2014, 69, 279–293. [Google Scholar] [CrossRef]
- Walsh, G.; Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006, 24, 1241–1252. [Google Scholar] [CrossRef]
- Zhu, A.; Hurst, R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation 2002, 9, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.A.; Moons, S.J.; Timmermans, S.B.P.E.; de Jong, H.; Boltje, T.J.; Büll, C. Sialic acid O-acetylation: From biosynthesis to roles in health and disease. J. Biol. Chem. 2021, 97, 100906. [Google Scholar] [CrossRef] [PubMed]
- Hermeling, S.; Crommelin, D.J.A.; Schellekens, H.; Jiskoot, W. Structure-immunogenicity relationships of therapeutic proteins. Pharm. Res. 2004, 21, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Bassett, R.; Lispi, M.; Ceccarelli, D.; Grimaldi, L.; Mancinelli, M.; Martelli, F.; Van Dorsselaer, A. Analytical identification of additional impurities in urinary-derived gonadotrophins. Reprod. BioMed. Online 2009, 19, 300–313. [Google Scholar] [CrossRef]
- Lispi, M.; Bassett, R.; Crisci, C.; Mancinelli, M.; Martelli, F.; Ceccarelli, D.; De Bellis, C.; Mendola, D. Comparative assessment of the consistency and quality of a highly purified FSH extracted from human urine (urofollitropin) and a recombinant human FSH (follitropin α). Reprod. BioMed. Online 2006, 13, 179–193. [Google Scholar] [CrossRef]
- Meher, B.R.; Dixit, A.; Bousfield, G.R.; Lushington, G.H. Glycosylation Effects on FSH-FSHR Interaction Dynamics: A Case Study of Different FSH Glycoforms by Molecular Dynamics Simulations. PLoS ONE 2015, 10, e0137897. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Dias, J.A.; He, X. Structural biology of glycoprotein hormones and their receptors: Insights to signalling. Mol. Cell. Endocrinol. 2014, 382, 424–451. [Google Scholar] [CrossRef]
- Barrios-De-Tomasi, J.; Timossi, C.; Merchant, H.; Quintanar, A.; Avalos, J.M.; Andersen, C.Y.; Ulloa-Aguirre, A. Assessment of the in vitro and in vivo biological activities of the human follicle-stimulating isohormones. Mol. Cell. Endocrinol. 2002, 186, 189–198. [Google Scholar] [CrossRef]
- Asian-Pacific Biotech News. An Exclusive on LG Life Sciences. Available online: https://www.asiabiotech.com/12/1203/0022_0028.pdf (accessed on 14 June 2022).
- Barakhoeva, Z.; Vovk, L.; Fetisova, Y.; Marilova, N.; Ovchinnikova, M.; Tischenko, M.; Scherbatyuk, Y.; Kolotovkina, A.; Miskun, A.; Kasyanova, G.; et al. A multicenter, randomized, phase III study comparing the efficacy and safety of follitropin alpha biosimilar and the original follitropin alpha. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 241, 6–12. [Google Scholar] [CrossRef]
- Drugs.com. Follitrope. Available online: https://www.drugs.com/international/follitrope.html. (accessed on 14 June 2022).
- Generics and Biosimilar Initiative. Folisurge Summary of Product Characteristics. Available online: https://www.gabionline.net/biosimilars/general/Similar-biologics-approved-and-marketed-in-India (accessed on 14 June 2022).
- Hu, L.; Zhang, S.; Quan, S.; Lv, J.; Qian, W.; Huang, Y.; Lu, W.; Sun, Y. Efficacy and safety of recombinant human follicle-stimulating hormone in patients undergoing in vitro fertilization-embryo transfer. Aging 2020, 12, 4918–4930. [Google Scholar] [CrossRef]
- Medsintez Plant. The First Russian Medicine for Infertility, Primapur® by Medsintez Plant, Will Reach Clinics in the New Year. Available online: http://www.medsintez.com/en/novosti/199-the-first-russian-medicine-for-infertility-primapur-by-medsintez-plant-will-reach-clinics-in-the-new-year (accessed on 14 June 2022).
- PR Newswire. China Recombinant Human Follitropin Market Report 2018–2022. Featuring Merck Serono (GONAL-f) & GeneScience Pharmaceuticals Co., Ltd. (Jinsaiheng). Available online: https://www.prnewswire.com/news-releases/china-recombinant-human-follitropin-market-report-2018-2022-featuring-merck-serono-gonal-f-genescience-pharmaceuticals-co-ltd-jinsaiheng-300776822.html (accessed on 14 June 2022).
- Budani, M.C.; Fensore, S.; Di Marzio, M.; Tiboni, G.M. Efficacy and safety of follitropin alpha biosimilars compared to their reference product: A meta-analysis. Gynecol. Endocrinol. 2020, 37, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Vitt, U.A.; Nayudu, P.L.; Rose, U.M.; Kloosterboer, H.J. Embryonic development after follicle culture is influenced by follicle-stimulating hormone isoelectric point range. Biol. Reprod. 2001, 65, 1542–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.; Westergaard, L.; van Wely, M. FSH isoform composition of commercial gonadotrophin preparations: A neglected aspect? Reprod BioMed. Online 2004, 9, 231–236. [Google Scholar] [CrossRef]
- Heikinheimo, O.; Bitzer, J.; Garcia Rodriguez, L. Real-world research and the role of observational data in the field of gynaecology—A practical review. Eur. J. Contracept. Reprod. Health Care 2017, 22, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Benchaib, M.; Grynberg, M.; Cedrin-Durnerin, I.; Raguideau, F.; Lennon, H.; Castello-Bridoux, C.; Paillet, S.; Porte, F.; Verpillat, P.; Van Hille, B.; et al. Effectiveness and treatment cost of assisted reproduction technology for women stimulated by gonadotropin in France: A cohort study using the National Health Database. Hum. Reprod. 2021, 36, deab126.022. [Google Scholar] [CrossRef]
- Iegemiddelverk, S. Decision on Admission to the Exchange List for Follitropin Alfa. Available online: https://legemiddelverket.no/ (accessed on 14 June 2022).
- Australian Government Department of Health. Therapeutic Goods Administration. Australian Public Assessment Report for Follitropin Alfa. Available online: https://www.tga.gov.au/sites/default/files/auspar-follitropin-alfa-rch-160408.docx (accessed on 14 June 2022).
- Sinegubova, M.; Vorobiev, I.; Klishin, A.; Eremin, D.; Orlova, N.; Orlova, N.; Polzikov, M. Purification Process of a Recombinant Human Follicle Stimulating Hormone Biosimilar (Primapur®) to Yield a Pharmaceutical Product with High Batch-to-Batch Consistency. Pharmaceutics 2022, 14, 96. [Google Scholar] [CrossRef]
- Pignataro, M.F.; Herrera, M.G.; DoderClick or tap here to enter text.o, V.I. Evaluation of Peptide/Protein Self-Assembly and Aggregation by Spectroscopic Methods. Molecules 2020, 25, 4854. [Google Scholar] [CrossRef]

| Product | Country/Company of Origin | Identifier | Batch Number * |
|---|---|---|---|
| GONAL-f | European Union; Merck Healthcare KGaA | B1 | BA057111 |
| B2 | BA064607 | ||
| Primapur | Russia; iVFarma, LLC, Russia | B1 | 0011019A102 |
| B2 | 0010220B02 | ||
| Jin Sai Heng | China; Genescience Pharmaceuticals Co., Ltd. | B1 | 201812049 |
| B2 | 055201910044 | ||
| Corneumon | Mexico; Laboratorios Corne, SA de CV | - | RFU19002 |
| Follitrope | South Korea; LG Life Sciences | B1 | RFV19009 |
| B2 | RFV199005 | ||
| Folisurge | India; Intas Pharmaceuticals Ltd. | B1 | 7070091 |
| B2 | 7150015 | ||
| B3 | 7150014 | ||
| B4 | 7070093 |
| Glycosylation Site | Index | GONAL-f® | Corneumon® | Primapur® | Jin Sai Heng® | |||
|---|---|---|---|---|---|---|---|---|
| BA057111 | BA064607 | RFU19002 | 0011019A102 | 0010220B02 | 201812049 | 55201910044 | ||
| Asn52-α | NGNA index | 0.8 | 0.9 | 0.1 | 0.2 | 0.9 | 4.5 | 4.0 |
| Acetyl index | 1.9 | 1.6 | 1.6 | 1.7 | 1.0 | 35.0 | 33.2 | |
| Asn78-α | NGNA index | 1.0 | 1.2 | 0.2 | 0.3 | 1.0 | 5.5 | 4.6 |
| Acetyl index | 3.9 | 3.5 | 3.6 | 4.0 | 2.6 | 44.7 | 43.0 | |
| Asn7-β | NGNA index | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Acetyl index | 2.7 | 2.3 | 1.7 | 1.7 | 1.2 | 28.3 | 29.1 | |
| Asn24-β | NGNA index | 0.5 | 0.7 | 0.1 | 0.2 | 0.7 | 3.1 | 2.8 |
| Acetyl index | 5.4 | 4.7 | 3.1 | 5.3 | 2.8 | 58.0 | 57.8 | |
| Average | NGNA index | 0.6 | 0.7 | 0.1 | 0.2 | 0.6 | 3.3 | 2.8 |
| Acetyl index | 3.5 | 3.0 | 2.5 | 3.2 | 1.9 | 41.5 | 40.8 | |
| Glycosylation Site | Index | GONAL-f® | Follitrope® | Folisurge® | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BA057111 | BA064607 | Korea | Korea | India | India | India | India | |||
| RFV19009 | RFV19005 | 7070091 | 7150015 | 7150014 | 7070093 | |||||
| Asn52-α | NGNA index | 0.6 | 0.7 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Acetyl index | 2.0 | 1.5 | 1.2 | 1.3 | 6.0 | 5.7 | 4.6 | 4.9 | ||
| Asn78-α | NGNA index | 0.9 | 1.1 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | |
| Acetyl index | 2.7 | 2.1 | 2.4 | 2.4 | 7.6 | 7.5 | 6.0 | 6.4 | ||
| Asn7-β | NGNA index | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Acetyl index | 2.5 | 1.9 | 1.3 | 1.3 | 8.3 | 8.4 | 7.5 | 7.6 | ||
| Asn24-β | NGNA index | 0.4 | 0.4 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | |
| Acetyl index | 5.6 | 4.2 | 3.1 | 3.0 | 16.1 | 15.4 | 13.7 | 14.4 | ||
| Average | NGNA index | 0.5 | 0.5 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | |
| Acetyl index | 3.2 | 2.4 | 2.0 | 2.0 | 9.5 | 9.2 | 8.0 | 8.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzi, L.; Sepe, N.; Migliaccio, W.; Lanzoni, L.; Iozzino, L.; D’Angelo, F.; Colarusso, L.; Montenegro, S.; Palmese, A.; D’Hooghe, T.; et al. Comparative Assessment of the Structural Features of Originator Recombinant Human Follitropin Alfa Versus Recombinant Human Follitropin Alfa Biosimilar Preparations Approved in Non-European Regions. Int. J. Mol. Sci. 2022, 23, 6762. https://doi.org/10.3390/ijms23126762
Manzi L, Sepe N, Migliaccio W, Lanzoni L, Iozzino L, D’Angelo F, Colarusso L, Montenegro S, Palmese A, D’Hooghe T, et al. Comparative Assessment of the Structural Features of Originator Recombinant Human Follitropin Alfa Versus Recombinant Human Follitropin Alfa Biosimilar Preparations Approved in Non-European Regions. International Journal of Molecular Sciences. 2022; 23(12):6762. https://doi.org/10.3390/ijms23126762
Chicago/Turabian StyleManzi, Lucio, Nunzio Sepe, Walter Migliaccio, Ludovica Lanzoni, Luisa Iozzino, Fabrizia D’Angelo, Lucia Colarusso, Susana Montenegro, Angelo Palmese, Thomas D’Hooghe, and et al. 2022. "Comparative Assessment of the Structural Features of Originator Recombinant Human Follitropin Alfa Versus Recombinant Human Follitropin Alfa Biosimilar Preparations Approved in Non-European Regions" International Journal of Molecular Sciences 23, no. 12: 6762. https://doi.org/10.3390/ijms23126762
APA StyleManzi, L., Sepe, N., Migliaccio, W., Lanzoni, L., Iozzino, L., D’Angelo, F., Colarusso, L., Montenegro, S., Palmese, A., D’Hooghe, T., Ulloa-Aguirre, A., Koloda, Y., & Lispi, M. (2022). Comparative Assessment of the Structural Features of Originator Recombinant Human Follitropin Alfa Versus Recombinant Human Follitropin Alfa Biosimilar Preparations Approved in Non-European Regions. International Journal of Molecular Sciences, 23(12), 6762. https://doi.org/10.3390/ijms23126762






